Abstract
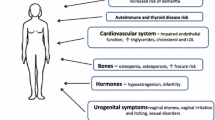
Premature ovarian insufficiency (POI) is a condition in which a woman experiences premature decline in ovarian function before the age of 40 years, manifested by menstrual disorders, decreased fertility, and possibly postmenopausal symptoms such as insomnia, hot flashes, and osteoporosis, and is one of the predominant clinical syndromes leading to female infertility. Genetic, immunologic, iatrogenic and other factors, alone or in combination, have been reported to trigger POI, yet the etiology remains unknown in most cases. The main methods currently used clinically to ameliorate menopausal symptoms due to hypoestrogenemia in POI patients are hormone replacement therapy, while the primary methods available to address infertility in POI patients are oocyte donation and cryopreservation techniques, both of which have limitations to some degree. In recent years, researchers have continued to explore more efficient and safe therapies, and have achieved impressive results in preclinical trials. In this article, we will mainly review the three most popular therapies and their related signaling pathways published in the past ten years, with the aim of providing ideas for clinical applications.

Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Rebar RW, Keator CS. Expanding our knowledge of premature ovarian insufficiency. Fertil Steril. 2021;115(2):328–9. https://doi.org/10.1016/j.fertnstert.2020.09.145.
Anderson RA, et al. Anti-Müllerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: a systematic review. Hum Reprod Update. 2022;28(3):417–34. https://doi.org/10.1093/humupd/dmac004.
European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937. https://doi.org/10.1093/humrep/dew027.
Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22(4):403–11. https://doi.org/10.1080/13697137.2019.1574738.
De Vos M, Devroey P, Fauser BCJM. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–21. https://doi.org/10.1016/S0140-6736(10)60355-8.
Silvén H, et al. Incidence and familial risk of premature ovarian insufficiency in the Finnish female population. Hum Reprod. 2022;37(5):1030–6. https://doi.org/10.1093/humrep/deac014.
de Carvalho BR, de Rosa e Silva ACJS, Rosa e Silva JC, dos Reis RM, Ferriani RA, de Silva Sá MF. “Ovarian reserve evaluation: state of the art. J Assist Reprod Genet. 2008;25(7):311–22. https://doi.org/10.1007/s10815-008-9241-2.
Bates GW. Is hope on the horizon for premature ovarian insufficiency? Fertil Steril. 2018;109(5):800–1. https://doi.org/10.1016/j.fertnstert.2018.02.129.
Vujovic S. Aetiology of premature ovarian failure. Menopause Int. 2009;15(2):72–5. https://doi.org/10.1258/mi.2009.009020.
Takahashi A, Yousif A, Hong L, Chefetz II. Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell. J Mol Med. 2021;99(5):637–50. https://doi.org/10.1007/s00109-021-02055-5.
Silvén H, et al. Association of genetic disorders and congenital malformations with premature ovarian insufficiency: a nationwide register-based study. Hum Reprod. 2023;38(6):1224–30. https://doi.org/10.1093/humrep/dead066.
Heddar A, et al. Genetic landscape of a large cohort of Primary Ovarian Insufficiency: New genes and pathways and implications for personalized medicine. EBioMedicine. 2022;84:104246. https://doi.org/10.1016/j.ebiom.2022.104246.
Laml T, Preyer O, Umek W, Hengstschlager M, Hanzal H. Genetic disorders in premature ovarian failure. Hum Reprod Update. 2002;8(5):483–91. https://doi.org/10.1093/humupd/8.5.483.
Abir R, Fisch B, Nahum R, Orvieto R, Nitke S, Ben Rafael Z. Turner’s syndrome and fertility: current status and possible putative prospects. Hum Reprod Update. 2001;7(6):603–10. https://doi.org/10.1093/humupd/7.6.603.
Bondy CA, Bondy and Turner Syndrome Study Group. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92(1):10–25. https://doi.org/10.1210/jc.2006-1374.
Biswas L, Tyc K, El Yakoubi W, Morgan K, Xing J, Schindler K. Meiosis interrupted: the genetics of female infertility via meiotic failure. Reproduction. 2021;161(2):R13–35. https://doi.org/10.1530/REP-20-0422.
