Abstract
Introduction
Our objective was to perform a systematic review of the outcomes of various frostbite treatments to determine which treatments are effective. We also planned to perform meta-analyses of the outcomes of individual treatments for which suitable data were available.
Main Body
We performed a systematic review and meta-analyses in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. We searched PubMed, Cochrane Trials, and EMBase to identify primary references from January 1, 1900, to June 18, 2022. After eliminating duplicates, we screened abstracts to identify eligible studies containing information on treatment and outcomes of Grade 2 to 4 frostbite. We performed meta-analyses of groups of articles that provided sufficient data. We registered our review in the prospective registry of systematic reviews PROSPERO (Nr. 293,693).
We identified 4,835 potentially relevant studies. We excluded 4,610 studies after abstract screening. We evaluated the full text of the remaining 225 studies, excluding 154. Ultimately, we included 71 articles with 978 cases of frostbite originating from 1 randomized controlled trial, 20 cohort studies and 51 case reports. We found wide variations in classifications of treatments and outcomes. The two meta-analyses we performed both found that patients treated with thrombolytics within 24 h had better outcomes than patients treated with other modalities. The one randomized controlled trial found that the prostacyclin analog iloprost was beneficial in severe frostbite if administered within 48 h.
Conclusions
Iloprost and thrombolysis may be beneficial for treating frostbite. The effectiveness of other commonly used treatments has not been validated. More prospective data from clinical trials or an international registry may help to inform optimal treatment.
Similar content being viewed by others
Background
Frostbite is a local tissue injury caused by cold exposure with freezing [1, 2]. After tissue reaches subfreezing temperatures, intra- and extracellular ice formation cause electrolyte and pH shifts as well as cell membrane disruption, resulting in cell death and tissue destruction [3]. When tissue rewarms, reperfusion injury can cause inflammation, vasoconstriction, thrombus formation, endothelial damage, edema, and ischemia with further tissue damage [4]. Clinically, frostbite presents with a wide spectrum of injury ranging from no loss of tissue to extensive necrosis requiring amputations [1, 2].
Regardless of treatment, meticulous wound care with delayed debridement is critical [5]. Acute treatment often aims to reverse vasoconstriction and thrombosis to limit progression of injury. Although there is only one published randomized controlled trial studying the relative effectiveness of various frostbite treatments, there are many retrospective studies, case reports, and case series, reporting the results of various treatments with varying rates of tissue salvage [6, 7]. Pharmacologic treatment can use thrombolytics, such as recombinant tissue plasminogen activator (tPA), vasodilators, such as iloprost, a systemic prostacyclin analog, [8,9,10] and nifedipine, as well as phosphodiesterase inhibitors, such as sildenafil [7]. Regional anesthesia with peripheral nerve blocks [11], surgical sympathectomies [12] and colloid infusions [13] have also been used for their vasodilatory effects. Adjunctive pharmacologic treatments can include cyclooxygenase inhibitors, such as acetylsalicylic acid (ASA), and ibuprofen [5, 14], platelet inhibitors such as clopidogrel [15], anticoagulation with heparin or low-molecular weight heparin (LMWH), [16, 17] and hyperbaric oxygen therapy (HBOT) [18]. Medications for pain relief include opiates and other drugs without significant anti-inflammatory effect [5, 7].
Thus far, no study has systematically evaluated the outcome of frostbite treatment based on different treatment strategies. Here, we aimed to systematically evaluate the accessible literature regarding the effectiveness of various approaches to treating frostbite. Where sufficient data existed, we compiled meta-analyses for individual treatment methods.
Methods
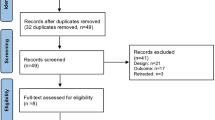
We performed a systematic review and meta-analyses in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA—www.prisma-statement.org). We registered our review in the prospective registry of systematic reviews, PROSPERO (293,693) [19]. Figure 1 shows the article selection process.
Search strategy, and study selection
We used PubMed (search terms "Cold Injury"[Mesh] OR frostbit*[tiab] OR cold injur*[tiab]), Cochrane Trials (frostbit*:ab,ti,kw OR cold injur*:ab,ti,kw OR MeSH descriptor: [Cold Injury] explode all trees), and EMBase (frostbit*:ab,ti OR 'cold injur*':ab,ti) to identify primary references from January 1, 1900 to June 18, 2022. After elimination of duplicates, we reviewed the citations for eligible studies selecting studies based on the abstracts. We then read the full texts of the selected studies to identify those suitable for inclusion in the review. We assessed methodologic quality using the ROBIS tool [20] to minimize risk of bias.
Eligibility criteria
We identified studies that contained information about treatments and outcomes of Grade 2 to 4 frostbite injuries [21]. We included only articles in which specific outcomes for specific treatments could be determined. We screened articles in English, French, German, Italian, and Spanish. We excluded data from patients with severe comorbidities (American Society of Anesthesiologists Physical Status Class ≥ 3) [22]. Although the ASA classification is typically used for preoperative evaluation, we used the system to exclude patients with comorbidities that could affect frostbite treatment outcomes. We also excluded reports of patients with flash freezing injuries caused by accidental skin exposure to cryogenic materials, such as dry ice and liquid nitrogen. We excluded data from pediatric cases (< 18 years of age) if age was reported. In two studies, it was not possible to exclude pediatric data [23, 24]. We excluded abstracts, presentations, conference proceedings, and reviews.
Data extraction and classification
One reviewer (IR) extracted the data from the selected studies. For each study the following information was extracted and summarized: sex, age, body part(s) involved, frostbite grade [21], prehospital treatment, in-hospital treatment, and outcome.
