Abstract
Premature ovarian insufficiency (POI) is a condition in which the quantity of follicles and the quality of oocytes gradually decrease. This results in an estrogen secretion disorder and abnormal follicle development, which can lead to related diseases, early onset of menopause, sexual dysfunction, and an increased risk of cardiovascular issues, osteoporosis, and depression, among others. This disease significantly impacts the physical and mental health and overall quality of life of affected women. Factors such as genetic abnormalities, oophorectomy, radiotherapy for malignancy, idiopathic conditions, and an unhealthy lifestyle, including smoking, can accelerate the depletion of the follicular pool and the onset of menopause. Extensive research has been conducted on the detrimental effects of tobacco smoke on the ovaries. This article aims to review the advancements in understanding the impact of tobacco smoke on POI, both in vivo and in vitro. Furthermore, we explore the potential adverse effects of common toxicants found in tobacco smoke, such as polycyclic aromatic hydrocarbons (PAHs), heavy metals like cadmium, alkaloids like nicotine and its major metabolite cotinine, benzo[a]pyrene, and aromatic amines. In addition to discussing the toxicants, this article also reviews the complications associated with POI and the current state of research and application of treatment methods. These findings will contribute to the development of more precise treatments for POI, offering theoretical support for enhancing the long-term quality of life for women affected by this condition.
Similar content being viewed by others
Introduction
Premature ovarian insufficiency (POI) has emerged as a significant public health concern and a leading cause of fertility issues. As the quality of oocytes diminishes, and the quantity of follicles declines significantly, the female reproductive system undergoes an accelerated aging process, resulting in irregular menstruation and early onset of menopause. This, in turn, leads to ovarian dysfunction and infertility. Disorders in estrogen secretion and abnormal follicular development can further trigger various related health conditions, significantly impacting the overall quality of life for affected women. Genetic abnormalities, oophorectomy, radiotherapy for malignancies, idiopathic conditions, and unhealthy lifestyle choices such as smoking can expedite the depletion of the follicular pool and the onset of menopause [1].
The ovary, as a critical female reproductive organ, plays a pivotal role in ovulation and the secretion of sex hormones necessary for the subsequent growth, development, and maturation of follicles [2]. A woman is born with a fixed number of follicles in her ovaries, which gradually develop and mature, ultimately being ovulated during puberty [3]. This specific development process involves several key stages: gastrulation → ectodermal cell formation → primordial germ cells → germ cells → oogonia → primary oocytes → secondary oocytes → Graafian follicles → mature oocytes. The depletion of the follicle pool signals the onset of menopause, marking the conclusion of the follicle growth process. Diminished oocyte quality disrupts the intricate sequence of maturation, ovulation, fertilization, implantation, and early embryonic development, ultimately resulting in compromised reproductive health and infertility.
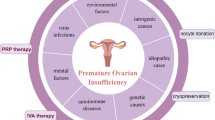
According to the European Society of Human Reproduction and Embryology Guidelines (ESHRE) (European Society for Human et al. 2016), clinical symptoms of oligomenorrhea or amenorrhea persisting for at least 4 months, with intervals exceeding 4 weeks, and supported by multiple tests and examinations revealing a follicle-stimulating hormone (FSH) level greater than 25 IU/L, can define the current presence of POI. The prevalence of POI in females under 40 years of age is approximately 1% (European Society for Human et al. 2016). However, the causes and pathogenesis of POI remain unclear. The progression of POI can be categorized into four stages: the normal stage, the latent stage, the abnormal biochemical stage, and the clinical development stage. Premature ovarian failure (POF) represents the final stage of POI. Ovarian insufficiency can manifest as secondary amenorrhea, menstrual irregularities, reduced fertility, and symptoms of estrogen deficiency (European Society for Human et al. 2016). POI not only impacts women's fertility, causing them to enter menopause prematurely or experience sexual dysfunction, but it also elevates the risk of cardiovascular problems, osteoporosis, depression, and other diseases affecting physical and mental health (see Fig. 1). Extending the female reproductive lifespan reduces the risk of bone injuries and type 2 diabetes but increases the incidence of hormone-sensitive malignancies.
POI is an idiopathic disorder that originates from various factors, including autoimmune diseases [4], hereditary factors [5], environmental factors [6], iatrogenic factors [7] such as chemotherapy, radiotherapy, and surgery, and mitochondrial abnormalities [8]. Currently, approximately a quarter of patients diagnosed with POI have a relatively clear cause. Compounds like phthalates, bisphenol A, pesticides, and tobacco have been found to have toxic effects on ovarian function, leading to increased follicular exhaustion and early menopause. These detrimental effects have been observed at various stages from fertilized egg development to adulthood, with different mechanisms operating at each stage. However, the etiology of more than 75% of POI cases remains undetermined [9]. Notably, smoking has adverse effects on female fertility and is a recognized risk factor for POI [10]. Factors and genes related to sex hormones and the menstrual cycle appear to exert a more pronounced influence on smoking habits and cessation in women compared to men, underscoring the importance of considering gender differences in providing tobacco dependence interventions [11]. This article primarily aims to review the potential pathways through which tobacco smoke, both active and passive exposure, can cause ovarian damage. Additionally, it will explore the complications associated with POI and propose treatment plans.
Effects of smoking on ovarian function and research status (Fig. 2)
The effect of smoking on ovarian function
Environmental factors appear to be significant determinants of ovarian reserve and may play a role in premenopause during the prenatal period or adulthood [12]. Environmental toxins can exert deleterious effects by disrupting the process of communication and nutrient flow from granulosa cells to oocytes. Cigarette smoke contains nearly 5,000 toxic and harmful substances, which are believed to be mutagenic and carcinogenic. Smoking is a recognized risk factor for POI [13,14,15]. Tobacco smoke disrupts folliculogenesis and development, increasing apoptosis or autophagy, DNA damage, and abnormal connections between oocytes and granulosa cells that are associated with ovarian follicle death [16]. Human granulosa cells cultured in vitro showed reduced production of estradiol and progesterone when exposed to tobacco smoke [17]. Granulosa cells are the main components of ovarian somatic cells and play a vital role in follicular growth or atresia. Studies have indicated a significant increase in primordial follicle depletion and antral follicle apoptosis after exposure to tobacco smoke. There were indications of persistent oxidative stress in ovarian tissue and undamaged oocytes exposed to tobacco smoke, with marked increases in mitochondrial ROS and lipid peroxidation levels and CYP2E1 detoxification enzymes. This results in reduced fertilization potential and the appearance of whole oocyte dysfunction [18]. Women who smoke suffer from weakened ovarian function, disturbances in hormonal synthesis and metabolism, followed by reduced fertility, early menopause, and a decrease in ovarian reserve. In recent years, the global smoking rate has been declining, but the likelihood of smoking among women of childbearing age has been significantly increasing worldwide, with nearly 33.3% of this age group having a history of smoking [3].