Yang Q, Mumusoglu S, Qin Y, Sun Y, Hsueh AJ. A kaleidoscopic view of ovarian genes associated with premature ovarian insufficiency and senescence. FASEB J. 2021;35(8):e21753. https://doi.org/10.1096/fj.202100756R.
Hu M, et al. PRC1-mediated epigenetic programming is required to generate the ovarian reserve. Nat Commun. 2022;13(1):4510. https://doi.org/10.1038/s41467-022-31759-6.
Roy S, et al. Jumonji domain-containing protein-3 (JMJD3/Kdm6b) is critical for normal ovarian function and female fertility. Endocrinology. 2022;163(5):bqac047. https://doi.org/10.1210/endocr/bqac047.
Jackson-Cook C. A hypothesis: Could telomere length and/or epigenetic alterations contribute to infertility in females with Turner syndrome? Am J Med Genet. 2019;181(1):116–24. https://doi.org/10.1002/ajmg.c.31684.
Vichinsartvichai P. Primary ovarian insufficiency associated with autosomal abnormalities: from chromosome to genome-wide and beyond. Menopause. 2016;23(7):806–15. https://doi.org/10.1097/GME.0000000000000603.
Hanna CW, et al. MLL2 conveys transcription-independent H3K4 trimethylation in oocytes. Nat Struct Mol Biol. 2018;25(1):73–82. https://doi.org/10.1038/s41594-017-0013-5.
Szeliga A, et al. Autoimmune diseases in patients with premature ovarian insufficiency—our current state of knowledge. Int J Mol Sci. 2021;22(5):5. https://doi.org/10.3390/ijms22052594.
Chen C-W, Huang Y-L, Tzeng C-R, Huang R-L, Chen C-H. Idiopathic low ovarian reserve is associated with more frequent positive thyroid peroxidase antibodies. Thyroid. 2017;27(9):1194–200. https://doi.org/10.1089/thy.2017.0139.
Luo W, Mao P, Zhang L, Chen X, Yang Z. “Assessment of ovarian reserve by serum anti-Müllerian hormone in patients with systemic lupus erythematosus: a meta-analysis. Ann Palliat Med. 2020;9(2):207–15. https://doi.org/10.21037/apm.2020.02.11.
Nguyen Q-N, Zerafa N, Findlay JK, Hickey M, Hutt KJ. DNA repair in primordial follicle oocytes following cisplatin treatment. J Assist Reprod Genet. 2021;38(6):1405–17. https://doi.org/10.1007/s10815-021-02184-3.
Nguyen Q-N, et al. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018;9(6):618. https://doi.org/10.1038/s41419-018-0633-7.
Wang Q, Stringer JM, Liu J, Hutt KJ. Evaluation of mitochondria in oocytes following γ-irradiation. Sci Rep. 2019;9(1):19941. https://doi.org/10.1038/s41598-019-56423-w.
Leung CT, et al. Low-dose radiation can cause epigenetic alterations associated with impairments in both male and female reproductive cells. Front Genet. 2021;12:710143. https://doi.org/10.3389/fgene.2021.710143.
Kaufman FR, Donnell GN, Roe TF, Kogut MD. Gonadal function in patients with galactosaemia. J Inherit Metab Dis. 1986;9(2):140–6. https://doi.org/10.1007/BF01799450.
Forges T, Monnier-Barbarino P, Leheup B, Jouvet P. Pathophysiology of impaired ovarian function in galactosaemia. Hum Reprod Update. 2006;12(5):573–84. https://doi.org/10.1093/humupd/dml031.
Menezo Y, Dale B, Elder K. The negative impact of the environment on methylation/epigenetic marking in gametes and embryos: A plea for action to protect the fertility of future generations. Mol Reprod Dev. 2019;86(10):1273–82. https://doi.org/10.1002/mrd.23116.
Turkyilmaz A, et al. Whole-exome sequencing reveals new potential genes and variants in patients with premature ovarian insufficiency. J Assist Reprod Genet. 2022;39(3):695–710. https://doi.org/10.1007/s10815-022-02408-0.
