Abstract
Background
Epidemiological studies regarding the correlation between anti-Müllerian hormone (AMH) and insulin resistance (IR) in polycystic ovarian syndrome (PCOS) remain inconsistent. The primary aim of this study was to determine the correlations between AMH and IR in patients with PCOS and to explore the selected factors that influence the correlations.
Methods
We conducted systemic searches of online databases (PubMed, Science Direct, Taylor and Francis, Scopus, and ProQuest) from inception to December 20, 2023 and manual searches of the associated bibliographies to identify relevant studies. We then performed subgroup and sensitivity analyses to explore the sources of heterogeneity, followed by a publication bias risk assessment of the included studies using the Joanna Briggs Institute critical appraisal tool. We used a random-effects model to estimate the pooled correlations between AMH and the homeostatic model assessment for insulin resistance (HOMA-IR) in patients with polycystic ovarian syndrome (PCOS).
Results
Of the 4835 articles identified, 22 eligible relevant studies from three regions were included and identified as low risk of bias. The random-effects pooled correlation estimate was 0.089 (95% confidence interval [CI]: −0.040, 0.215), with substantial heterogeneity (I2 = 87%; τ2 = 0.0475, p < .001). Subgroup analyses showed that the study region did not influence the correlation estimates, and sensitivity analysis showed no significant alteration in the pooled correlation estimate or 95% CI values. No publication bias was observed.
Conclusion
There was a weak, statistically insignificant correlation between AMH and HOMA-IR in patients with PCOS. The correlation estimates did not vary according to the study participants’ regions.
Similar content being viewed by others
Background
Polycystic ovarian syndrome (PCOS) is a highly prevalent endocrine disorder that affects 4–20% of reproductive-age women globally [1]. Metabolism plays an important role in the long-term sequelae of the condition. Central obesity, decreased glucose tolerance, and/or dyslipidemia are the most frequent metabolic abnormalities in PCOS, all of which are related to insulin resistance (IR) [2].
IR is a condition in which target organs fail to respond properly to insulin. It is a common metabolic derangement occurring in PCOS and is seen in all of the disease phenotypes [3]. Epigenetic changes such as DNA methylation, histone status, and miRNA expression are among the several factors that are hypothesized to play a role in the development of IR in PCOS patients [3]. Apart from that, environmental factors, dietary changes, inflammation, and vitamin D deficiency can also have an impact on insulin sensitivity in these patients [3]. IR leads to compensatory hyperinsulinemia, which stimulates the transcription of the gonadotropin-releasing hormone gene in the hypothalamus. As a result, there is an increase in luteinizing hormone pulse frequency at the hypophysis, which subsequently elevates androgen synthesis by the ovary. Hyperinsulinemia also increases androgenic production by directly stimulating the ovary to produce androgens and decreasing sex hormone–binding globulin synthesis by the liver. Hyperandrogenism may in turn worsen IR, creating a vicious cycle of IR–hyperinsulinemia–hyperandrogenemia in PCOS [3,4,5,6].
Anti-Müllerian hormone (AMH), also known as Müllerian-inhibiting substance, is a 140-kDa dimeric glycoprotein that belongs to transforming growth factor–β family [7]. It is secreted by the granulosa cells of growing ovarian follicles from the primary to small antral stages [7]. The hormone is well known for its role as a marker of ovarian reserve [8], and its potential role as a surrogate marker for the diagnosis of PCOS [9, 10]. AMH is thought to play an important role in the etiology of the disease because it can inhibit the formation of primary follicles and their recruitment, contributing to follicular arrest [11].
Many studies have investigated the correlation between AMH and IR in PCOS, but the results have been inconsistent. Despite an increasing number of intervention studies assessing the impact of AMH and IR on PCOS, there remains a lack of solid data indicating a causal relationship between AMH and IR. Although several studies have found a strong positive association between AMH and IR, others have found a negative correlation. Thus, the objective of this systematic review and meta-analysis (SRMA) was to quantitatively summarize the current evidence to determine whether levels of AMH correlate with IR in PCOS. Knowledge of the relationship between AMH and IR may contribute to a better understanding of the pathophysiology of PCOS and its metabolic complications. Furthermore, the finding of a significant correlation would increase the plausibility of a biological link between AMH and IR in PCOS and suggest a potential avenue for PCOS treatment. To the best of our knowledge, this is the first SRMA to investigate the relationship between AMH and IR in PCOS.