We classified frostbite using the system described by Cauchy et al. in 2001 [21]. It was a challenge to synthesize data from a century of literature with a diverse array of frostbite classifications. We attempted to ensure consistency by applying this uniform classification to earlier studies and to studies that used alternative classifications, classifying frostbite cases based on the published images and descriptions.
We focused on treatments that might influence the pathogenesis of frostbite: vasodilation (iloprost, sympathectomy, and colloid solution infusion), inhibition of platelet aggregation (ASA, ibuprofen, and clopidogrel), anticoagulation (heparin and LMWH), thrombolysis (tPA), and optimization of tissue oxygenation (HBOT). We classified interventions such as wound care and analgesic treatment with opiates and other drugs without significant anti-inflammatory effects as conservative treatment.
Data analysis
We used multiple methods to quantify the outcomes of frostbite treatments, because there was significant heterogeneity of data reporting among the studies.
(Modified) Hennepin Score
The Hennepin Score is primarily used for research purposes, offering an objective means of evaluating frostbite severity and facilitating outcome comparisons across various studies [25]. A numeric value is assigned to each frostbitten phalanx, digit, toe, and limb at risk, as indicated by low or no perfusion on a triple-phase bone scan (99mTc scintigraphy). This defines the tissue-at-risk score (R). If an article did not report this method, we based R on a modified Hennepin Score by assessing the clinical appearance of the extremities. We then estimated the amputation score (A), using the same method. The difference between R and A is the tissue salvage score: (S): S = R – A. The ratio between S and R gives the tissue salvage rate (TSR), expressed as a percentage (S / R × 100).
Digit salvage rate
The digit salvage rate uses the ratio of the digits at risk (DR] and digits amputated (DA] expressed as a percentage: (1-(DA/DR) × 100) [8].
Phalanx salvage rate
The phalanx salvage rate uses the ratio of the phalanges at risk (PR) and the phalanges amputated (PA) expressed as percentage: (1-PA/PR) × 100 [26].
Amputation score
Whenever other metrics were not applicable or not available to quantify frostbite treatment outcomes, we analyzed the frequency of cases resulting in amputation. In this approach, treatment success is evaluated by monitoring the number of patients who ultimately undergo amputation as a result of frostbite compared to the total number of patients with frostbite in a given study. Although it does not provide as much detail as other metrics, it still provides insight into the effectiveness of various treatments.
Statistical analysis
When feasible, we conducted meta-analyses to evaluate various treatments using both fixed effects and random effects models. This was achievable when at least two studies examined similar treatments (for instance, administering the same substance in the intervention group) in patient populations with comparable characteristics. In the first meta-analysis, we analyzed the salvage rate using the Hennepin Score. We estimated the between-study variance (τ2) using the DerSimonian-Laird method. In the second meta-analysis, we analyzed the risk ratio of amputation probability of a digit at risk, estimating τ2 using the Paule-Mandel method. We estimated heterogeneity using the statistic I2. We used R version 4.0.4 statistical software with meta and metasens libraries for these analyses [27, 28].
Results
After eliminating duplicate entries, we found 4,835 studies that were potentially relevant (Fig. 1). We then assessed the abstracts excluding 4,610 articles, primarily for lack of relevance or insufficient data. We obtained the full texts of the remaining 225 studies for further evaluation. We rejected 154 studies that failed to meet our eligibility criteria, primarily because they lacked adequate information about treatments or outcomes. Our selection procedure led to the inclusion of 978 frostbite cases from the remaining 71 articles.
Table 1 displays the baseline demographics, with men representing 78% of the cases, women 17%, and 5% not specifying sex. Upper and lower extremities were affected equally. A total of 873 cases were from studies that reported treatments across different patient cohorts (Additional file 1: eTables 2–5), while the remaining 105 cases were reported in 51 case reports and case series (Additional file 1: eTable 1).
Table 2 shows the treatment outcomes of cohort studies, grouped according to the methods used to quantify the outcomes of different frostbite treatments. Table 2 presents the treatment outcomes from cohort studies, organized according to the methods employed to measure the outcomes of various frostbite treatments. Patients receiving thrombolysis, iloprost, or a combination experienced higher tissue and digit salvage rates than other patients.. The Yukon frostbite protocol led to an increased digit salvage rate (overall 80%, with 100% for grade 2 -3 and 50% for grade 4 frostbite) compared to historical controls (31% for grade 2, 67% for grade 3 and 98%–100% for grade 4 frostbite) in patients with grade 2–4 frostbite treated with iloprost and patients with grade 4 frostbite who also received tPA and heparin [10, 21].
Among the 20 studies in our review, only seven included more than one treatment modality [8, 24, 26, 29,30,31,32]. Six studies compared patients treated with and without thrombolysis.
We conducted two meta-analyses. The first used the two studies that measured treatment outcomes with the Hennepin Score (Tissue Salvage Rate (TSR)) (Fig. 2), [24, 31]. The second used two studies that calculated digit salvage rates (DSR) as outcome measures [30, 32]. In both cases, thrombolytics significantly improved tissue (Fig. 2A) or digit (Fig. 2B) salvage rates, regardless of whether a common effects model or a random effects model was applied. The first meta-analysis reported a mean TSR difference of 25% (95% CI 8% – 42%) for both models, while the second showed a risk ratio of 5 (95% CI 3 – 7) for both models. Two studies allowed only binary distinctions of treatment success (amputated or not amputated) [26, 29] A combined meta-analysis was not feasible because of differences in patient populations (grade 2 vs. grade 3–4 frostbite).