Lindh-Åstrand L, Hoffmann M, Järvstråt L, Fredriksson M, Hammar M, Spetz Holm A-C. Hormone therapy might be underutilized in women with early menopause. Hum Reprod. 2015;30(4):848–52. https://doi.org/10.1093/humrep/dev017.
Sare GM, Gray LJ, Bath PMW. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29(16):2031–41. https://doi.org/10.1093/eurheartj/ehn299.
Pinelli S, Basile S. Fertility preservation: current and future perspectives for oncologic patients at risk for Iatrogenic premature ovarian insufficiency. Biomed Res Int. 2018;2018:6465903. https://doi.org/10.1155/2018/6465903.
Ruan X, et al. Ovarian tissue cryopreservation and transplantation prevents iatrogenic premature ovarian insufficiency: first 10 cases in China. Climacteric. 2020;23(6):574–80. https://doi.org/10.1080/13697137.2020.1767569.
Soares M, et al. Eliminating malignant cells from cryopreserved ovarian tissue is possible in leukaemia patients. Br J Haematol. 2017;178(2):231–9. https://doi.org/10.1111/bjh.14657.
Dolmans M-M, Hossay C, Nguyen TYT, Poirot C. Fertility preservation: how to preserve ovarian function in children, adolescents and adults. J Clin Med. 2021;10(22):5247. https://doi.org/10.3390/jcm10225247.
Kim SS, Battaglia DE, Soules MR. The future of human ovarian cryopreservation and transplantation: fertility and beyond. Fertil Steril. 2001;75(6):1049–56. https://doi.org/10.1016/s0015-0282(01)01790-3.
Baker V. Life plans and family-building options for women with primary ovarian insufficiency. Semin Reprod Med. 2011;29(4):362–72. https://doi.org/10.1055/s-0031-1280921.
Tong Y, et al. The trends and hotspots in premature ovarian insufficiency therapy from 2000 to 2022. Int J Environ Res Public Health. 2022;19(18):11728. https://doi.org/10.3390/ijerph191811728.
Maidarti M, Anderson RA, Telfer EE. Crosstalk between PTEN/PI3K/Akt signalling and DNA damage in the oocyte: implications for primordial follicle activation, oocyte quality and ageing. Cells. 2020;9(1):200. https://doi.org/10.3390/cells9010200.
Zhou J, Peng X, Mei S. Autophagy in ovarian follicular development and atresia. Int J Biol Sci. 2019;15(4):726–37. https://doi.org/10.7150/ijbs.30369.
Mi X, Jiao W, Yang Y, Qin Y, Chen Z-J, Zhao S. HGF secreted by mesenchymal stromal cells promotes primordial follicle activation by increasing the activity of the PI3K-AKT signaling pathway. Stem Cell Rev Rep. 2022;18(5):1834–50. https://doi.org/10.1007/s12015-022-10335-x.
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215–8. https://doi.org/10.1126/science.1086336.
Liu L, et al. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134(1):199–209. https://doi.org/10.1242/dev.02667.
Wang B, et al. Analysis of FOXO3 mutation in 114 Chinese women with premature ovarian failure. Reprod Biomed Online. 2010;20(4):499–503. https://doi.org/10.1016/j.rbmo.2010.01.008.
Zhang B-F, et al. The role of AKT and FOXO3 in preventing ovarian toxicity induced by cyclophosphamide”. PLoS One. 2018;13(8):e0201136. https://doi.org/10.1371/journal.pone.0201136.
Thanatsis N, Kaponis A, Koika V, Georgopoulos NA, Decavalas GO. Reduced Foxo3a, FoxL2, and p27 mRNA expression in human ovarian tissue in premature ovarian insufficiency. Hormones (Athens). 2019;18(4):409–15. https://doi.org/10.1007/s42000-019-00134-4.
Cheng Y, Kim J, Li XX, Hsueh AJ. Promotion of ovarian follicle growth following mTOR activation: synergistic effects of AKT stimulators”. PLoS One. 2015;10(2):e0117769. https://doi.org/10.1371/journal.pone.0117769.
Adhikari D, et al. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15(12):765–70. https://doi.org/10.1093/molehr/gap092.