Research has shown that various pollutants can impact all gametogenesis processes and may have adverse effects on ovarian function in women post-birth [19, 20]. It has been documented that exposure to cigarette smoke can lead to a reduction of approximately 20% in women's ovarian reserve (Zenzes 2000). Some women exposed to cigarette smoke may even experience premature menopause [21]. Moreover, a different set of studies has revealed that mothers who smoked during pregnancy had a significantly decreased quantity of oocytes and somatic cells in the ovaries of their female offspring [22], leading to reduced fertility and an earlier onset of menopause [23]. In a study utilizing a mouse model of chronic obstructive pulmonary disease induced by cigarette smoke, female mice exposed to direct nasal smoke showed signs of primordial follicle depletion, sinus follicle oocyte apoptosis, induced oxidative stress, and decreased follicular ovulation [18]. Smoking exposure has been linked to a reduction in the follicle reserve in ovaries and alterations in estrus status and serum hormone levels [16].
Previous research has demonstrated that women exposed to cigarette smoke experience a significant reduction in ovarian volume compared to women of the same age who were not exposed to cigarette smoke [24]. Upon microscopic examination, a pronounced decrease in the quantity of follicles was observed, primarily affecting primordial follicles, without a notable impact on primary, secondary, or antral follicles. Cigarette smoke contains various toxic substances, including PAHs, heavy metals such as cadmium, alkaloids like nicotine and its main metabolite cotinine, benzo[a]pyrene, and aromatic amines. Polycyclic hydrocarbons present in tobacco smoke may potentially harm ovarian germ cells, leading to follicular dysfunction and decreased blood estrogen levels [13, 25]. Increased oxidative stress, heightened apoptosis, alterations in the relationship between granulosa cells and oocytes, impaired oocyte nuclear function, and compromised luteal cell integrity may collectively contribute to disruptions in follicular development, possibly representing the mechanisms behind tobacco-induced damage to reproductive function [26]. In vitro studies have revealed that alkaloids found in tobacco, such as nicotine, cotinine, and anabacin, inhibit the conversion of androstenedione to estrogen, consequently leading to reduced estrogen levels in the bloodstream [27, 28]. Animal studies have indicated that smoking can impact the functioning of the hypothalamic-pituitary axis [29], leading to rapid hormonal imbalances release [13, 30]. Exposure to tobacco smoke does not induce apoptosis of Bcl-2/Bax-associated granules but activates the Atg autophagy pathway, ultimately resulting in follicular loss in mice. Smoking disrupts the menstrual cycle, reduces the efficacy of hormone replacement therapy, and increases the likelihood of experiencing side effects. Research suggests that the deleterious effects of smoking on ovarian function may be irreversible.
The literature suggests that increased smoking is associated with early menopause [31, 32], and that women who smoke enter menopause one year earlier than non-smoking women [13]. Genetically mediated increases in alcohol consumption and smoking are associated with early age at natural menopause (ANM). For each additional cigarette smoked per day, ANM was reduced by about 2.5 weeks [33]. The occurrence of premature menopause may be related to the amount of smoking, and the sooner women quit smoking, the more likely they are to avoid early menopause [34]. Some studies suggest that both passive and active smoking are associated with early menopause [35], while others report that only active smoking has this effect [35, 36]
The mechanism of common toxins in tobacco smoke affecting ovarian development and function
The effect of cadmium on ovarian development and function and its possible mechanisms
Cadmium, a heavy metal commonly found in plastic products, soil, animals, and food such as meat and fruit, is considered a major environmental pollutant. However, the most common modes of exposure to cadmium are still believed to be from tobacco smoke pollution. Cadmium has a biological half-life as long as 15 to 30 years and is slowly excreted from the body [37]. It inhibits the production of steroid hormones, such as progesterone, which is essential for follicular growth [38, 39].
Different concentrations of cadmium can have a dual effect on steroidogenesis. Previous studies have detected cadmium in the follicular fluid and oocytes of smokers [40, 41]. When the concentration of cadmium in female follicular fluid reaches 6.73 ± 0.31 μg/L, it interferes with the growth and development of follicles, increases the number of follicular atresia, and can further lead to ovulation failure, implantation disorders, spontaneous abortion, and birth defects [42, 43]. mRNA level studies have shown that cadmium can cause ovarian development disorders in rats by downregulating the expression of the SCF/c-kit gene and its related microRNA factors [44].
In rodents, under the electron microscope, cadmium particles are deposited in the ovary, destroying the structure and function of the ovary. This damage affects the endoplasmic reticulum, mitochondria, Golgi apparatus, endometrium, and other cellular organs. It also degrades the corpus luteum and disrupts the arrangement of follicles and corpus luteum, increasing the number of atretic follicles [42, 45, 46].
The effect of benzo[a]pyrene on ovarian development and function and its possible mechanisms
Benzo[a]pyrene can be detected in human serum and follicular fluid. It is found at higher concentrations in the follicular fluid of women exposed to tobacco smoke, with its levels being positively correlated with exposure [47]. Furthermore, the concentration of benzo[a]pyrene in the lung tissue of smokers is approximately twice as high as that in non-smokers [48]. The study revealed that the concentration of benzo[a]pyrene (B[a]P) was negatively correlated with the level of E2 in the medium. A decrease in E2 may result in insufficient follicular growth and development. E2 primarily exerts its effects through estrogen receptor alpha (ERa) or beta (ERb). Granulosa cells predominantly express ERb, a receptor that regulates granulosa cell growth and the effects of FSH. Animal experiments demonstrated that when rats were exposed to high levels of B[a]P, both E2 secretion and cumulus cell proliferation significantly decreased [47].