Meta-analyses of studies comparing thrombolysis and conservative treatment. Mean difference of the salvage rate of the Hennepin Frostbite Score (A) and risk ratio of the amputation probability of a digit at risk (B). The between-study variance τ2 was estimated by means of DerSimonian-Laird (A) and Paule-Mandel (B) methods. P-value refers to the heterogeneity test. I2, heterogeneity statistic; τ2, between-study variance; CI, confidence interval; MD, mean difference; N, numerosity; RR, risk ratio; SD, standard deviation; SR, salvage rate; tPA, tissue plasminogen activator
Table 3 shows the number of individual cases for each treatment. Because of variations in treatments among articles, there were generally few articles for each treatment. The only published prospective, controlled, randomized study [8] reported significantly better outcomes for patients treated with iloprost. This was a small study (n = 47) (Table 4).
Discussion
We found considerable variability in treatments and outcome classifications. We were often only able to describe outcome data instead of reporting quantitative results. Also, older treatments such as surgical sympathectomy have become obsolete with the advent of newer regional anesthesia techniques such as peripheral nerve blocks for temporary pharmacologic sympatholysis.
Our results suggest that thrombolysis or intravenous iloprost is effective when administered promptly to treat severe frostbite. For grade 3–4 frostbite the Wilderness Medical Society frostbite guidelines recommend the use of intravenous iloprost within 48 h of injury, and thrombolysis within 24 h of injury [5]. The Helsinki protocol recommends the use of tPA for patients with grade 3–4 frostbite presenting within 48 h of injury with angiographic evidence of thrombosis [9]. Patients with contraindications to thrombolysis (platelet count < 100 × 109/L, hematocrit < 30%), signs of vasospasm on angiography, or poor response to thrombolysis should be treated with iloprost. A retrospective analysis found an 81% tissue salvage rate using the Hennepin score for 20 patients with grade 3–4 frostbite treated using the Helsinki protocol [9]. The Yukon protocol recommends treatment of patients with grade 3–4 frostbite presenting within 72 h of injury with iloprost [10]. Patients with grade 4 frostbite presenting within 24 h of injury, should also receive tPA.
Iloprost is a synthetic prostaglandin I2 that has been used to treat frostbite [33]. Like other prostacyclins, it inhibits platelet aggregation and promotes vasodilation [34]. Iloprost may stimulate the release of endogenous tissue plasminogen activator or counteract its inhibitory effects [35]. Iloprost reduces vasoconstriction induced by thromboxane A2 [36], and may reduce oxidative stress from free radicals, moderating reperfusion injury [37, 38]. The effect on platelet aggregation may be reversed within two hours), but prostacyclin effects may disrupt the vicious cycle of activated platelets and leukocytes that damages endothelium [35, 39].
Thrombolytics work by binding to fibrin within a thrombus and activating plasminogen, causing local fibrinolysis and inhibiting blood clot formation. [40] A systematic review found comparable limb salvage rates for frostbite patients treated with intra-arterial and intravenous thrombolysis, (76% vs. 77%) [41]. Use of thrombolytics is associated with a risk of hemorrhage. A study of bleeding complications in patients with severe frostbite treated using intravenous tPA found that 8% of patients developed bleeding that necessitated changes in management [42].
Recommendations to treat of frostbite with low molecular weight dextran, ibuprofen, or topical aloe vera are based on mechanistic reasoning or animal studies, rather than clinical data. The limited number of reports on these treatments precluded us from drawing conclusions about their clinical effectiveness. Low molecular weight dextran, thought to reduce blood viscosity and inhibit thrombus formation, has demonstrated decreased necrosis in animal models of frostbite [13, 43, 44], but is not available in many countries. Nonsteroidal anti-inflammatory drugs (NSAIDs) block the effects of cyclo-oxygenases, decreasing the production of prostaglandins and thromboxane, mediators that can cause increased vasoconstriction, ischemia, and inflammatory tissue damage [3, 45]. An experimental rabbit frostbite model showed improved tissue survival in animals treated with ASA. [14] Because aspirin causes irreversible inhibition of cyclooxygenase that could disrupt wound healing, some authors have suggested that ibuprofen might be preferred as a treatment for frostbite [5, 46]. There are no head-to-head comparison studies. Topical aloe vera reduces formation of prostaglandins and thromboxane and has been shown to increase tissue survival in an experimental frostbite rabbit model [14], but has not been studied in humans.
A study of patients with grade 3–4 frostbite treated with HBOT, aspirin, and iloprost suggested a possible benefit of HBOT [18]. This multicenter prospective single-arm study compared outcomes with those of a historical cohort treated with aspirin and iloprost, but not HBOT and found that the group receiving HBOT had a significantly higher number of preserved tissue segments per patient. The reduction in amputation rates was also more pronounced in patients treated with HBOT.
Theoretically, hypobaric hypoxia at high altitudes might exacerbate the risk and severity of frostbite. A retrospective study reported that frostbite severity increased disproportionately at altitudes above 5,200 m [47]. These data are confounded by colder temperatures and stronger winds at higher elevations.
Among the various methods to measure frostbite injuries, we regard the Hennepin score as the most precise. The original report of the Hennepin score found a negative correlation of S and R (correlation coefficient, − 0.14, p = 0.001) in frostbite patients who underwent Tc-99 m three-phase bone scans of the affected extremities, with high consistency between evaluators (correlation coefficient: 0.93) [25].
Limitations
The reported treatments exhibited considerable variations with a wide array of medications administered at different doses and frequencies. The studies were heterogeneous, with various frostbite classification systems. Our retrospective classification may have introduced errors.