Adhikari D, et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19(3):397–410. https://doi.org/10.1093/hmg/ddp483.
Zhang J, et al. The protective effects of human umbilical cord mesenchymal stem cells on damaged ovarian function: A comparative study. Biosci Trends. 2016;10(4):265–76. https://doi.org/10.5582/bst.2016.01125.
Reddy P, et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18(15):2813–24. https://doi.org/10.1093/hmg/ddp217.
John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321(1):197–204. https://doi.org/10.1016/j.ydbio.2008.06.017.
Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21(8):886–97. https://doi.org/10.1101/gad.1536007.
Zhai J, et al. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab. 2016;101(11):4405–12. https://doi.org/10.1210/jc.2016-1589.
Hsueh AJW, Kawamura K. Hippo signaling disruption and ovarian follicle activation in infertile patients. Fertil Steril. 2020;114(3):458–64. https://doi.org/10.1016/j.fertnstert.2020.07.031.
Lee HN, Chang EM. Primordial follicle activation as new treatment for primary ovarian insufficiency. Clin Exp Reprod Med. 2019;46(2):43–9. https://doi.org/10.5653/cerm.2019.46.2.43.
Grosbois J, Demeestere I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum Reprod. 2018;33(9):1705–14. https://doi.org/10.1093/humrep/dey250.
Reddy P, Deguchi M, Cheng Y, Hsueh AJW. Actin cytoskeleton regulates Hippo signaling. PLoS One. 2013;8(9):e73763. https://doi.org/10.1371/journal.pone.0073763.
Liu J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. https://doi.org/10.1038/s41392-021-00762-6.
Chen C, et al. Protective effects of puerarin on premature ovarian failure via regulation of Wnt/β-catenin signaling pathway and oxidative stress. Reprod Sci. 2021;28(4):982–90. https://doi.org/10.1007/s43032-020-00325-0.
Fan H-Y, O’Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol. 2010;24(8):1529–42. https://doi.org/10.1210/me.2010-0141.
Chen B, et al. Mutation analysis of the WNT4 gene in Han Chinese women with premature ovarian failure. Reprod Biol Endocrinol. 2011;9:75. https://doi.org/10.1186/1477-7827-9-75.
Boyer A, et al. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010;24(8):3010–25. https://doi.org/10.1096/fj.09-145789.
Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397(6718):405–9. https://doi.org/10.1038/17068.
Abedini A, et al. WNT5a is required for normal ovarian follicle development and antagonizes gonadotropin responsiveness in granulosa cells by suppressing canonical WNT signaling. FASEB J. 2016;30(4):1534–47. https://doi.org/10.1096/fj.15-280313.
Santibañez JF, Quintanilla M, Bernabeu C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2011;121(6):233–51. https://doi.org/10.1042/CS20110086.
Tiotiu D, et al. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum Reprod. 2010;25(6):1581–7. https://doi.org/10.1093/humrep/deq073.
Rossetti R, et al. Fundamental role of BMP15 in human ovarian folliculogenesis revealed by null and missense mutations associated with primary ovarian insufficiency. Hum Mutat. 2020;41(5):983–97. https://doi.org/10.1002/humu.23988.
Park MJ, et al. Prediction of ovarian aging using ovarian expression of BMP15, GDF9, and C-KIT. Exp Biol Med (Maywood). 2020;245(8):711–9. https://doi.org/10.1177/1535370220915826.
Durlinger AL, et al. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–96. https://doi.org/10.1210/endo.140.12.7204.
Gruijters MJG, Visser JA, Durlinger ALL, Themmen APN. Anti-Müllerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003;211(1–2):85–90. https://doi.org/10.1016/j.mce.2003.09.024.
Gleicher N, et al. Clinical relevance of combined FSH and AMH observations in infertile women. J Clin Endocrinol Metab. 2013;98(5):2136–45. https://doi.org/10.1210/jc.2013-1051.
Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol. 2008;28(23):7001–11. https://doi.org/10.1128/MCB.00732-08.
Panay N, et al. Premature ovarian insufficiency: an International Menopause Society White Paper. Climacteric. 2020;23(5):426–46. https://doi.org/10.1080/13697137.2020.1804547.
