Abstract
Breast cancer is one of the frequent tumors that seriously endanger the physical and mental well-being in women. F-box and WD repeat domain-containing 7 (FBXW7) is a neoplastic repressor. Serving as a substrate recognition element for ubiquitin ligase, FBXW7 participates in the ubiquitin–proteasome system and is typically in charge of the ubiquitination and destruction of crucial oncogenic proteins, further performing a paramount role in cell differentiation, apoptosis and metabolic processes. Low levels of FBXW7 cause abnormal stability of pertinent substrates, mutations and/or deletions in the FBXW7 gene have been reported to correlate with breast cancer malignant progression and chemoresistance. Given the lack of an effective solution to breast cancer's clinical drug resistance dilemma, elucidating FBXW7's mechanism of action could provide a theoretical basis for targeted drug exploration. Therefore, in this review, we focused on FBXW7's role in a range of breast cancer malignant behaviors and summarized the pertinent cellular targets, signaling pathways, as well as the mechanisms regulating FBXW7 expression. We also proposed novel perspectives for the exploitation of alternative therapies and specific tumor markers for breast cancer by therapeutic strategies aiming at FBXW7.
Similar content being viewed by others
Introduction
Breast cancer is the most prominent type of cancer in females globally, with the second highest fatality rate [1]. Chemotherapy remains the backbone of therapy for advanced breast cancer because advanced patients make up most breast cancer patients, but this comes with side effects such as drug resistance and organ damage, as well as a higher risk of metastasis and recurrence [2]. It is apparent that metastasis and chemotherapy resistance has substantially harmed the survival of patients with breast cancer, and better-targeted therapeutic options are required to arrest its progression.
The Skp1-Cullin1-F-box (SCF) complex's substrate receptor, F-box and WD repeat domain-containing 7 (FBXW7), is a member of the FBXW family of F-box proteins [3]. 13 encoding exons and 4 untranslated introns make up the roughly 210 kb-long human FBXW7 gene, located at 4q31q.3 chromosome, which is absent in ~ 30% cancers [4]. There are 3 different FBXW7 isoforms: FBXW7α, FBXW7β, and FBXW7γ, with distinct exons created via selective splicing. The isoforms have different cellular localizations. The nucleoplasm, cytoplasm, and nucleolus are the respective locations of FBXW7α, FBXW7β, and FBXW7γ [5] (Fig. 1). The following functionally significant domains are found in the regions shared by all FBXW7 subtypes: the D domain, which promotes the formation of FBXW7 dimers; the WD40 domain, which recognizes substrates; and the F-box domain, which connects with the SCF group subunits RING-finger protein 1 (RBX1), Cullin1 (CUL1), and S-phase kinase-associated protein 1 (Skp1). The SCF complex acts as a ubiquitin ligase (E3) to ubiquitinate proteins and initiate proteasomal degradation along with ubiquitin-activating enzymes (E1) and ubiquitin-conjugating enzymes (E2) [6]. FBXW7 may recognize substrates that have been phosphorylated at specific residues through the conserved Cdc4 phospho-degron (CPD) motif. FBXW7 employs the phosphorylation of substrates, which is mediated mostly by Glycogen Synthase Kinase 3 beta (GSK3β), to encourage substrate recruitment, ubiquitination, and proteasomal degradation [7]. By encouraging the ubiquitination and destruction of associated oncogenic proteases such c-Myc, Kruppel-like factor 5 (KLF5), c-Jun, NOTCH, cyclin E, mechanistic target of rapamycin (mTOR), and Aurora A, FBXW7 inhibited the formation of tumors [8,9,10,11,12,13,14] (Fig. 2). Recent evidence has connected abnormal FBXW7 expression to breast cancer growth, metastasis, and drug resistance [15,16,17]. Research in mouse models confirmed that FBXW7 deletion causes mammary epithelial cell degeneration with invasive cancer transformation and that the number of transplanted tumor nodules in the lungs of FBXW7 gene-deficient mice is significantly increased [18, 19]. Additionally, inactivating FBXW7 leads to a drop in double-stranded RNA (dsRNA) in mice tumor cells, triggering an altered immune microenvironment and anti-PD-1 resistance [20]. Breast cancer development is affected by associated regulators, FBXW7, and protein substrates crosstalk at the molecular level. Therefore, to break the clinical resistance bottleneck and achieve an early targeted diagnosis, it is necessary to elucidate FBXW7's biological functions in breast cancer. In this review, we highlight the functional role of FBXW7 in the spectrum of malignant behaviors in breast cancer, ubiquitination targets, and the mechanisms of FBXW7 expression. Furthermore, we also summarize breast cancer therapeutic strategies targeting FBXW7, providing novel perspectives for the exploration and innovation of clinically targeted agents and specific diagnostic markers.