Many studies were low quality. There was only one randomized controlled trial. Direct medication comparisons were scarce. There was only one multicenter study. Our analysis could not account for coexisting injuries or conditions such as trauma, volume depletion, or hypothermia. We were also unable to control for other potential confounders, such as mechanical damage to the injury site, the method of rewarming and the quality of wound care.
Conclusions
Iloprost and thrombolysis may be beneficial for treating frostbite. The effectiveness of other commonly used treatments has not been validated. More prospective data from clinical trials or international registry may help to inform optimal treatment. Because there is a low incidence of severe frostbite at any single institution, conducting multicenter trials, or establishing international registries could help reach higher levels of evidence.
Availability of data and materials
Data derived from existing literature can be found within the main article as well as in the supplementary tables. The source data can be obtained from the respective publications cited in the reference section.
Abbreviations
- ASA:
-
Acetylsalicylic acid
- CI:
-
Confidence interval
- DA:
-
Digits amputated
- DR:
-
Digits at risk
- DSR:
-
Digit salvage rate
- HBOT:
-
Hyperbaric oxygen therapy
- LMWH:
-
Low-molecular weight heparin
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- PA:
-
Phalanges amputated
- PR:
-
Phalanges at risk
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analysis
- PROSPERO:
-
Prospective registry of systematic reviews
- RCT:
-
Randomized controlled trial
- ROBIS:
-
Risk of bias in systematic reviews
- tPA:
-
Tissue plasminogen activator
- TSR:
-
Tissue salvage rate
References
Cauchy E, Zafren K, Imray C. Frostbite. In: Brugger H, Festi L, Zafren K, Paal P, Strapazzon G, eds. Mountain Emergency Medicine. Edra; 2021:253–267:chap Frostbite.
Freer L, Handford C, Imray C. Frosbite. In: Auerbach PS, ed. Auerbach's Wilderness Medicine. 7th ed. Elsevier; 2017:197–222:chap Frostbite.
Regli IB, Strapazzon G, Falla M, Oberhammer R, Brugger H. Long-term sequelae of frostbite-a scoping review. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18189655.
Manson PN, Jesudass R, Marzella L, Bulkley GB, Im MJ, Narayan KK. Evidence for an early free radical-mediated reperfusion injury in frostbite. Free Radical Biol Med. 1991;10(1):7–11. https://doi.org/10.1016/0891-5849(91)90015-u.
McIntosh SE, Freer L, Grissom CK, et al. Wilderness medical society clinical practice guidelines for the prevention and treatment of frostbite: 2019 update. Wilderness Environ Med. 2019;30(4s):S19-s32. https://doi.org/10.1016/j.wem.2019.05.002.
Sheridan RL, Goverman JM, Walker TG. Diagnosis and treatment of frostbite. N Engl J Med. 2022;386(23):2213–20. https://doi.org/10.1056/NEJMra1800868.
Cauchy E, Davis CB, Pasquier M, Meyer EF, Hackett PH. A new proposal for management of severe frostbite in the austere environment. Wilderness Environ Med. 2016;27(1):92–9. https://doi.org/10.1016/j.wem.2015.11.014.
Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite letter randomized controlled trial. New England J Med. 2011;364(2):189–90. https://doi.org/10.1056/NEJMc1000538.
Lindford A, Valtonen J, Hult M, et al. The evolution of the Helsinki frostbite management protocol. Burns : J Int Soc Burn Injuries. 2017;43(7):1455–63. https://doi.org/10.1016/j.burns.2017.04.016.
Poole A, Gauthier J, MacLennan M. Management of severe frostbite with iloprost, alteplase and heparin: a Yukon case series. CMAJ Open. 2021;9(2):E585–91. https://doi.org/10.9778/cmajo.20200214.
Pasquier M, Ruffinen GZ, Brugger H, Paal P. Pre-hospital wrist block for digital frostbite injuries. High Alt Med Biol. 2012;13(1):65–6. https://doi.org/10.1089/ham.2011.1072.
Shumacker HBJ, Kilman JW. Sympathectomy in the treatment of frostbite. Arch Surg. 1964;89(3):575–84. https://doi.org/10.1001/archsurg.1964.01320030165029.
Kapur BM, Gulati SM, Talwar JR. Low molecular dextran in the management of frostbite in monkeys. Indian J Med Res. 1968;56(11):1675–81.
Heggers JP, Robson MC, Manavalen K, et al. Experimental and clinical observations on frostbite. Ann Emerg Med. 1987;16(9):1056–62. https://doi.org/10.1016/s0196-0644(87)80758-8.
Garg I, Baladron Zanetti MJ, Kendi AT. Bone scan in evaluation of bone viability in severe frostbite of the hand. Indian J Nucl Med Oct-Dec. 2017;32(4):367–8. https://doi.org/10.4103/ijnm.IJNM_53_17.
Theis FV, O’Connor WR, Wahl FJ. ANTICOAGULANTS IN ACUTE FROSTBITE. J Am Med Assoc. 1951;146(11):992–5. https://doi.org/10.1001/jama.1951.03670110012004.
Hödl S. Zur Therapie der Erfrierungen (2005) Wiener Medizinische Wochenschrift 2005/04/01 155(7): 199–203. doi: https://doi.org/10.1007/s10354-005-0165-5
Magnan M-A, Gayet-Ageron A, Louge P, et al. Hyperbaric oxygen therapy with iloprost improves digit salvage in severe frostbite compared to iloprost alone. Medicina (Kaunas). 2021;57(11):1284. https://doi.org/10.3390/medicina57111284.