Machura P, et al. Premature ovarian insufficiency - hormone replacement therapy and management of long-term consequences. Prz Menopauzalny. 2018;17(3):135–8. https://doi.org/10.5114/pm.2018.78559.
Piccioni P, et al. Hormonal replacement therapy after stem cell transplantation. Maturitas. 2004;49(4):327–33. https://doi.org/10.1016/j.maturitas.2004.02.015.
Madalinska JB, et al. The impact of hormone replacement therapy on menopausal symptoms in younger high-risk women after prophylactic salpingo-oophorectomy. J Clin Oncol. 2006;24(22):3576–82. https://doi.org/10.1200/JCO.2005.05.1896.
Baber RJ, Panay N, Fenton A. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19(2):109–50. https://doi.org/10.3109/13697137.2015.1129166.
Furness S, Roberts H, Marjoribanks J, Lethaby A, Hickey M, Farquhar C. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst Rev. 2004;3 https://doi.org/10.1002/14651858.CD000402.pub2
Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–81. https://doi.org/10.1001/jama.288.7.872.
Barrett-Connor E, Stuenkel CA. Hormone replacement therapy (HRT)–risks and benefits. Int J Epidemiol. 2001;30(3):423–6. https://doi.org/10.1093/ije/30.3.423.
Lutjen P, Trounson A, Leeton J, Findlay J, Wood C, Renou P. The establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failure. Nature. 1984;307(5947):5947. https://doi.org/10.1038/307174a0.
Klein JU, Sauer MV. Ethics in egg donation: past, present, and future. Semin Reprod Med. 2010;28(4):322–8. https://doi.org/10.1055/s-0030-1255180.
Klitzman RL, Sauer MV. Kamakahi vs ASRM and the future of compensation for human eggs. Am J Obstet Gynecol. 2015;213(2):186-187.e1. https://doi.org/10.1016/j.ajog.2015.03.046.
Frith L, Blyth E, Farrand A. UK gamete donors’ reflections on the removal of anonymity: implications for recruitment. Hum Reprod. 2007;22(6):1675–80. https://doi.org/10.1093/humrep/dem061.
ÖzgülÖzdemir RB, et al. Mesenchymal stem cells: a potential treatment approach for refractory chronic spontaneous urticaria. Stem Cell Rev Rep. 2021;17(3):911–22. https://doi.org/10.1007/s12015-020-10059-w.
Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–48. https://doi.org/10.1007/s00018-019-03125-1.
Lai D, Wang F, Yao X, Zhang Q, Wu X, Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155. https://doi.org/10.1186/s12967-015-0516-y.
Park H-S, et al. Human BM-MSC secretome enhances human granulosa cell proliferation and steroidogenesis and restores ovarian function in primary ovarian insufficiency mouse model. Sci Rep. 2021;11(1):4525. https://doi.org/10.1038/s41598-021-84216-7.
Lee H-J, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25(22):3198–204. https://doi.org/10.1200/JCO.2006.10.3028.
Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10(4):353–63. https://doi.org/10.1080/14653240802035926.
Kilic S, Pinarli F, Ozogul C, Tasdemir N, NazSarac G, Delibasi T. Protection from cyclophosphamide-induced ovarian damage with bone marrow-derived mesenchymal stem cells during puberty. Gynecol Endocrinol. 2014;30(2):135–40. https://doi.org/10.3109/09513590.2013.860127.
Gabr H, Rateb MA, El Sissy MH, Ahmed Seddiek H, Ali Abdelhameed Gouda S. The effect of bone marrow-derived mesenchymal stem cells on chemotherapy induced ovarian failure in albino rats. Microsc Res Techniq. 2016;79(10):938–47. https://doi.org/10.1002/jemt.22725.
Song K, Cai H, Zhang D, Huang R, Sun D, He Y. Effects of human adipose-derived mesenchymal stem cells combined with estrogen on regulatory T cells in patients with premature ovarian insufficiency. Int Immunopharmacol. 2018;55:257–62. https://doi.org/10.1016/j.intimp.2017.12.026.