In rodent studies, benzo[a]pyrene (B[a]P) and other PAHs were shown to reduce external follicular growth [47], while in vivo exposure resulted in the loss of primordial follicles through apoptosis induction. In utero exposure to B[a]P led to a decreased quantity of primordial follicles and infertility in mice [49]. Earlier studies have indicated that B[a]P damaged DNA in human granulosa cells. However, recent research has shown that B[a]P is more concentrated in the follicular fluid compared to serum, suggesting that it may be preferentially accumulated in the ovary, leading to ovarian toxicity and selective destruction of preantral follicles, including primitive and primary follicles [47].
The effect of alkaloids (nicotine and its major metabolite cotinine) on ovarian development and function and its possible mechanisms
The content of alkaloids in tobacco smoke is relatively high (2010), with nicotine being the primary alkaloid, along with its main metabolites, nornicotine and cotinine. Nicotine exhibits a certain degree of anti-estrogenic effects, further disrupting the balance of reproductive and hormonal systems, and reducing the likelihood of successful pregnancy in both healthy women and those undergoing assisted reproduction [50]. Smoking can exacerbate the symptoms of premenstrual syndrome due to nicotine's impact on neural circuits, which increases sensitivity to environmental stressors [51]. Nicotine in cigarettes activates the sympathetic adrenal system, leading to increased synthesis and release of norepinephrine and adrenaline. These effects can result in an elevated heart rate, increased blood glucose levels, weight loss, and enhanced stimulation of the hypothalamic–pituitary–adrenal (HPA) and hypothalamic-pituitary-thyroid (HPT) axes, ultimately leading to elevated plasma cortisol and thyroid hormone concentrations [52]. Cotinine, with a biological half-life estimated to be approximately 16–19 h [53], serves as a valuable marker of cigarette smoke exposure due to its extended half-life [54]. Potential targets for these toxins include the nervous system, kidneys, liver, heart, and reproductive organs. Estrogen and progesterone, both essential for follicle growth, oocyte maturation, and ovulation, are steroid hormones that require synthesis from cholesterol. Nicotine can influence basal luteosterol production in human granulosa cells but has no significant effect on hCG-promoted progesterone release. Studies have indicated that nicotine and its metabolites cause human luteal insufficiency by regulating the prostaglandin (PG) system, severely inhibiting progesterone release. This inhibition of progesterone by tobacco smoke alkaloids appears to be associated with growth arrest and/or apoptosis of steroidogenic cells [55].
When nicotine was administered to oocytes at low levels (< 0.5 mM), there was no significant influence on their maturation. However, exposure to higher concentrations (5 mM) led to significant changes in subsequent meiosis, resulting in abnormal chromosomal configurations [56]. Animals exposed to high doses of nicotine exhibited an increased proportion of granulosa cells undergoing apoptosis in the follicles, further inhibiting follicle growth. Follicle growth is a crucial process in ovulation and fertilization, and nicotine-induced apoptosis of granulosa cells may be a significant mechanism in the context of cigarette smoke and fertility-related diseases.
The ovary expresses transcripts from different subunits of nAChR, and studies have shown that the expression levels of some transcripts are significantly altered after exposure to nicotine. Various cell types of different origins and functions express nicotine receptors, including nAChR, within the granulosa cells of the adult rat ovary. Some cells synthesize and release acetylcholine [57, 58]. The pharmacological properties of nAChRs promote a highly tissue-specific response to circulating levels of nicotine ligands. Through their interaction with nAChRs, several ligands can modulate the activity of specific nAChR isoforms, influencing biological functions such as cell proliferation, apoptosis, migration, and signal transduction [58].
The effects of polycyclic aromatic hydrocarbons on ovarian development and function and their possible mechanisms
PAHs in tobacco smoke are believed to exert significant toxic effects on the ovary. Studies have demonstrated that women who smoke exhibit lower pregnancy rates per menstrual cycle and experience early menopause [13]. In experimental research, various methods of exposing adult mice to various PAHs have induced ovarian tumorigenesis [59,60,61]. Epidemiological studies have revealed an elevated risk of developing epithelial ovarian cancer in women who smoke [62, 63].
PAHs are highly mutagenic and can lead to conditions such as mammalian primordial folliculopenia, follicular atresia, and overall ovarian toxicity. Exposure of ovaries to PAHs in vitro has been shown to increase the expression of Bcl-2, while the protein expression of pro-apoptotic markers such as Bax and active caspase 3 remains unchanged, with no significant alteration in the level of apoptosis. One specific PAH, 9,10-dimethylbenzo(a)anthracene-3,4-dihydrodiol (DMMA-DHD), is known to activate the aryl hydrocarbon receptor (AHR) present in human ovarian and reproductive cells [64]. This activation further induces fetal germ cell apoptosis by directly stimulating Bax expression [65]. Early exposure of fetal ovaries to PAHs during pregnancy has been associated with reduced ovarian germ cell proliferation [66]. Tobacco smoke accelerates oocyte atresia and impairs oocyte quality, which are the two primary factors contributing to the shortened female reproductive lifespan.
The effects of other harmful substances on ovarian development and function and their possible mechanisms
Various chemically active molecules, such as carbon dioxide, hydrogen peroxide, and nitrogen oxides, containing reactive oxygen derivatives and free radicals, are present in tobacco smoke. Apoptosis in follicle cells significantly increased due to oxidative stress, and a glutathione culture demonstrated that antioxidants can prevent excessive cell death [3]. When examining human ovarian cells, the primary toxic effects of smoking were observed to be oxidative stress and DNA damage in granulosa cells and cumulus cells of oocytes. This damage further resulted in impaired ovarian function, including improper maturation of oocytes, compromised binding of gonadotropins to their receptors, and an increase in the thickness of the transparent zone, which could reduce fertilization ability [67,68,69,70].
Both enzymatic and non-enzymatic antioxidants were found to be reduced in smokers [71]. The study also revealed that the antioxidant capacity of follicular fluid is generally low. High levels of nitric oxide (NO) produced by oocytes or follicular somatic cells play a crucial role in oocyte maturation and physiological processes. A decrease in NO levels can lead to high concentrations of free radicals causing cellular damage [72, 73]. Melatonin, known as N-acetyl-5-methoxytryptamine, can be derived from various tissues, such as the nervous system, immune system, and reproductive system [74, 75]. It serves as both a neurohormone and an antioxidant molecule. Melatonin and its metabolites exert their effects by scavenging reactive oxygen and reactive nitrogen species (RNS) [76],El-Sokkary et al. 2006). Treatment with different concentrations of melatonin in smokers can lead to a significant decrease in the apoptotic index of follicle cells, while simultaneously increasing tissue antioxidant activity [77].