Materials and methods
Design and protocol development
The protocol for this SRMA was registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration No. CRD42021255383; Appendix A). We used the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines to report the SRMA results [12].
Eligibility criteria
Two investigators (M.Z.M.M. and A.M.J.) independently screened all titles and abstracts from the initial search and full-text articles identified during the first-stage screening. We included studies from inception to December 20, 2023, reporting primary data for Pearson’s correlations between AMH and homeostatic model assessment for insulin resistance (HOMA-IR) in PCOS. The searches were conducted in English, and only articles published in English were selected. Observational studies, such as cross-sectional, cohort, or longitudinal studies, were eligible for inclusion if they reported target populations of reproductive-age women diagnosed with PCOS according to the Rotterdam criteria. We excluded experimental (randomized and nonrandomized) trials, case reports, ecological studies, case reports, studies that did not involve human participants (animal and in vitro studies), book chapters, narrative reviews, and protocol studies.
Data source and search strategy
Two investigators (M.Z.M.M. and A.M.J.) extensively searched online international databases to which our institutional library subscribed (PubMed, ScienceDirect, Taylor and Francis, Scopus, and ProQuest) from inception to December 20, 2023. We used the following MeSH terms and text words linked to AMH, HOMA-IR, and PCOS: “Müllerian-inhibiting factor,” “anti Müllerian hormone,” “Müllerian-inhibitory substance,” “resistance, insulin,” “insulin sensitivity,” “homeostatic model assessment for insulin resistance,” “insulin resistance,” “Müllerian regression factor,” “ovary syndrome, polycystic,” “syndrome, polycystic ovary,” “Stein–Leventhal syndrome,” “polycystic ovarian syndrome,” “sclerocystic ovarian degeneration,” and “sclerocystic ovary.” We tested the search strategy in PubMed and further refined it based on its efficacy in retrieving relevant studies from each database. To identify other relevant research, we conducted forward and backward reference chaining of the included studies and searched the reference lists of the included papers. We applied an OR Boolean operator to connect all MeSH terms to maximize the sensitivity of the literature search (Appendix B).
Selection process
Two reviewers (M.Z.M.M. and A.M.J.) conducted an independent preliminary screening of the titles, abstracts, and selected articles that potentially met the inclusion criteria. We used Microsoft Excel 365 to sort the data and then retrieved and reassessed the full texts that met the eligibility criteria. To avoid bias in the study selection, we conducted the eligibility assessment in duplicate and independently. Discrepancies were resolved through discussion and consensus between the reviewers and the third author (N.S.). All three authors were in complete agreement with the final decision and documented detailed reasons for the exclusion of sources.
Data extraction
We downloaded the search results from each database and then imported them into the Zotero software using the Zotero web connector. We removed duplicate articles using Zotero software, exported the search results in Microsoft Excel.csv format, and later converted them to .xlsx format.
Two reviewers (M.Z.M.M. and A.M.J.) conducted a preliminary screening of titles and abstracts to identify potential articles of interest. The full texts of the potentially eligible studies were retrieved and reassessed according to the inclusion/exclusion criteria. To avoid bias in the study selection process, the reviewers independently assessed eligibility in duplicate and resolved conflicts regarding study identification through discussion with the third researcher (N.S.) to reach 100% agreement on the final decision. We prepared a detailed report explaining why studies were excluded following the full-text review.
After the studies were identified, two investigators (M.Z.M.M. and A.M.J.) abstracted data from the included studies using a standardized predesign and prepiloted electronic data abstraction Microsoft Excel form to assess the study quality and synthesize the evidence. We conducted data abstraction independently to minimize the risk of errors. The abstracted information included the author’s name, publication year, country, region, study design, study subjects, PCOS criteria used, method/platform for AMH measurement, AMH value, HOMA-IR value, and Pearson’s correlation coefficient (r) for AMH and HOMA-IR in PCOS.
In cases in which there were multiple publications of the same study, we extracted the most complete and up-to-date data from each publication. We then analyzed the data after eliminating overlaps in the extracted data. We report the literature search and screening outputs using a PRISMA flow diagram.