Regli IB, Oberhammer R, Zafren K, Strapazzon G, Brugger H. Frostbite: Systematic Review on treatment and outcome. PROSPERO 2021 CRD42021293693
Whiting P, Savović J, Higgins JP, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. https://doi.org/10.1016/j.jclinepi.2015.06.005.
Cauchy E, Chetaille E, Marchand V, Marsigny B. Retrospective study of 70 cases of severe frostbite lesions: a proposed new classification scheme. Wilderness Environ Med Winter. 2001;12(4):248–55. https://doi.org/10.1580/1080-6032(2001)012[0248:rsocos]2.0.co;2.
Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status - historical perspectives and modern developments. Anaesthesia. 2019;74(3):373–9. https://doi.org/10.1111/anae.14569.
Gonzaga T, Jenabzadeh K, Anderson CP, Mohr WJ, Endorf FW, Ahrenholz DH. Use of Intra-arterial thrombolytic therapy for acute treatment of frostbite in 62 patients with review of thrombolytic therapy in frostbite. J Burn Care & Res : Official Public Am Burn Assoc. 2016;37(4):e323-34. https://doi.org/10.1097/bcr.0000000000000245.
Nygaard RM, Lacey AM, Lemere A, et al. Time matters in severe frostbite: assessment of limb/digit salvage on the individual patient level. J Burn Care & Research : Official Publication Am Burn Assoc. 2017;38(1):53–9. https://doi.org/10.1097/bcr.0000000000000426.
Nygaard RM, Whitley AB, Fey RM, Wagner AL. The hennepin score: quantification of frostbite management efficacy. J Burn Care & Res : Official Publication Am Burn Assoc. 2016;37(4):e317-22. https://doi.org/10.1097/bcr.0000000000000277.
Paine RE, Turner EN, Kloda D, Falank C, Chung B, Carter DW. Protocoled thrombolytic therapy for frostbite improves phalangeal salvage rates. Burns & Trauma. 2020. https://doi.org/10.1093/burnst/tkaa008.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. https://doi.org/10.1136/ebmental-2019-300117.
Schwarzer G, Carpenter J, Rücker G. Meta-Analysis with R. 2015.
Heard J, Shamrock A, Galet C, Pape KO, Laroia S, Wibbenmeyer L. Thrombolytic use in management of frostbite injuries: eight year retrospective review at a single institution. J Burn Care Res. 2020;41(3):722–6. https://doi.org/10.1093/jbcr/iraa028.
Bruen KJ, Ballard JR, Morris SE, Cochran A, Edelman LS, Saffle JR. 2007 Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Archiv Surg Chicago, Ill. 1960;142(6):546–51.
Dole M, Endorf FW, Gayken J, Fey R, Nygaard RM. Early mobilization in lower extremity frostbite injury: preliminary experience at a single burn center. J Burn Care & Res: Official Publication Am Burn Assoc. 2018;39(3):339–44. https://doi.org/10.1097/bcr.0000000000000590.
Patel N, Srinivasa DR, Srinivasa RN, et al. Intra-arterial thrombolysis for extremity frostbite decreases digital amputation rates and hospital length of stay. Cardiovasc Intervent Radiol. 2017;40(12):1824–31. https://doi.org/10.1007/s00270-017-1729-7.
Groechenig E. Treatment of frostbite with iloprost. Lancet (London, England). 1994;344(8930):1152–3. https://doi.org/10.1016/s0140-6736(94)90657-2.
Kaukinen S, Ylitalo P, Pessi T, Vapaatalo H. Hemodynamic effects of iloprost, a prostacyclin analog. Clin Pharmacol Ther. 1984;36(4):464–9. https://doi.org/10.1038/clpt.1984.205.
Grant SM, Iloprost GKL. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs. 1992;43(6):889–924.
Norel X. Prostanoid receptors in the human vascular wall. ScientificWorldJournal. 2007;7:1359–74. https://doi.org/10.1100/tsw.2007.184.
Iriz E, Iriz A, Take G, et al. Iloprost and vitamin C attenuates acute myocardial injury induced by suprarenal aortic ischemia-reperfusion in rabbits. Bratisl Lek Listy. 2015;116(10):627–31. https://doi.org/10.4149/bll_2015_121.
Erre GL, Passiu G. Antioxidant effect of Iloprost: current knowledge and therapeutic implications for systemic sclerosis. Reumatismo. 2009;61(2):90–7. https://doi.org/10.4081/reumatismo.2009.90.
Schermuly RT, Schulz A, Ghofrani HA, et al. Comparison of pharmacokinetics and vasodilatory effect of nebulized and infused iloprost in experimental pulmonary hypertension: rapid tolerance development. J Aerosol Med Fall. 2006;19(3):353–63. https://doi.org/10.1089/jam.2006.19.353.
Collen D, Lijnen HR. The tissue-type plasminogen activator story. Arterioscler Thromb Vasc Biol. 2009;29(8):1151–5. https://doi.org/10.1161/atvbaha.108.179655.
Drinane J, Kotamarti VS, O’Connor C, et al. Thrombolytic salvage of threatened frostbitten extremities and digits: a systematic review. J Burn Care & Res : Official Publication Am Burn Assoc. 2019;40(5):541–9. https://doi.org/10.1093/jbcr/irz097.
Murphy J, Endorf FW, Winters MK, et al. Bleeding complications in patients with severe frostbite injury. J Burn Care & Res: Official Publication Am Burn Assoc. 2022. https://doi.org/10.1093/jbcr/irac180.
Martínez Villén G, García Bescos G, Rodriguez Sosa V, Morandeira García JR. Effects of haemodilution and rewarming with regard to digital amputation in frostbite injury: an experimental study in the rabbit. J Hand Surgery (Edinburgh, Scotland). 2002;27(3):224–8. https://doi.org/10.1054/jhsb.2001.0743.