Previous studies have identified more than 50 mutations in multiple genes, including LHCGR, FSHR, NR5A1 [78], NOBOX [79], FIGLA, BMP15 [80], FOXL2, STAG3, NANOS3 [81], as contributing factors in idiopathic POIs. The pathogenic factors of POI [82,83,84] involve the interplay of genetic changes and environmental factors, and the precise pathogenesis remains unclear.
Complications of POI (Table S1)
Impaired fertility represents the most distressing consequence of POI, imposing a significant psychological burden on young women. The presence of impaired fertility and the fear of premature aging are linked to a loss of emotional control, diminished self-esteem, social anxiety, and an increased incidence of depressive symptoms [85]. Currently, there are no clearly effective interventions to enhance residual ovarian function, with the primary approach being the use of donated eggs to improve the conception rate among women with POI (van Kasteren and Schoemaker 1999; [86]. Nevertheless, infertility associated with POI is not an absolute condition. In about 25% of patients diagnosed with POI, ovulation may still occur, potentially leading to pregnancy and childbirth. Furthermore, it is worth noting that women with idiopathic POI who conceive naturally do not exhibit a higher incidence of obstetric complications or neonatal risks when compared to the general population [86].
Cardiovascular disease and stroke stand out as the leading causes of reduced life expectancy among untreated POI patients compared to age-matched controls (Archer 2009; [86, 87]. This primary cause may be attributed to the diminished vascular endothelial function observed in POI patients, which in turn contributes to the development of atherosclerosis [88]. Estrogen proves to be advantageous for cholesterol metabolism, capable of reducing the incidence of atherosclerosis, and alleviating coronary artery constriction through catecholamine regulation [89]. Furthermore, endothelial function demonstrated significant improvement following estrogen therapy [90]. A consistent body of research supports that, in patients with POI, the benefits of estrogen therapy far outweigh any associated risks [91]. Women who experience premature menopause due to bilateral oophorectomy for medical reasons exhibit a substantial cognitive decline [92, 93], and the earlier the surgical intervention, the more rapid the cognitive decline tends to be [94]. An increased formation of long-term neuritic plaques amplifies the risk of Alzheimer's disease neuropathy [92, 93]. It should be noted that while supplemental estrogen therapy leads to an improvement in the degree of cognitive decline, it does not reverse the neuropathology associated with Alzheimer's disease [92, 93].
Bone mineral density (BMD) experiences a significant decrease in patients with POI [88, 95]. Lifestyle modifications, such as engaging in weight-bearing exercise, increasing calcium intake, supplementing with vitamin D, and refraining from smoking, promote overall bone health [86]. It is currently recommended to guide women with POI to consider estrogen replacement therapy prior to natural menopause, with the aim of reducing the risk of chronic diseases such as cardiovascular disease, osteoporosis, and neurocognitive impairment [86, 87].
Treatment of POI and prospects for treatment
Hormone replacement therapy (HRT), which is considered a physiological replacement for estrogen, is currently a common treatment for POI. However, it does not fully restore ovarian function. Therefore, there are various new methods under review for addressing this issue. These methods include stem cell activation, in vitro activation (IVA), mitochondrial activation, exosome therapy, biomaterial strategies, and intraovarian perfusion of platelet-rich plasma (PRP).
Stem cell activation can occur through the release of paracrine bioactive molecules, such as cytokines, regulatory factors, growth factors, and signal peptides. These molecules can have a positive impact on neighboring cells, improving ovarian function by exerting anti-inflammatory, anti-apoptotic, anti-angiogenic, anti-fibrotic, and immune-regulating functions [96]. In stem cell research, the primary focus has been on various types of stem cells, including bone marrow stem cells (BMSCs) [97,98,99,100], umbilical cord mesenchymal stem cells (UC-MSCs) [101, 102], menstrual blood-derived mesenchymal stem cells (MenSCs) [103, 104], human amniotic fluid stem cells (AFSCs) [105, 106], placenta-derived mesenchymal stem cells (PMSCs) [107,108,109,110], amniotic mesenchymal stem cells (AMSCs) [111, 112], and adipose-derived stem cells (ADSCs) [113, 114]. In addition, some researchers have proposed that induced oogonial stem celled (iOSC) may provide a new direction for the treatment of POI [115] (Table 1).
The tumorigenicity, immunogenicity, and heterogeneity of stem cells currently limit their applications, with a particular emphasis on addressing tumorigenicity. One emerging approach under investigation involves the intraovarian infusion of PRP as a novel treatment method for POI. In this method, activated platelets stimulate angiogenesis, control inflammation, and promote anabolism by releasing a multitude of hormones and growth factors, thereby facilitating tissue healing and regeneration [116]. The potential risks associated with this approach primarily include intense cellular proliferative events, which may pose a risk of malignancy, as well as the potential for infection and unknown adverse effects on the embryo. Most current studies on the treatment of POI are based on animal models. However, significant differences exist between humans and animals, underscoring the need for more precise experiments in the future to enhance the safety and efficacy of these new technologies in human applications.
Conclusion
In conclusion, POI can manifest through various mechanisms, including reduced peak follicle quantity, accelerated follicle depletion due to apoptosis, or follicle dysfunction [117]. This review offers a comprehensive understanding of the impact of tobacco smoke on POI, particularly the effects and potential mechanisms of various harmful substances such as PAHs, heavy metals (cadmium), alkaloids (nicotine and its primary metabolite, cotinine), and benzo[a]pyrene on ovarian development. Furthermore, it consolidates the range of complications associated with POI and highlights current treatment methods, thereby providing valuable insights for further exploration into the prevention and management of this condition.
References
Soares S, Melo MA. Cigarette smoking and reproductive function. 2008; 20(3):281–91 https://doi.org/10.1097/GCO.0b013e3282fc9c1e
Mikhael S, Punjala-Patel A, Gavrilova-Jordan L. Hypothalamic-pituitary-ovarian axis disorders impacting female fertility. Biomedicines. 2019; 7(1) https://doi.org/10.3390/biomedicines7010005.
Camlin N, McLaughlin E, Holt J. Through the smoke: use of in vivo and in vitro cigarette smoking models to elucidate its effect on female fertility. 2014; 281(3):266–75:https://doi.org/10.1016/j.taap.2014.10.010.