Methodological quality assessment
Two authors (M.Z.M.M. and A.M.J.) independently performed the quality assessment using the Joanna Briggs Institute (JBI) Critical Appraisal for Cross-Sectional Studies [13] checklist, which consists of eight questions for assessing specific domains of cross-sectional studies to determine the potential risk of bias; questions can be answered with “yes,” “no,” “unclear,” or “not applicable” (Appendix C). We resolved any disagreement through discussion with the third review author (N.S.). Finally, we summed the scores and converted them to percentages. We classified the risk of bias in each study as high (scores > 50%), moderate (50–69%), or low (≥ 70%) [14]. We included only low-risk studies in this SRMA.
Data synthesis and statistical analysis
We summarized the descriptions of the original studies using tables and forest plots based on oligo-ovulatory and anovulatory subjects according to Zhang et al. [15]. We entered the data into a Microsoft Excel file before we performed statistical analysis using the Rstudio metacor package [16] (version February 2, 2022) in R (version 4.1.3) [17].
Before pooling the correlation estimates using the inverse variance method, we applied Fisher z-transformations to the correlations. We considered a random-effects model the most appropriate method for computing the summary effect size in the presence of heterogeneity. Therefore, we used a random-effects model with Hartung–Knapp adjustment to estimate the pooled correlation with a 95% confidence interval (CI).
Heterogeneity assessment
To determine the heterogeneity among the included studies, we used forest plots, tau-squared (τ2), Higgins I-squared (I2), and Cochrane’s Q test p values [18]. We used Schmidt–Hunter estimation to estimate the τ2 values and the τ2 CIs using the Q-profile method. The τ2 and p values from the Cochrane’s Q test revealed only the presence versus absence of heterogeneity but did not explain the extent of heterogeneity [19]. We interpreted the τ2 values by their CIs, and the Cochrane’s Q test explained the significance of the p values. If the τ2 CI did not contain zero and the p value from the Cochrane’s Q test was significant (p < .001), some between-study heterogeneity existed [20]. The amount of heterogeneity in the meta-analysis can be estimated using I2. An I2 value less than 25% indicates low heterogeneity, a value of 25–75% indicates moderate heterogeneity, and a value of 75% or higher indicates substantial heterogeneity [21].
Subgroup and sensitivity analysis
To explore the possible causes of heterogeneity, we also conducted subgroup analyses according to region. The random-effects pooled correlation estimate corresponded to the 95% CI, and we reported the within-group and between-group heterogeneity. A p value for this test of < 0.10 indicated a statistically significant subgroup effect. We performed a sensitivity analysis using the leave-one-out method to assess the impact of each study on the pooled results by removing one study at a time from the analysis. We used Egger’s test, Begg’s test, and visual inspection of the symmetry in the funnel plots to evaluate publication bias. The level of significance was set at p < .05 for the Egger’s and Begg’s tests [22].
Results
Study selection
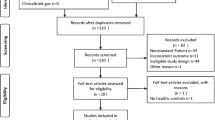
We identified 4835 articles through electronic databases and manual searches. After removing duplicates, we screened 3978 titles and abstracts for relevance, yielding 59 full-text articles. After screening the full text of 59 articles, we rejected 37 studies with incorrect statistical data, studies that were irrelevant to this review, and non-English articles. The SMRA covered a final sample of 22 studies (Fig. 1).
Study characteristics
Table 1 describes the characteristics of the included studies. The studies were published between 2004 and 2023 in 11 countries across three regions, and they included 3,028 PCOS patients. The largest proportions of studies came from Asia (13 studies, 59.1%), Europe (8 studies, 36.4%), and North America (1 study, 4.5%). Most studies (18) were cross-sectional studies, and the remaining four studies were case-control studies. The sample sizes ranged from 12 to 293 PCOS subjects of reproductive age.
All involved studies used the Rotterdam criteria to diagnose PCOS. Most of the selected studies measured AMH levels using the enzyme-linked immunosorbent assay method, and only two studies measured serum AMH levels using the electrochemiluminescence immunoassay method. We assessed IR according to the HOMA-IR method, and we conducted the correlation analyses using Pearson’s correlation coefficient (r) for all included studies. Among the studies, one used oligo-ovulatory and anovulatory subjects. Zhang et al. divided the subjects into oligo-ovulatory and anovulatory subjects [15].
Quality assessment
We assessed the quality of the articles using the JBI checklist.43 Each question was applied to each of the 22 articles, and the answer to each question was given as “yes” or “no.” The overall risk is specified at the bottom of Table 2, with the scores summed as percentages. All included studies achieved a > 50% score and were identified as having a moderate to low risk of bias. Two authors (M.Z.M.M. and A.M.J.) independently evaluated the risk and quality of each study, and any confusion was resolved through a consensus team meeting.