Webster DR, Bonn G. Low-molecular-weight dextran in the treatment of experimental frostbite. Can J Surg J Can de chirurgie. 1965;8(4):423–7.
Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17(6):275–342. https://doi.org/10.1007/s10787-009-0016-x.
Robson MC, DelBeccaro EJ, Heggers JP, Loy GL. Increasing dermal perfusion after burning by decreasing thromboxane production. J Trauma. 1980;20(9):722–5. https://doi.org/10.1097/00005373-198009000-00002.
Hashmi MA, Rashid M, Haleem A, Bokhari SA, Hussain T. Frostbite: epidemiology at high altitude in the Karakoram mountains. Ann R Coll Surg Engl. 1998;80(2):91–5.
Wexler A, Zavala S. The use of thrombolytic therapy in the treatment of frostbite injury. J Burn Care & Res: Official Publication Am Burn Assoc. 2017;38(5):e877–81. https://doi.org/10.1097/bcr.0000000000000512.
Tavri S, Ganguli S, Bryan RG, et al. Catheter-directed intraarterial thrombolysis as part of a multidisciplinary management protocol of frostbite injury. J Vascular Interventional Radiol: JVIR. 2016;27(8):1228–35. https://doi.org/10.1016/j.jvir.2016.04.027.
Johnson AR, Jensen HL, Peltier G, DelaCruz E. Efficacy of intravenous tissue plasminogen activator in frostbite patients and presentation of a treatment protocol for frostbite patients. Foot Ankle Spec. 2011;4(6):344–8. https://doi.org/10.1177/1938640011422596.
Zhang N, Yu X, Zhao J, Shi K, Yu J. Management and outcome of feet deep frostbite injury (iii and iv degrees): a series report of 36 cases. Int J Lower Extremity Wounds. 2020. https://doi.org/10.1177/1534734620941479.
Woo EK, Lee JW, Hur GY, et al. Proposed treatment protocol for frostbite: a retrospective analysis of 17 cases based on a 3-year single-institution experience. Arch Plast Surg. 2013;40(5):510–6. https://doi.org/10.5999/aps.2013.40.5.510.
Jones LM, Coffey RA, Natwa MP, Bailey JK. The use of intravenous tPA for the treatment of severe frostbite. Burns : J Int Soc Burn Injuries. 2017;43(5):1088–96. https://doi.org/10.1016/j.burns.2017.01.013.
Ghumman A, St Denis-Katz H, Ashton R, Wherrett C, Malic C. Treatment of frostbite with hyperbaric oxygen therapy: a single center’s experience of 22 cases. Wounds : a Compendium of Clin Res Practice. 2019;31(12):322–5.
Carmichael H, Michel S, Smith TM, Duffy PS, Wiktor AJ, Lambert WA. Remote delivery of thrombolytics prior to transfer to a regional burn center for tissue salvage in frostbite: a single-center experience of 199 patients. J Burn Care & Res: Official Publication Am Burn Assoc. 2022;43(1):54–60. https://doi.org/10.1093/jbcr/irab041.
Zhao JC, Fan X, Yu JA, Zhang XH, Shi K, Hong L. Deep frostbite: clinical characteristics and outcomes in northeastern China. J Tissue Viability. 2020;29(2):110–5. https://doi.org/10.1016/j.jtv.2020.01.006.
Ibrahim AE, Goverman J, Sarhane KA, Donofrio J, Walker TG, Fagan SP. The emerging role of tissue plasminogen activator in the management of severe frostbite. J Burn Care & Res: Official Publication Am Burn Assoc. 2015;36(2):e62-6. https://doi.org/10.1097/bcr.0000000000000135.
Joseph NM, Alfonso N, Hirschfeld AG. Compartment syndrome following use of tissue plasminogen activator for frostbite in the setting of concomitant diaphyseal tibia fracture. OTA Int. 2020;3(2):e079–e079. https://doi.org/10.1097/OI9.0000000000000079.
Gross EA, Moore JC. Using thrombolytics in frostbite injury. J Emerg Trauma Shock. 2012;5(3):267–71. https://doi.org/10.4103/0974-2700.99709.
Saemi AM, Johnson JM, Morris CS. Treatment of bilateral hand frostbite using transcatheter arterial thrombolysis after papaverine infusion. Cardiovasc Intervent Radiol. 2009;32(6):1280–3. https://doi.org/10.1007/s00270-009-9584-9.
Masters T, Omodt S, Gayken J, et al. Microangiography to Monitor treatment outcomes following severe frostbite injury to the hands. J Burn Care & Res: Official Publication Am Burn Assoc. 2018;39(1):162–7. https://doi.org/10.1097/bcr.0000000000000526.
Higdon B, Youngman L, Regehr M, Chiou A. Deep frostbite treated with hyperbaric oxygen and thrombolytic therapies. Wounds : a Compendium of Clin Res Practice. 2015;27(8):215–23.
MacLennan M, Poole A, Gauthier J. Use of fluorescence to visualize response to iloprost treatment for frostbite. CMAJ Can Med Assoc. 2021. https://doi.org/10.1503/cmaj.202258.
Jud P, Hafner F, Brodmann M. Frostbite of the hands after paragliding: a chilling experience. Lancet (London, England). 2019;394:2282. https://doi.org/10.1016/s0140-6736(19)32960-5.