Ge W, Li L, Dyce PW, De Felici M, Shen W. Establishment and depletion of the ovarian reserve: physiology and impact of environmental chemicals. Cell Mol Life Sci. 2019;76(9):1729–46. https://doi.org/10.1007/s00018-019-03028-1.
Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808. https://doi.org/10.1093/humupd/dmv036.
Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76. https://doi.org/10.1001/jama.2021.4788.
Ben-Aharon I, Bar-Joseph H, Tzarfaty G, et al. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol. 2010;8:20. https://doi.org/10.1186/1477-7827-8-20.
Rudnicka E, Kruszewska J, Klicka K, et al. Premature ovarian insufficiency - aetiopathology, epidemiology, and diagnostic evaluation. 2018; 17(3):105–108 https://doi.org/10.5114/pm.2018.78550
Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. 2006;1:9. https://doi.org/10.1186/1750-1172-1-9.
Chang SH, Kim CS, Lee KS, et al. Premenopausal factors influencing premature ovarian failure and early menopause. Maturitas. 2007;58(1):19–30. https://doi.org/10.1016/j.maturitas.2007.04.001.
Torchalla I, Okoli CT, Bottorff JL, Qu A, Poole N, Greaves L. Smoking cessation programs targeted to women: a systematic review. Women Health. 2012;52(1):32–54. https://doi.org/10.1080/03630242.2011.637611.
Richardson MC, Guo M, Fauser BC, Macklon NS. Environmental and developmental origins of ovarian reserve. Hum Reprod Update. 2014;20(3):353–69. https://doi.org/10.1093/humupd/dmt057.
Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas. 2000;35(1):3–9. https://doi.org/10.1016/s0378-5122(00)00092-x.
Kinney A, Kline J, Levin B. Alcohol, caffeine and smoking in relation to age at menopause. Maturitas. 2006;54(1):27–38. https://doi.org/10.1016/j.maturitas.2005.10.001.
Sun L, Tan L, Yang F, et al. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause. 2012;19(2):126–32. https://doi.org/10.1097/gme.0b013e318224f9ac.
Li F, Wang Y, Xu M, et al. Single-nucleus RNA Sequencing reveals the mechanism of cigarette smoke exposure on diminished ovarian reserve in mice. Ecotoxicol Environ Saf. 2022;245: 114093. https://doi.org/10.1016/j.ecoenv.2022.114093.
Vidal J, VandeVoort C, Marcus C, Lazarewicz N, Conley AJ. In vitro exposure to environmental tobacco smoke induces CYP1B1 expression in human luteinized granulosa cells. 2006; 22(4):731–7 https://doi.org/10.1016/j.reprotox.2006.06.001
Sobinoff A, Beckett E, Jarnicki A, et al. Scrambled and fried: cigarette smoke exposure causes antral follicle destruction and oocyte dysfunction through oxidative stress. 2013;271(2):156–67. https://doi.org/10.1016/j.taap.2013.05.009.
Fowler P, Dorà N, McFerran H, et al. In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. 2008;14(5):269–80. https://doi.org/10.1093/molehr/gan020.
Hunt P, Lawson C, Gieske M, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. 2012;109(43):17525–30. https://doi.org/10.1073/pnas.1207854109.
Baron J, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. 1990; 162(2):502–14:https://doi.org/10.1016/0002-9378(90)90420-c.
Fowler P, Childs A, Courant F, et al. In utero exposure to cigarette smoke dysregulates human fetal ovarian developmental signalling. 2014;29(7):1471–89. https://doi.org/10.1093/humrep/deu117.
Gold E, Crawford S, Avis N, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. 2013;178(1):70–83. https://doi.org/10.1093/aje/kws421.
Li F, Ding J, Cong Y, et al. Trichostatin A alleviated ovarian tissue damage caused by cigarette smoke exposure. 2020;93:89–98. https://doi.org/10.1016/j.reprotox.2020.01.006.
Jurisicova A, Taniuchi A, Li H, et al. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J Clin Invest. 2007;117(12):3971–8. https://doi.org/10.1172/JCI28493.
Erdem Guzel E, Kaya N, Tektemur A, et al. Chronic effects of maternal tobacco-smoke exposure and/or alpha-lipoic acid treatment on reproductive parameters in female rat offspring. Syst Biol Reprod Med. 2020;66(6):387–99. https://doi.org/10.1080/19396368.2020.1815248.
Barbieri RL, Gochberg J, Ryan KJ. Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. J Clin Invest. 1986;77(6):1727–33. https://doi.org/10.1172/JCI112494.
Shiverick KT, Salafia C. Cigarette smoking and pregnancy I: ovarian, uterine and placental effects. Placenta. 1999;20(4):265–72. https://doi.org/10.1053/plac.1998.0377.
Tziomalos K, Charsoulis F. Endocrine effects of tobacco smoking. Clin Endocrinol (Oxf). 2004;61(6):664–74. https://doi.org/10.1111/j.1365-2265.2004.02161.x.
McLean BK, Rubel A, Nikitovitch-Winer MB. The differential effects of exposure to tobacco smoke on the secretion of luteinizing hormone and prolactin in the proestrous rat. Endocrinology. 1977;100(6):1566–70. https://doi.org/10.1210/endo-100-6-1566.
Aiken CE, Tarry-Adkins JL, Penfold NC, Dearden L, Ozanne SE. Decreased ovarian reserve, dysregulation of mitochondrial biogenesis, and increased lipid peroxidation in female mouse offspring exposed to an obesogenic maternal diet. FASEB J. 2016;30(4):1548–56. https://doi.org/10.1096/fj.15-280800.
Pittman DL, Cobb J, Schimenti KJ, et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1(5):697–705. https://doi.org/10.1016/s1097-2765(00)80069-6.
Ruth KS, Day FR, Hussain J, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596(7872):393–7. https://doi.org/10.1038/s41586-021-03779-7.
Mikkelsen TF, Graff-Iversen S, Sundby J, Bjertness E. Early menopause, association with tobacco smoking, coffee consumption and other lifestyle factors: a cross-sectional study. BMC Public Health. 2007;7:149. https://doi.org/10.1186/1471-2458-7-149.
Everson RB, Sandler DP, Wilcox AJ, Schreinemachers D, Shore DL, Weinberg C. Effect of passive exposure to smoking on age at natural menopause. Br Med J (Clin Res Ed). 1986;293(6550):792. https://doi.org/10.1136/bmj.293.6550.792.
Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med (Maywood). 2004;229(5):383–92. https://doi.org/10.1177/153537020422900506.
Samuel JB, Stanley JA, Princess RA, Shanthi P, Sebastian MS. Gestational cadmium exposure-induced ovotoxicity delays puberty through oxidative stress and impaired steroid hormone levels. J Med Toxicol. 2011;7(3):195–204. https://doi.org/10.1007/s13181-011-0143-9.
Baird DD, Wilcox AJ. Cigarette smoking associated with delayed conception. JAMA. 1985; 253(20):2979–83 3999259
Piasek M, Laskey JW. Effects of in vitro cadmium exposure on ovarian steroidogenesis in rats. J Appl Toxicol. 1999. https://doi.org/10.1002/(SICI)1099-1263(199905/06)19:3<211::AID-JAT568>3.0.CO;2-4.
Singh A, Chattopadhyay R, Chakravarty B. Chaudhury KJRt. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. 2013;42:116–24. https://doi.org/10.1016/j.reprotox.2013.08.005.
Zenzes M, Krishnan S, Krishnan B, Zhang H, Casper RJF. Cadmium accumulation in follicular fluid of women in in vitro fertilization-embryo transfer is higher in smokers. 1995; 64(3):599–603 https://doi.org/10.1016/s0015-0282(16)57799-1
Wang Y, Wang X, Wang Y, et al. Effect of cadmium on cellular ultrastructure in mouse ovary. 2015;39(5):324–8. https://doi.org/10.3109/01913123.2015.1027436.
Zhang W, Wu T, Zhang C, Luo L, Xie M. Huang HJTl. Cadmium exposure in newborn rats ovary induces developmental disorders of primordial follicles and the differential expression of SCF/c-kit gene. 2017;280:20–8. https://doi.org/10.1016/j.toxlet.2017.08.004.
Weng S, Wang W, Li Y, et al. Continuous cadmium exposure from weaning to maturity induces downregulation of ovarian follicle development-related SCF/c-kit gene expression and the corresponding changes of DNA methylation/microRNA pattern. 2014;225(3):367–77. https://doi.org/10.1016/j.toxlet.2014.01.012.
Nna V, Usman U, Ofutet E, Owu DU, Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride - induced oxidative stress in the uterus and ovaries of female Wistar rats. 2017; 102:143–155 https://doi.org/10.1016/j.fct.2017.02.010.
Samuel J, Stanley J, Princess R, Shanthi P, Sebastian MS. Gestational cadmium exposure-induced ovotoxicity delays puberty through oxidative stress and impaired steroid hormone levels. 2011; 7(3):195–204 https://doi.org/10.1007/s13181-011-0143-9
Neal M, Zhu J, Holloway A, Foster WJ. Follicle growth is inhibited by benzo-[a]-pyrene, at concentrations representative of human exposure, in an isolated rat follicle culture assay. 2007; 22(4):961–7 https://doi.org/10.1093/humrep/del487.
Goldman R, Enewold L, Pellizzari E, et al. Smoking increases carcinogenic polycyclic aromatic hydrocarbons in human lung tissue. 2001; 61(17):6367–71 11522627
Lim J, Lawson G, Nakamura B, et al. Glutathione-deficient mice have increased sensitivity to transplacental benzo[a]pyrene-induced premature ovarian failure and ovarian tumorigenesis. 20013; 73(2):908–17 https://doi.org/10.1158/0008-5472.Can-12-3636.
Jandikova H, Duskova M, Starka L. The influence of smoking and cessation on the human reproductive hormonal balance. Physiol Res. 2017;66(Suppl 3):S323–31. https://doi.org/10.33549/physiolres.93372.
Choi SH, Hamidovic A. Association between smoking and premenstrual syndrome: a meta-analysis. Front Psychiatry. 2020;11:575526 https://doi.org/10.3389/fpsyt.2020.575526.
Berlin I. Endocrine and metabolic effects of smoking cessation. Curr Med Res Opin. 2009;25(2):527–34. https://doi.org/10.1185/03007990802707626.
Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78(6):696–8. https://doi.org/10.2105/ajph.78.6.696.
Benowitz NL, Kuyt F, Jacob P 3rd, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34(5):604–11. https://doi.org/10.1038/clpt.1983.222.
Miceli F, Minici F, Tropea A, et al. Effects of nicotine on human luteal cells in vitro: a possible role on reproductive outcome for smoking women. Biol Reprod. 2005;72(3):628–32. https://doi.org/10.1095/biolreprod.104.032318.
Racowsky C, Hendricks RC, Baldwin KV. Direct effects of nicotine on the meiotic maturation of hamster oocytes. Reprod Toxicol. 1989;3(1):13–21. https://doi.org/10.1016/0890-6238(89)90033-6.
Petrik J, Gerstein H, Cesta C, Kellenberger L, Alfaidy N, Holloway AJE. Effects of rosiglitazone on ovarian function and fertility in animals with reduced fertility following fetal and neonatal exposure to nicotine. 2009;36(2):281–90. https://doi.org/10.1007/s12020-009-9229-4.
Zhang B, Madden P, Gu J, et al. Uncovering the transcriptomic and epigenomic landscape of nicotinic receptor genes in non-neuronal tissues. 2017;18(1):439. https://doi.org/10.1186/s12864-017-3813-4.
Biancifiori C, Bonser GM, Caschera F. Ovarian and mammary tumours in intact C3Hb virgin mice following a limited dose of four carcinogenic chemicals. Br J Cancer. 1961;15(2):270–83. https://doi.org/10.1038/bjc.1961.34.
Krarup T. 9:10-dimethyl-1:2-benzantracene induced ovarian tumours in mice. Acta Pathol Microbiol Scand. 1967;70(2):241–8. https://doi.org/10.1111/j.1699-0463.1967.tb01287.x.
Taguchi O, Michael SD, Nishizuka Y. Rapid induction of ovarian granulosa cell tumors by 7,12-dimethylbenz(a)anthracene in neonatally estrogenized mice. Cancer Res. 1988; 48(2):425–9 3121173
Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol. 2010;171(1):45–53. https://doi.org/10.1093/aje/kwp314.
Terry PD, Miller AB, Jones JG, Rohan TE. Cigarette smoking and the risk of invasive epithelial ovarian cancer in a prospective cohort study. Eur J Cancer. 2003;39(8):1157–64. https://doi.org/10.1016/s0959-8049(03)00195-3.