Meta-analysis correlation of AMH in PCOS and IR
Substantial statistical heterogeneity existed among the individual study estimates (I2 = 87%; τ2 = 0.0475, p < .001). Therefore, we used a random-effects model for the meta-analysis. The overall correlation estimate was 0.089 (95% CI: −0.040, 0.215), which we considered to be a weak correlation (Fig. 2).
Subgroup and sensitivity analyses
To identify the sources of heterogeneity among the studies, we performed subgroup and sensitivity analyses. Different races and ethnicities may contribute to variations in AMH and IR due to various genetic and environmental factors [44]. The pooled correlation between AMH and HOMA-IR in PCOS patients in Europe [0.099 (95% CI: -0.147, 0.333)] was slightly lower than that in Asia [0.116 (95% CI: -0.050, 0.277]; Fig. 3). The heterogeneity was significant in these two regions: (I2 = 85%; τ2 = 0.0437, p < .001) and (I2 = 82%; τ2 = 0.0321, p < .001), respectively. Although the heterogeneity is significant in these two regions, the pooled correlation did not cause significant variation in this study. Subgroup analyses for other types of possible heterogeneity, such as body mass index (BMI), weight, PCOS phenotype, and age, could not be performed because of inadequate studies and data.
To identify the possible sources of heterogeneity in the pooled meta-analysis of the correlation between AMH and IR in patients with PCOS, we conducted a leave-one-out influential analysis. This analysis showed that the overall prevalence was strong and did not depend on a single study (Fig. 4). In patients with PCOS, the pooled correlation between AMH and IR ranged from 0.06 (95% CI: 0.060, 0.180) to 0.11 (95% CI: ?0.020, 0.230).
Publication bias
To assess the publication bias of the included studies, we used Begg’s and Egger’s tests. We found no evidence of publication bias in the overall meta-analysis of the correlation between AMH and HOMA-IR in patients with PCOS (Begg’s test, p = .177; Egger’s test, p = .216). The symmetry of the funnel plot was in agreement with the results of Egger’s tests (Fig. 5). We searched unpublished or gray literature using Google scholar and a web-based search to reduce publication bias.
Discussion
Although many studies have been conducted regarding the relationship between AMH and IR in PCOS, the findings are conflicting. In this SRMA, we identified a weak correlation between serum AMH and HOMA-IR in patients with PCOS. It is known that both parameters play an important role in the pathophysiology of the disease. However, the results of our SRMA led us to conclude that changes in AMH levels have no significant influence on IR in patients with PCOS. This means that no reduction in AMH level will improve IR in patients with PCOS. Similarly, treating IR will not change AMH levels in PCOS.
We observed no significant variation in the pooled correlation estimate when we conducted subgroup analyses according to region, although different regions may have various genetic and environmental factors that could affect AMH levels [44,45,46]. In Europe, the subgroup analysis revealed a slightly lower pooled effect estimate (0.099 [95% CI: 0.147, 0.333]) compared with Asia (0.116 [95% CI: −0.050, 0.277]). This subgroup analysis should be judged with caution because of the small number of studies from Asia (n = 13) and Europe (n = 8). Because only one study was conducted in North America, we could not compare the effect estimates with the North American region. Subgroup analysis by BMI and phenotypes may provide valuable data for the study, as different PCOS phenotypes and BMI have been reported to have different degrees of IR incidence [47]. Previous studies have shown that PCOS patients of hyperandrogenic phenotypes were prone to develop IR compared to the other phenotypes [48, 49]. In turn, IR and excessive BMI may exacerbate the symptoms of hyperandrogenism [50]. However, we could not compare the correlation estimates between different PCOS phenotypes and different classifications of BMI because of limited data.
Among the studies included in the review, we found that various cutoffs for HOMA-IR were used as IR indicators. One study used a cutoff value of > 3.0 [6], and other studies used cutoff values of > 2.5 and > 2.14, based on their population HOMA-IR cutoffs [23, 28]. The variability in HOMA-IR cutoffs may reflect different correlations between AMH and HOMA-IR in patients with PCOS across the studies, as a lower HOMA-IR cutoff will include more subjects with PCOS diagnosed as IR as compared with different studies using higher HOMA-IR values [46].