Sen Halicioglu B, Saadat KASM, Tuglu MI. “Adipose-derived mesenchymal stem cell transplantation in chemotherapy-induced premature ovarian insufficiency: the role of connexin and pannexin. Reprod Sci. 2022;29(4):1316–31. https://doi.org/10.1007/s43032-021-00718-9.
Hong L, et al. Protective effects of human umbilical cord mesenchymal stem cell-derived conditioned medium on ovarian damage. J Mol Cell Biol. 2020;12(5):372–85. https://doi.org/10.1093/jmcb/mjz105.
Liu T, et al. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 2014;23(13):1548–57. https://doi.org/10.1089/scd.2013.0371.
Cho J, et al. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab Invest. 2021;101(3):304–17. https://doi.org/10.1038/s41374-020-00513-1.
Ling L, et al. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther. 2019;10(1):46. https://doi.org/10.1186/s13287-019-1136-x.
Li J, et al. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Res Ther. 2018;9(1):81. https://doi.org/10.1186/s13287-018-0819-z.
Bahrehbar K, RezazadehValojerdi M, Esfandiari F, Fathi R, Hassani S-N, Baharvand H. Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure. World J Stem Cells. 2020;12(8):857–78. https://doi.org/10.4252/wjsc.v12.i8.857.
Lai D, Wang F, Dong Z, Zhang Q. Skin-derived mesenchymal stem cells help restore function to ovaries in a premature ovarian failure mouse model. PLoS One. 2014;9(5):e98749. https://doi.org/10.1371/journal.pone.0098749.
Feng X, et al. Effects of human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation in situ on primary ovarian insufficiency in SD rats. Reprod Sci. 2020;27(7):1502–12. https://doi.org/10.1007/s43032-020-00147-0.
Fazekasova H, Lechler R, Langford K, Lombardi G. Placenta-derived MSCs are partially immunogenic and less immunomodulatory than bone marrow-derived MSCs. J Tissue Eng Regen Med. 2011;5(9):684–94. https://doi.org/10.1002/term.362.
Kretlow JD, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. https://doi.org/10.1186/1471-2121-9-60.
Ding C, et al. EGF released from human placental mesenchymal stem cells improves premature ovarian insufficiency via NRF2/HO-1 activation. Aging (Albany NY). 2020;12(3):2992–3009. https://doi.org/10.18632/aging.102794.
Liu J, et al. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol Cells. 2014;37(12):865–72. https://doi.org/10.14348/molcells.2014.0145.
Ding L, et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci China Life Sci. 2018;61(12):1554–65. https://doi.org/10.1007/s11427-017-9272-2.
Fu X, et al. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. 2017;8(1):187. https://doi.org/10.1186/s13287-017-0641-z.
Park H-S, et al. Towards cell free therapy of premature ovarian insufficiency: human bone marrow mesenchymal stem cells secretome enhances angiogenesis in human ovarian microvascular endothelial cells. HSOA J Stem Cells Res Dev Ther. 2019;5(2):019. https://doi.org/10.24966/srdt-2060/100019.
Deng T, et al. Human umbilical cord mesenchymal stem cells improve ovarian function in chemotherapy-induced premature ovarian failure mice through inhibiting apoptosis and inflammation via a paracrine mechanism. Reprod Sci. 2021;28(6):1718–32. https://doi.org/10.1007/s43032-021-00499-1.
Jiao W, et al. Mesenchymal stem cells combined with autocrosslinked hyaluronic acid improve mouse ovarian function by activating the PI3K-AKT pathway in a paracrine manner. Stem Cell Res Ther. 2022;13(1):49. https://doi.org/10.1186/s13287-022-02724-3.
Zhao Y, et al. Human umbilical cord mesenchymal stem cells restore the ovarian metabolome and rescue premature ovarian insufficiency in mice. Stem Cell Res Ther. 2020;11(1):466. https://doi.org/10.1186/s13287-020-01972-5.
Awwad J, Farra C, Hannoun A, Abou-Abdallah M, Isaacson K, Ghazeeri G. Idiopathic premature ovarian failure: what is the most suitable ovarian stimulation protocol? Clin Exp Obstet Gynecol. 2013;40(3):327–30.