Khorram O, Garthwaite M, Golos T. Uterine and ovarian aryl hydrocarbon receptor (AHR) and aryl hydrocarbon receptor nuclear translocator (ARNT) mRNA expression in benign and malignant gynaecological conditions. 2002; 8(1):75–80 https://doi.org/10.1093/molehr/8.1.75.
Matikainen T, Moriyama T, Morita Y, et al. Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. 2002;143(2):615–20. https://doi.org/10.1210/endo.143.2.8624.
Anderson R, McIlwain L, Coutts S, Kinnell H, Fowler P, Childs AJ. Activation of the aryl hydrocarbon receptor by a component of cigarette smoke reduces germ cell proliferation in the human fetal ovary. 2014; 20(1):42–8:https://doi.org/10.1093/molehr/gat059.
Alviggi C, Cariati F, Conforti A, et al. The effect of FT500 Plus(®) on ovarian stimulation in PCOS women. 2016; 59:40–4; https://doi.org/10.1016/j.reprotox.2015.10.014.
Budani M, Carletti E, Tiboni GJZ. Cigarette smoke is associated with altered expression of antioxidant enzymes in granulosa cells from women undergoing in vitro fertilization. 2017;25(3):296–303. https://doi.org/10.1017/s0967199417000132.
Shiloh H, Lahav-Baratz S, Koifman M, et al. The impact of cigarette smoking on zona pellucida thickness of oocytes and embryos prior to transfer into the uterine cavity. 2004;19(1):157–9. https://doi.org/10.1093/humrep/deh029.
Sinkó I, Mórocz M, Zádori J, Kokavszky K, Raskó I. Effect of cigarette smoking on DNA damage of human cumulus cells analyzed by comet assay. 2005; 20(1):65–71 https://doi.org/10.1016/j.reprotox.2004.12.007
Mohamed M, Sulaiman S, Jaafar H, Sirajudeen KN. Antioxidant protective effect of honey in cigarette smoke-induced testicular damage in rats. 2011; 12(9):5508–21 https://doi.org/10.3390/ijms12095508.
Cui M, Wang X, Tang D, Zhang J, Liu Y, Zeng SJT. Acetylation of H4K12 in porcine oocytes during in vitro aging: potential role of ooplasmic reactive oxygen species. 2011; 75(4):638–46 https://doi.org/10.1016/j.theriogenology.2010.09.031.
Xu Z, Dai F, Chen J, et al. Experimental research into the potential therapeutic effect of GYY4137 on Ovariectomy-induced osteoporosis. 2018; 23:47 https://doi.org/10.1186/s11658-018-0114-0.PMID: 30305826
Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz JJT. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. 2006; 228:333–43; https://doi.org/10.1016/j.tox.2006.09.018.
Reiter R, Tan D, Korkmaz A, Rosales-Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. 2014; 20(2):293–307 https://doi.org/10.1093/humupd/dmt054.
El-Sokkary G, Cuzzocrea S, Reiter RJT. Effect of chronic nicotine administration on the rat lung and liver: beneficial role of melatonin. 2007;239:60–7. https://doi.org/10.1016/j.tox.2007.06.092.
Kole E, Ozkan S, Eraldemir C, et al. Effects of melatonin on ovarian reserve in cigarette smoking: an experimental study. 2020;16(6):1376–86. https://doi.org/10.5114/aoms.2019.89409.
Lourenço D, Brauner R, Lin L, et al. Mutations in NR5A1 associated with ovarian insufficiency. 2009; 360(12):1200–10 doi:https://doi.org/10.1056/NEJMoa0806228.
Qin Y, Choi Y, Zhao H, Simpson J, Chen Z, Rajkovic A. NOBOX homeobox mutation causes premature ovarian failure. 2007; 81(3):576–81 https://doi.org/10.1086/519496.
Chand A, Ponnampalam A, Harris S, Winship I, Shelling AJF. Mutational analysis of BMP15 and GDF9 as candidate genes for premature ovarian failure. 2006; 86(4):1009–12:https://doi.org/10.1016/j.fertnstert.2006.02.107.
Wu X, Wang B, Dong Z, et al. A NANOS3 mutation linked to protein degradation causes premature ovarian insufficiency. 2013; 4:e825 https://doi.org/10.1038/cddis.2013.368
Caburet S, Arboleda V, Llano E, et al. Mutant cohesin in premature ovarian failure. 2014;370(10):943–9. https://doi.org/10.1056/NEJMoa1309635.
Fonseca D, Patiño L, Suárez Y, et al. Next generation sequencing in women affected by nonsyndromic premature ovarian failure displays new potential causative genes and mutations. 2015;104(1):154-62.e2. https://doi.org/10.1016/j.fertnstert.2015.04.016.
Tucker E, Grover S, Bachelot A, Touraine P, Sinclair AH. Premature ovarian insufficiency: new perspectives on genetic cause and phenotypic spectrum. 2016; 37(6):609–635 https://doi.org/10.1210/er.2016-1047
Schmidt P, Cardoso G, Ross J, Haq N, Rubinow D, Bondy CJJ. Shyness, social anxiety, and impaired self-esteem in Turner syndrome and premature ovarian failure. 2006; 295(12):1374–6 https://doi.org/10.1001/jama.295.12.1374
Webber L, Davies M, Anderson R, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. 2016;31(5):926–37. https://doi.org/10.1093/humrep/dew027.
Rocca W, Grossardt B, Miller V, Shuster L, Brown RJM. Premature menopause or early menopause and risk of ischemic stroke. 2012;19(3):272–7. https://doi.org/10.1097/gme.0b013e31822a9937.
Sullivan S, Sarrel P, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. 2016; 106(7):1588–1599 https://doi.org/10.1016/j.fertnstert.2016.09.046
Sarrel P, Lindsay D, Rosano G, Poole-Wilson PA. Angina and normal coronary arteries in women: gynecologic findings. 1992; 167(2):467–71 https://doi.org/10.1016/s0002-9378(11)91431-8
Kalantaridou S, Naka K, Papanikolaou E, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. 2004;89(8):3907–13. https://doi.org/10.1210/jc.2004-0015.
Moen M, Rees M, Brincat M, et al. EMAS position statement: Managing the menopause in women with a past history of endometriosis. 2010;67(1):94–7. https://doi.org/10.1016/j.maturitas.2010.04.018.