We acknowledge the limitations of this SRMA. Despite a thorough search strategy, some studies might not have been included. Because of the limited number of studies, it was not possible to assess publication bias across demographic, metabolic, and endocrine parameters, which limited our ability to perform subgroup analyses and attenuated the power of the analyses. A further limitation of this study is the lack of a standardized scale to assess the quality of the included studies. We found statistically significant heterogeneity in most analyses (approximately 80%). This limitation, which has been observed in other meta-analyses of epidemiological studies, may result from unreported factors. By using a random effect model for statistical interpretation, the findings will not be affected by the high degree of heterogeneity, and reliable and more efficient estimates are provided when there is a high degree of heterogeneity [51, 52]. The other causes of potential biases across the studies were possibly the different sample sizes and anthropometry of the study subjects [53].
We could not examine the heterogeneity effect of different age groups in this SRMA because all studies involved young adults. None of the studies were race specific, which leaves room for this variation to be examined.
Conclusion
To the best of our knowledge, this is the first SRMA to examine the correlation between AMH and IR in patients with PCOS. Our SRMA suggests there is limited or no evidence that high serum AMH levels in patients with PCOS are causally linked to the development of IR. A high level of heterogeneity was potentially caused by different PCOS phenotypes, different BMI classifications, variation in environmental factors and genetics across regions, and different age groups. Subgroup analysis of these factors may reduce the degree of heterogeneity. Future studies on the relationship between AMH and IR in PCOS with alternative interventions may be needed to enhance our understanding.
Data availability
The data for this meta-analysis were retrieved from published articles and are available from the author upon request.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- CI:
-
Confidence interval
- ECLIA:
-
Electrochemiluminescence immunoassay
- ELISA:
-
Enzyme-linked immunosorbent assay
- HA:
-
Hyperandrogenism
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- IR:
-
Insulin resistance
- JBI:
-
Joanna Briggs Institute
- OA:
-
Oligomenorrhea
- PCOM:
-
Polycystic ovarian morphology
- PCOS:
-
Polycystic ovarian syndrome
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- SRMA:
-
Systematic review and meta-analysis
References
Deswal R, Narwal V, Dang A, Pundir CS. The prevalence of polycystic ovary syndrome: a brief systematic review. J Hum Reprod Sci. 2020;13(4):261.
Allahbadia GN, Merchant R. Polycystic ovary syndrome and impact on health. Middle East Fertil Soc J. 2011;16(1):19–37.
Zhao H, Zhang J, Cheng X, Nie X, He B. Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J Ovarian Res. 2023;16(1):9.
Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev. 2015;36(5):487–525.
Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. International journal of reproductive medicine. 2014;2014.
13, Azziz R, Carmina E, Chen Z, Dunaif A, Laven J, Legro RS, et al. Polycystic ovary syndrome. Nat Reviews Disease Primers. 2016;2:16057.
Rzeszowska M, Leszcz A, Putowski L, Hałabiś M, Tkaczuk-Włach J, Kotarski J et al. Anti-Müllerian hormone: structure, properties and appliance. Ginekologia Polska. 2016;87(9).
Moolhuijsen LM, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105(11):3361–73.
Köninger A, Koch L, Edimiris P, Enekwe A, Nagarajah J, Kasimir-Bauer S, et al. Anti-mullerian hormone: an indicator for the severity of polycystic ovarian syndrome. Arch Gynecol Obstet. 2014;290:1023–30.
Teede H, Misso M, Tassone EC, Dewailly D, Ng EH, Azziz R, et al. Anti-Müllerian hormone in PCOS: a review informing international guidelines. Trends Endocrinol Metabolism. 2019;30(7):467–78.
Garg D, Tal R. The role of AMH in the pathophysiology of polycystic ovarian syndrome. Reprod Biomed Online. 2016;33(1):15–28.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int Surg J. 2021;88:105906.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: systematic reviews of etiology and risk. Joanna Briggs institute reviewer’s manual. Joanna Briggs Inst. 2017;5:217–69.
Akhtar S, Nasir JA, Ali A, Asghar M, Majeed R, Sarwar A. Prevalence of type-2 diabetes and prediabetes in Malaysia: a systematic review and meta-analysis. PLoS ONE. 2022;17(1):e0263139.
Zhang F, Du J, Wang B, Wen H, Jia X, Chen H et al. Dynamics of hormonal profile and anti-mullerian hormone during spontaneous ovulation in PCOS women with oligomenorrhea. Biomed Res. 2017;28(6).