Cui L, et al. hUMSCs regulate the differentiation of ovarian stromal cells via TGF-β1/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats. Stem Cell Res Ther. 2020;11(1):386. https://doi.org/10.1186/s13287-020-01904-3.
El-Derany MO, Said RS, El-Demerdash E. Bone marrow-derived mesenchymal stem cells reverse radiotherapy-induced premature ovarian failure: emphasis on signal integration of TGF-β, Wnt/β-catenin and hippo pathways. Stem Cell Rev Rep. 2021;17(4):1429–45. https://doi.org/10.1007/s12015-021-10135-9.
Edessy M, Hosni H, Shady Y, Waf Y, Bakr S, Kamel M. Autologous stem cells therapy, The first baby of idiopathic premature ovarian failure. Acta Med Int. 2016;3(1):19. https://doi.org/10.5530/ami.2016.1.7.
Igboeli P, et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J Med Case Rep. 2020;14(1):108. https://doi.org/10.1186/s13256-020-02426-5.
Yan L, et al. Clinical analysis of human umbilical cord mesenchymal stem cell allotransplantation in patients with premature ovarian insufficiency. Cell Proliferat. 2020;53(12):e12938. https://doi.org/10.1111/cpr.12938.
Mashayekhi M, et al. Evaluation of safety, feasibility and efficacy of intra-ovarian transplantation of autologous adipose derived mesenchymal stromal cells in idiopathic premature ovarian failure patients: non-randomized clinical trial, phase I, first in human. J Ovarian Res. 2021;14(1):5. https://doi.org/10.1186/s13048-020-00743-3.
Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–8. https://doi.org/10.1097/00008505-200110000-00002.
Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150–63.
Sharara FI, Lelea L-L, Rahman S, Klebanoff JS, Moawad GN. A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J Assist Reprod Genet. 2021;38(5):1003–12. https://doi.org/10.1007/s10815-021-02146-9.
Sills ES, Rickers NS, Li X, Palermo GD. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol. 2018;34(9):756–60. https://doi.org/10.1080/09513590.2018.1445219.
Tu S, Wu WJ, Wang J, Cerione RA. Epidermal growth factor-dependent regulation of Cdc42 is mediated by the Src tyrosine kinase. J Biol Chem. 2003;278(49):49293–300. https://doi.org/10.1074/jbc.M307021200.
Yan H, et al. CDC42 controls the activation of primordial follicles by regulating PI3K signaling in mouse oocytes. BMC Biol. 2018;16(1):73. https://doi.org/10.1186/s12915-018-0541-4.
Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006;131(6):1007–15. https://doi.org/10.1530/rep.1.00978.
El Bakly W, et al. Optimized platelet rich plasma releasate (O-rPRP) repairs galactosemia-induced ovarian follicular loss in rats by activating mTOR signaling and inhibiting apoptosis. Heliyon. 2020;6(9):e05006. https://doi.org/10.1016/j.heliyon.2020.e05006.
Sfakianoudis K, et al. Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. J Clin Med. 2018;8(1):1. https://doi.org/10.3390/jcm8010001.
Pantos K, et al. A Case Series on Natural Conceptions Resulting in Ongoing Pregnancies in Menopausal and Prematurely Menopausal Women Following Platelet-Rich Plasma Treatment. Cell Transplant. 2019;28(9–10):1333–40. https://doi.org/10.1177/0963689719859539.
Sfakianoudis K, et al. Reactivating ovarian function through autologous platelet-rich plasma intraovarian infusion: pilot data on premature ovarian insufficiency, perimenopausal, menopausal, and poor responder women. J Clin Med. 2020;9(6):1809. https://doi.org/10.3390/jcm9061809.
Cakiroglu Y, et al. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY). 2020;12(11):10211–22. https://doi.org/10.18632/aging.103403.
Sabouni R, Tarrab R, Kalaji D, Abbassi H. A new approach of using platelet-rich autologous plasma to increase the ovarian reservoir in a Syrian patient with ovarian insufficiency: A case report. Ann Med Surg (Lond). 2022;73:103149. https://doi.org/10.1016/j.amsu.2021.103149.