Roche-Nagle G, Murphy D, Collins A, Sheehan S. Frostbite: management options. Eur J Emerg Med: Official J Eur Soc for Emerg Med. 2008;15(3):173–5. https://doi.org/10.1097/MEJ.0b013e3282bf6ed0.
Pandey P, Vadlamudi R, Pradhan R, Pandey KR, Kumar A, Hackett P. Case report: severe frostbite in extreme altitude climbers-the kathmandu iloprost experience. Wilderness Environ Med. 2018;29(3):366–74. https://doi.org/10.1016/j.wem.2018.03.003.
Irarrázaval S, Besa P, Cauchy E, Pandey P, Vergara J. Case report of frostbite with delay in evacuation: field use of iloprost might have improved the outcome. High Alt Med Biol. 2018;19(4):382–7. https://doi.org/10.1089/ham.2018.0027.
Poole A, Gauthier J. Treatment of severe frostbite with iloprost in northern Canada. CMAJ : Can Med Assoc J. 2016;188:1255–8. https://doi.org/10.1503/cmaj.151252.
Barker JR, Haws MJ, Brown RE, Kucan JO, Moore WD. Magnetic resonance imaging of severe frostbite injuries. Ann Plast Surg. 1997;38(3):275–9. https://doi.org/10.1097/00000637-199703000-00015.
Folio LR, Arkin K, Butler WP. Frostbite in a mountain climber treated with hyperbaric oxygen: case report. Mil Med. 2007;172(5):560–3. https://doi.org/10.7205/milmed.172.5.560.
Kemper TC, de Jong VM, Anema HA, van den Brink A, van Hulst RA (2014) Frostbite of both first digits of the foot treated with delayed hyperbaric oxygen a case report and review of literature. Undersea Hyperbaric Med J 41: 65–70
Magnan DM, Gelsomino M, Louge P, Pignel R. Successful delayed hyperbaric oxygen therapy and iloprost treatment on severe frostbite at high altitude. High Altitude Med Biol. 2022. https://doi.org/10.1089/ham.2021.0172.
Dwivedi DA, Alasinga S, Singhal S, Malhotra VK, Kotwal A. Successful treatment of frostbite with hyperbaric oxygen treatment. Indian J Occupational Environ Med. 2015;19(2):121–2. https://doi.org/10.4103/0019-5278.165336.
Lansdorp CA, Roukema GR, Boonstra O, Dokter J, van der Vlies CH. Delayed treatment of frostbite with hyperbaric oxygen: a report of two cases. Undersea & Hyperbaric Med J Undersea and Hyperbaric Med Soc. 2017;44(4):365–9. https://doi.org/10.22462/7.8.2017.9.
Ali NMK, Green L, Buckwalter JA. effective management of frostbite with sympathetic blockade. Regional Anesthesia: J Neural Blockade in Obstetrics, Surg, & Pain Control. 1982;7(3):128. https://doi.org/10.1136/rapm-00115550-198207030-00011.
Punja K, Graham M, Cartotto R. Continuous infusion of epidural morphine in frostbite. J Burn Care & Rehabil. 1998;19(2):142–5. https://doi.org/10.1097/00004630-199803000-00012.
Campbell HH, Walker FG. Continuous epidural analgesia in the treatment of frostbite; a report of three cases. Can Med Assoc J. 1961;84(2):87–90.
Engkvist O. The effect of regional intravenous guanethidine block in acute frostbite: case report. Scandinavian J Plastic and Reconstruct Surg. 1986;20(2):243–5. https://doi.org/10.3109/02844318609006327.
Ekdahl M, Penninga L. Successful conservative treatment of severe frostbite lesions in a Greenlandic Inuit. BMJ Case Rep. 2017. https://doi.org/10.1136/bcr-2017-219672.
Lorentzen AK, Penninga L. Frostbite-a case series from arctic greenland. Wilderness Environ Med. 2018;29(3):392–400. https://doi.org/10.1016/j.wem.2018.03.001.
Xiao Y, Hao D, Xin Y, Jiang X. A Tibetan adolescent girl suffered frostbite on the journey of pilgrimage: a case report. Chinese J Traumatol. 2021. https://doi.org/10.1016/j.cjtee.2021.10.006.
Goertz O, Kapalschinski N, Hirsch T, et al. Drei fallberichte über erfrierungen. Der Unfallchirurg. 2011;114(7):634–8. https://doi.org/10.1007/s00113-010-1866-9.
Kroeger K, Janssen S, Niebel W. Frostbite in a mountaineer. VASA Zeitschrift fur Gefasskrankheiten. 2004;33(3):173–6. https://doi.org/10.1024/0301-1526.33.3.173.
Prommersberger KJ, van Schoonhoven J, Lanz U. Treatment of 3rd degree fingertip frostbite in a mountain climber with semi-occlusive dressings. Handchirurgie, Mikrochirurgie, plastische Chirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft fur Handchirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft fur Mikrochirurgie der Peripheren Nerven und Gefasse Mar 2001;33(2):95–100. doi:https://doi.org/10.1055/s-2001-12288
Aygit AC, Sankaya A. Imaging of frostbite injury by technetium-99m-sestamibi scintigraphy: a case report. Foot & Ankle Int. 2022;23(1):56–9. https://doi.org/10.1177/107110070202300111.
Erba P, Harbi P, Thacher T, Pries A, Ambrosio G, Raffoul W. Early detection of microcirculatory perfusion changes with a high resolution, real time laser Doppler imaging camera–frostbite case study. BMJ Case Rep. 2011. https://doi.org/10.1136/bcr.06.2011.4404.
Orak M, Üstündaǧ M, Güloǧlu C, Dogan H, Altunci Y. Frostbite a case report. Case Rep Clin Practice Rev. 2007;8:128–31.