Borenstein M, Hedges LV, Higgins JP. Rothstein hr. Introduction to meta-analysis. West Sussex, England: Wiley & Sons Ltd; 2009.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2022. https://www.r-project.org/. Accessed November 23, 2022.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol Methods. 2006;11(2):193.
Harrer M, Cuijpers P, Furukawa T, Ebert D. Doing meta-analysis with R: a hands-on guide. Volume 14. Chapman and Hall/CRC; 2021 Sep.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Gjerdevik M, Heuch I. Improving the error rates of the Begg and Mazumdar test for publication bias in fixed effects meta-analysis. BMC Med Res Methodol. 2014;14(1):1–6.
Shen SH, Shen SY, Liou TH, Hsu MI, Chang YC, Cheng CY, et al. Obesity and inflammatory biomarkers in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2015;192:66–71.
Feldman RA, O’Neill K, Butts SF, Dokras A. Antimüllerian hormone levels and cardiometabolic risk in young women with polycystic ovary syndrome. Fertil Steril. 2017;107(1):276–81.
La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Müllerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82(4):970–2.
Chun S. 1-h postprandial glucose level is related to the serum anti-Müllerian hormone level in women with polycystic ovary syndrome. Gynecol Endocrinol. 2015;31(10):815–8.
Caglar GS, Kahyaoglu I, Pabuccu R, Demirtas S, Seker R. Anti-mullerian hormone and insulin resistance in classic phenotype lean PCOS. Arch Gynecol Obstet. 2013;288:905–10.
Sahmay S, Mathyk BA, Sofiyeva N, Atakul N, Azemi A, Erel T. Serum AMH levels and insulin resistance in women with PCOS. Eur J Obstet Gynecol Reprod Biol. 2018;224:159–64.
Tokmak A, Kokanali D, Timur H, Kuntay Kokanali M, Yilmaz N. Association between anti-mullerian hormone and insulin resistance in non-obese adolescent females with polycystic ovary syndrome. Gynecol Endocrinol. 2016;32(11):926–30.
Skałba P, Cygal A, Madej P, Dąbkowska-Huć A, Sikora J, Martirosian G, et al. Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod. 2011;158(2):254–9.
Jun TJ, Jelani AM, Omar J, Rahim RA, Yaacob NM. Serum anti-müllerian hormone in polycystic ovary syndrome and its relationship with insulin resistance, lipid profile and adiponectin. Indian J Endocrinol Metab. 2020;24(2):191.
Tian X, Ruan X, Mueck AO, Wallwiener D, Wang J, Liu S, et al. Serum anti-Müllerian hormone and insulin resistance in the main phenotypes of non-obese polycystic ovarian syndrome women in China. Gynecol Endocrinol. 2014;30(11):836–9.
Chen MJ, Yang WS, Chen CL, Wu MY, Yang YS, Ho HN. The relationship between anti-Müllerian hormone, androgen and insulin resistance on the number of antral follicles in women with polycystic ovary syndrome. Hum Reprod. 2008;23(4):952–7.
Wiweko B, Indra I, Susanto C, Natadisastra M, Hestiantoro A. The correlation between serum AMH and HOMA-IR among PCOS phenotypes. BMC Res Notes. 2018;11(1):1–6.
Öztürk B, Gürbüz AS, Durak ZE, Öztürk HS. Dipeptidyl peptidase-4 and adenosine deaminase enzyme levels in polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(2):138–41.
Şahin AY, Baş F, YETİM Ç, Uçar A, Poyrazoğlu Ş, Bundak R, et al. Determination of insulin resistance and its relationship with hyperandrogenemia, anti-Müllerian hormone, inhibin A, inhibin B, and insulin-like peptide-3 levels in adolescent girls with polycystic ovary syndrome. Turkish J Med Sci. 2019;49(4):1117–25.
Gupta M, Yadav R, Mahey R, Agrawal A, Upadhyay A, Malhotra N, Bhatla N. Correlation of body mass index (BMI), anti-mullerian hormone (AMH), and insulin resistance among different polycystic ovary syndrome (PCOS) phenotypes–a cross-sectional study. Gynecol Endocrinol. 2019 May 12.