Navali N, et al. Intraovarian injection of autologous platelet-rich plasma improves therapeutic approaches in the patients with poor ovarian response: a before-after study. Int J Fertil Steril. 2022;16(2):90–4. https://doi.org/10.22074/IJFS.2021.533576.1154.
Hsu C-C, Hsu L, Hsu I, Chiu Y-J, Dorjee S. Live birth in woman with premature ovarian insufficiency receiving ovarian administration of platelet-rich plasma (PRP) in combination with gonadotropin: a case report. Front Endocrinol. 2020;11:50. https://doi.org/10.3389/fendo.2020.00050.
Li J, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA. 2010;107(22):10280–4. https://doi.org/10.1073/pnas.1001198107.
Suzuki N, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–15. https://doi.org/10.1093/humrep/deu353.
Kawamura K, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013;110(43):17474–9. https://doi.org/10.1073/pnas.1312830110.
Zhao B, Tumaneng K, Guan K-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):8. https://doi.org/10.1038/ncb2303.
Zhang L, et al. Autotransplantation of the ovarian cortex after in-vitro activation for infertility treatment: a shortened procedure. Hum Reprod. 2021;36(8):2134–47. https://doi.org/10.1093/humrep/deab143.
McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol Hum Reprod. 2014;20(8):736–44. https://doi.org/10.1093/molehr/gau037.
Kawamura K, Ishizuka B, Hsueh AJW. Drug-free in-vitro activation of follicles for infertility treatment in poor ovarian response patients with decreased ovarian reserve. Reprod Biomed Online. 2020;40(2):245–53. https://doi.org/10.1016/j.rbmo.2019.09.007.
Keikha F, et al. One side ovarian rejuvenation: a quasi-experimental study of the effect of the autologous platelet rich plasma in poor ovarian responders in IVF. Ethiop J Health Sci. 2022;32(6):1133–40. https://doi.org/10.4314/ejhs.v32i6.10.
Cakiroglu Y, et al. Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich plasma (PRP). Aging Albany NY. 2022;14(6):2513–23. https://doi.org/10.18632/aging.203972.
Imesch P, et al. Developmental potential of human oocytes matured in vitro followed by vitrification and activation. J Ovarian Res. 2013;6:30. https://doi.org/10.1186/1757-2215-6-30.
Fabregues F, et al. Pregnancy after drug-free in vitro activation of follicles and fresh tissue autotransplantation in primary ovarian insufficiency patient: a case report and literature review. J Ovarian Res. 2018;11(1):76. https://doi.org/10.1186/s13048-018-0447-3.
Lunding SA, et al. Biopsying, fragmentation and autotransplantation of fresh ovarian cortical tissue in infertile women with diminished ovarian reserve. Hum Reprod. 2019;34(10):1924–36. https://doi.org/10.1093/humrep/dez152.
Ferreri J, et al. Drug-free in-vitro activation of follicles and fresh tissue autotransplantation as a therapeutic option in patients with primary ovarian insufficiency. Reprod Biomed Online. 2020;40(2):254–60. https://doi.org/10.1016/j.rbmo.2019.11.009.
Zhai J, et al. Autotransplantation of the ovarian cortex after in-vitro activation for infertility treatment: a shortened procedure. Hum Reprod. 2021;36(8):2134–47. https://doi.org/10.1093/humrep/deab143.
Funding
This study was supported by grants from Research Fund for Shandong Provincial Natural Science Foundation (ZR2020QH042) and Lin He’s Academician Workstation of New Medicine and Clinical Translation in Jining Medical University (JYHL2021MS13).
Author information
Authors and Affiliations
Contributions
Kai Meng and Xuechun Ding contributed to the study conception and design. Xuechun Ding, Shenmin Lv, Zhipeng Guo and Xiaowei Gong wrote the manuscript. Kai Meng, Caiqin Wang and Xiaoyan Zhang contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors approved the final manuscript and the submission to this journal.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, X., Lv, S., Guo, Z. et al. Potential Therapeutic Options for Premature Ovarian Insufficiency: Experimental and Clinical Evidence. Reprod. Sci. 30, 3428–3442 (2023). https://doi.org/10.1007/s43032-023-01300-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01300-1