Bove R, Secor E, Chibnik L, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. 2014;82(3):222–9. https://doi.org/10.1212/wnl.0000000000000033.
Sarrel P, Sullivan S, Nelson LJF. Hormone replacement therapy in young women with surgical primary ovarian insufficiency. 2016; 106(7):1580–1587 https://doi.org/10.1016/j.fertnstert.2016.09.018
Phung T, Waltoft B, Laursen T, et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. 2010; 30(1):43–50 https://doi.org/10.1159/000314681.
Popat V, Calis K, Vanderhoof V, et al. Bone mineral density in estrogen-deficient young women. 2009;94(7):2277–83. https://doi.org/10.1210/jc.2008-1878.
Zhao Y, Chen S, Su P, et al. Using Mesenchymal Stem Cells to Treat Female Infertility: an update on female reproductive diseases. 2019;2019:9071720. https://doi.org/10.1155/2019/9071720.
Abd-Allah S, Shalaby S, Pasha H, et al. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. 2013; 15(1):64-75; https://doi.org/10.1016/j.jcyt.2012.08.001
Fu X, He Y, Wang X, et al. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. 2017; 8(1):187 https://doi.org/10.1186/s13287-017-0641-z.
Guo J, Gao X, Lin Z, et al. BMSCs reduce rat granulosa cell apoptosis induced by cisplatin and perimenopause. 2013;14:18. https://doi.org/10.1186/1471-2121-14-18.
Zhang Y, Le X, Zheng S, et al. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. 2022; 13(1):171 https://doi.org/10.1186/s13287-022-02855-7
Cui L, Bao H, Liu Z, et al. hUMSCs regulate the differentiation of ovarian stromal cells via TGF-β/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats. 2020; 11(1):386:https://doi.org/10.1186/s13287-020-01904-3.
Ding C, Zhu L, Shen H, et al. Exosomal miRNA-17–5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. 2020; 38(9):1137–1148:https://doi.org/10.1002/stem.3204.
Wang Z, Wang Y, Yang T, Li J, Yang X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. 2017; 8(1):11 https://doi.org/10.1186/s13287-016-0458-1
Yan Z, Guo F, Yuan Q, et al. Endometrial mesenchymal stem cells isolated from menstrual blood repaired epirubicin-induced damage to human ovarian granulosa cells by inhibiting the expression of Gadd45b in cell cycle pathway. 2019; 10(1):4 https://doi.org/10.1186/s13287-018-1101-0
Xiao G, Cheng C, Chiang Y, Cheng W, Liu I, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. 2016; 6:23120 doi:https://doi.org/10.1038/srep23120
Yu X, Wang N, Qiang R, et al. Human amniotic fluid stem cells possess the potential to differentiate into primordial follicle oocytes in vitro. 2014;90(4):73. https://doi.org/10.1095/biolreprod.113.112920.
Li H, Zhao W, Wang L, et al. Human placenta-derived mesenchymal stem cells inhibit apoptosis of granulosa cells induced by IRE1α pathway in autoimmune POF mice. 2019; 43(8):899–909 https://doi.org/10.1002/cbin.11165.
Seok J, Park H, Choi J, Lim J, Kim K, Kim GJA. Placenta-derived mesenchymal stem cells restore the ovary function in an ovariectomized rat model via an antioxidant effect. 2020; 9(7) https://doi.org/10.3390/antiox9070591
Yin N, Wang Y, Lu X, et al. hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. 2018; 9(1):37 https://doi.org/10.1186/s13287-018-0772-x
Zhang H, Luo Q, Lu X, et al. Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice. 2018;9(1):20. https://doi.org/10.1186/s13287-017-0745-5.
Ding C, Qian C, Hou S, et al. Exosomal miRNA-320a is released from hAMSCs and regulates SIRT4 to prevent reactive oxygen species generation in POI. 2020; 21:37–50 https://doi.org/10.1016/j.omtn.2020.05.013.
Ling L, Feng X, Wei T, et al. Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats. 2017; 8(1):283 https://doi.org/10.1186/s13287-017-0739-3.
Huang B, Lu J, Ding C, et al. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. 2018;9(1):216. https://doi.org/10.1186/s13287-018-0953-7.
Terraciano P, Garcez T, Ayres L, et al. Cell therapy for chemically induced ovarian failure in mice. 2014;2014: 720753. https://doi.org/10.1155/2014/720753.
Celik O, Ak M, Sahin E, Senturk S, Ugur K, Celik S, Celik N, Cengiz F, Muderris Iİ, Capar M, Sahin İ, Aydin S. Intra-ovarian stem cell transplantation in management of premature ovarian insufficiency: towards the induced Oogonial Stem Cell (iOSC). Cell Mol Biol (Noisy-le-grand). 2020; 66(1):114–121.
Cakiroglu Y, Saltik A, Yuceturk A, et al. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. 2020;12(11):10211–22. https://doi.org/10.18632/aging.103403.
Maclaran K, Panay N. Current concepts in premature ovarian insufficiency. Womens Health (Lond). 2015;11(2):169–82. https://doi.org/10.2217/whe.14.82.
How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. Publications and Reports of the Surgeon General, Atlanta (GA) 2010.
Archer DF. Premature menopause increases cardiovascular risk. 2009; 26–31: https://doi.org/10.1080/13697130903013452.
El-Sokkary G, Khidr B. Younes HJEjop. Role of melatonin in reducing hypoxia-induced oxidative stress and morphological changes in the liver of male mice. 2006;540:107–14. https://doi.org/10.1016/j.ejphar.2006.04.036.
European Society for Human R, Embryology Guideline Group on POI, Webber L, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016; 31(5):926–37:https://doi.org/10.1093/humrep/dew027.
van Kasteren Y, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. 1999; 5(5):483–92 https://doi.org/10.1093/humupd/5.5.483
Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos. 2000; 6(2):122–31 https://doi.org/10.1093/humupd/6.2.122
Declarations
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (81971459), and the Natural Science Foundation of Liaoning Province (No. 2020-MS-03).
Author information
Authors and Affiliations
Contributions
Ying Wang conceived and wrote the main manuscript. Jinghan Cui prepared figures and tables. All authors edited, revised and approved this review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table 1. Complications of POI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, J., Wang, Y. Premature ovarian insufficiency: a review on the role of tobacco smoke, its clinical harm, and treatment. J Ovarian Res 17, 8 (2024). https://doi.org/10.1186/s13048-023-01330-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-023-01330-y