Sharma M, Singh HV, Geethanjali G, Jain PK, Ranjan R. Correlation of anti mullerian hormone with Homeostatic Model Assessment-Insulin Resistance in polycystic ovarian syndrome and normally ovulating women. J Clin Diagn Res. 2019;13(11).
Fu H, Lin Y, Deng X, Wu L. Correlation between anti-mullerian hormone levels and antral follicle counts in polycystic ovary and metabolic syndromes. Syst Biol Reprod Med. 2021;67(2):112–20.
Sova H, Unkila-Kallio L, Tiitinen A, Hippeläinen M, Perheentupa A, Tinkanen H, et al. Hormone profiling, including anti-Müllerian hormone (AMH), for the diagnosis of polycystic ovary syndrome (PCOS) and characterization of PCOS phenotypes. Gynecol Endocrinol. 2019;35(7):595–600.
Woo HY, Kim KH, Rhee EJ, Park H, Lee MK. Differences of the association of anti-Müllerian hormone with clinical or biochemical characteristics between women with and without polycystic ovary syndrome. Endocr J. 2012;59(9):781–90.
Yue CY, Lu LK, Li M, Zhang QL, Ying CM. Threshold value of anti-mullerian hormone for the diagnosis of polycystic ovary syndrome in Chinese women. PLoS ONE. 2018;13(8):e0203129.
Han Zhao D, Zhou C, Liu L, Zhang. The relationship between insulin resistance and obesity and serum anti-mullerian hormone level in Chinese women with polycystic ovary syndrome: a retrospective, single-Center Cohort Study. Int J Womens Health. 2023;15:151–66. PMID: 36778752; PMCID: PMC9911904.
Tal R, Seifer DB. Potential mechanisms for racial and ethnic differences in antimüllerian hormone and ovarian reserve. Int J Endocrinol. 2013;2013:818912. https://doi.org/10.1155/2013/818912. Epub 2013 Nov 21. PMID: 24348557; PMCID: PMC3857838.
Kotlyar AM, Seifer DB. Ethnicity/race and age-specific variations of serum AMH in women—a review. Front Endocrinol. 2021;11:593216.
Gromski PS, Patil RS, Chougule SM, Bhomkar DA, Jirge PR, Nelson SM. Ethnic discordance in serum anti-Müllerian hormone in European and Indian healthy women and Indian infertile women. Reprod Biomed Online. 2022;45(5):979–86.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K et al. 2020. https://synthesismanual.jbi.global. Borzan V, Lerchbaum E, Missbrenner C, Heijboer AC, Goschnik M, Trummer C,. Risk of insulin resistance and metabolic syndrome in women with hyperandrogenemia: a comparison between PCOS phenotypes and beyond. Journal of Clinical Medicine. 2021;10(4):829.
Borzan V, Lerchbaum E, Missbrenner C, Heijboer AC, Goschnik M, Trummer C,. Risk of insulin resistance and metabolic syndrome in women with hyperandrogenemia: a comparison between PCOS phenotypes and beyond. Journal of Clinical Medicine. 2021;10(4):829.
Moghetti P, Tosi F, Bonin C, Di Sarra D, Fiers T, Kaufman JM, et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metabolism. 2013;98(4):E628–37.
Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–21.
Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De Francisco A, Quintela AG. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13(1):1–0.
Imrey PB. Limitations of Meta-analyses o of studies with high heterogeneity. JAMA Netw Open. 2020;3(1):e1919325. https://doi.org/10.1001/jamanetworkopen.2019.19325.
Faber J, Fonseca LM. How sample size influences research outcomes. Dent Press J Orthod. 2014;19:27–9.
Author information
Authors and Affiliations
Contributions
A.M.J. was responsible for the study conception and design. A.M.J. and M.Z.M.M. did the literature search, screening and selection of the articles as well as data analysis. N.S. contributed to the screening and selection of the articles. N.M.Y. thoroughly guided and assisted in the study design, methodology, statistics and data analysis. N.A.A.C.S. and H.A.I guided in the literature search and study methodology. M.Z.M.M. wrote the first draft of the study. All authors critically revised the manuscript. A.M.J. and M.Z.M.M. were responsible for the final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Md Muslim, M.Z., Mohammed Jelani, A., Shafii, N. et al. Correlation between anti-mullerian hormone with insulin resistance in polycystic ovarian syndrome: a systematic review and meta-analysis. J Ovarian Res 17, 106 (2024). https://doi.org/10.1186/s13048-024-01436-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-024-01436-x