Santapau A, Razola P, Tardin L, Andrés A, Prats E, Banzo J. The role of bone scanning in severe frostbite of the feet in a mountaineer. Rev Esp Med Nucl Imagen Mol. 2013;32(2):113–4. https://doi.org/10.1016/j.remn.2012.01.009.
Kayser B, Binzoni T, Hoppeler H, et al. A case of severe frostbite on Mt Blanc: a multi-technique approach. J Wilderness Med. 1993;4(2):167–74. https://doi.org/10.1580/0953-9859-4.2.167.
Welch GS, Gormly PJ, Lamb DW. Frostbite of the Hands. Hand. 1974;6(1):33–9. https://doi.org/10.1016/0072-968x_74_90007-2.
Erikson U, Pontén B. The possible value of arteriography supplemented by a vasodilator agent in the early assessment of tissue viability in frostbite. Injury. 1974;6(2):150–3. https://doi.org/10.1016/0020-1383(74)90008-4.
Gralino BJ, Porter JM, Rosch J. Angiography in the diagnosis and therapy of frostbite. Radiology. 1976;119(2):301–5. https://doi.org/10.1148/119.2.301.
Porter JM, Wesche DH, Rösch J, Baur GM. Intra-arterial sympathetic blockade in the treatment of clinical frostbite. Am J Surg. 1976;132(5):625–30. https://doi.org/10.1016/0002-9610(76)90359-7.
Glenn WWL, Maraist FB, Braaten OM. Treatment of frostbite with particular reference to the use of adrenocorticotrophic hormone (ACTH. New England J Med. 1952;247:191–200.
Gavrilin EV, Dunaevskiy GE, Antipov VB. Microwave treatment of cold injuries. J Emerg, Trauma, And Shock. 2021;14(2):108–10. https://doi.org/10.4103/JETS.JETS_142_20.
Ezquerra-Herrando L, Corella-Abenia E, Zamora-Rodríguez JM, Albareda-Albareda J, Banzo-Marraco J. Amputation level after frostbite: role of bone scan. A case report Cirugia y cirujanos. 2013;81(4):353–6.
Daniel NJ, Storn JM, Elder JH, Chevalier JI, Weinberg NE. Clinical utilization of a sous vide device in the acute rewarming of frostbitten extremities. Am J Emerg Med. 2022;52:200–2. https://doi.org/10.1016/j.ajem.2021.12.026.
Brown JR. A case of frostbite or “it takes more than two pairs of socks to keep your feet warm.” J R Army Med Corps. 1986;132(2):93–5. https://doi.org/10.1136/jramc-132-02-06.
Banzo J, Martínez Villén G, Abós MD, et al. Frostbite of the upper and lower limbs in an expert mountain climber: the value of bone scan in the prediction of amputation level. Revista espanola de medicina nuclear. 2002;21(5):366–9.
Mulgrew S, Khoo A, Oxenham T, James N. Cold finger: urban frostbite in the UK. BMJ Case Rep. 2013. https://doi.org/10.1136/bcr-11-2011-5167.
Poulakidas S, Cologne K, Kowal-Vern A. Treatment of frostbite with subatmospheric pressure therapy. J Burn Care & Res: Official Publication Am Burn Assoc. 2008;29(6):1012–4. https://doi.org/10.1097/BCR.0b013e31818ba0ad.
Russell KW, Imray CH, McIntosh SE, et al. Kite Skier’s Toe: An Unusual Case of Frostbite. Wilderness & Environmental Medicine. 2013;24(2):136–40. https://doi.org/10.1016/j.wem.2012.11.013.
Page RE, Robertson GA. Management of the frostbitten hand. Hand. 1983;15(2):185–91. https://doi.org/10.1016/S0072-968X(83)80012-6.
Brandão RA, St John JM, Langan TM, Schneekloth BJ, Burns PR. Acute compartment syndrome of the foot due to frostbite: literature review and case report. J Foot Ankle Surg: Official Publication Am College of Foot and Ankle Surg. 2018;57(2):382–7. https://doi.org/10.1053/j.jfas.2017.07.005.
Johnson-Arbor K. Digital frostbite. New England J Med. 2014. https://doi.org/10.1056/NEJMicm1310126.
Acknowledgements
We would like to thank Tomas del Capello for his competent advice in statistical matters.
Funding
The research was self-funded by the institutions where the authors work and did not receive any outside financial support.
Author information
Authors and Affiliations
Contributions
IR, HB, and GS conceived the study. IR searched and selected the studies and extracted and analyzed the data. RO and GS reviewed and reanalyzed the extracted data. All authors provided advice on methodology and data analysis. IR, KZ, and GS drafted the manuscript. All authors contributed substantially to the revision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This systematic review and meta-analysis did not involve primary data collection from human or animal subjects and therefore did not require ethics approval.
Consent for publication
Not applicable, as this study did not involve primary data collection from human or animal subjects.
Competing interests
The authors declare that they have no competing financial, professional, or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. eTable 1:
List of case reports. eTable 2: Patient cohort studies with outcomes quantified by the Hennepin score. eTable 3: Patient cohort studies with outcomes quantified by digit salvage rate. eTable 4: Patient cohort studies with outcomes quantified by phalanx salvage rate. eTable 5: Patient cohort studies with outcomes quantified by amputations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Regli, I.B., Oberhammer, R., Zafren, K. et al. Frostbite treatment: a systematic review with meta-analyses. Scand J Trauma Resusc Emerg Med 31, 96 (2023). https://doi.org/10.1186/s13049-023-01160-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-023-01160-3