Structure of the FBXW7 gene situated on human chromosome 4q31q.3 and protein isoforms of FBXW7. All FBXW7 isoforms have a locus structure with 10 exons (c1-c10) and 4 introns. Additionally, While FBXW7β and FBXW7γ are both encoded by a single exon (β1 and γ1), FBXW7α has four distinct exons (α1, α2, α3, and α4). Apart from the NH2 terminal section, all three isomers of FBXW7 share the D domain, the F-box domain, and the WD40 repeat domain. FBXW7α, FBXW7β, and FBXW7γ are present in the nucleoplasm, cytoplasm, and nucleolus, respectively. FBXW7, F-box and WD repeat domain-containing 7
SCFFBXW7-mediated ubiquitination and degradation of substrate proteins. Ubiquitin molecules and phosphorylation are denoted by Ub and P, respectively. Before being transported to E2, the ubiquitin molecule is first activated by E1 via a thioester bond with associated ATP hydrolysis. The SCF-type E3 ligase then identifies the phosphorylated substrate through the substrate receptor FBXW7 and adds ubiquitin from E2 to the substrate. Once enough ubiquitin chains have been attached to the substrate, they are transferred in an ATP-dependent manner to the 26S proteasome for hydrolysis. While the ubiquitin molecule circulates, the substrate protein is broken down into a brief peptide. FBXW7 substrates mainly include c-Myc, c-Jun, cyclin E, NOTCH, Aurora A, KLF5, and mTOR. FBXW7, F-box and WD repeat domain-containing 7. E1, ubiquitin-activating enzymes. E2, ubiquitin-conjugating enzymes. E3, ubiquitin ligase. SCF, Skp1-Cullin1-F-box. RBX1, RING-finger protein 1. CUL1, Cullin1. Skp1, S-phase kinase-associated protein 1. KLF5, Kruppel-like factor 5. mTOR, mechanistic target of rapamycin
The Regulation of FBXW7 Expression
FBXW7 regulation at transcriptional level
Given the identification of FBXW7 mutations and/or deletion in a range of malignancies, abnormal regulation of FBXW7 expression may be a trigger for carcinogenesis. FBXW7 has multiple transcriptional regulators, including the tumor suppressor p53, the transcriptional repressors RAN binding protein 10 (RANBP10) and HES family bHLH transcription factor 5 (HES5), the transcriptional activator CCAAT/enhancer-binding protein-delta (C/EBPδ) and 27-hydroxycholesterol (27-HC) [21,22,23,24,25] (Fig. 3). A p53 binding site (p53BS) has been reported on exon 1b of the FBXW7 gene. p53 activates FBXW7 transcription through p53BS, whereas p53 loss in breast cancer inhibits FBXW7 expression, increasing NOTCH5 receptor activation and chemotherapy resistance [26, 27]. 27-HC is an oxysterol that activates estrogen receptor-positive (ER +) breast cancer cell growth by stimulating ERα [28]. The transcriptional activity of FBXW7 is repressed by 27-HC, hence attenuating c-Myc turnover and promoting MCF-7 cells proliferation [25]. The leucine zipper protein family member C/EBPδ, generally stimulates substrate transcription and slows tumor growth. However, C/EBPδ appears to exert an opposite modulating effect on FBXW7 [29]. Most of these transcriptional elements negatively regulate the mRNA levels of FBXW7.
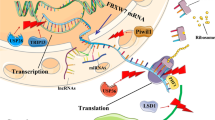
Mechanisms of intracellular FBXW7 regulation at the transcriptional, translational, and post-translational levels. (1) At the transcriptional level, C/EBPδ and HES5 inhibit the FBXW7 gene’s transcription. (2) During FBXW7 pre-mRNA splicing, three isoforms α, β, and γ are produced through alternative splicing. (3) At the translational level, ncRNAs control the expression levels of the FBXW7 mRNA and protein. MiRNAs first form RISC and then bind to the 3' UTR of FBXW7 and regulate its expression, while lncRNAs and circRNAs restore the expression levels of FBXW7 mRNA and protein by sponge-attracting miRNAs. (4) At the post-translational level, multiple upstream factors regulate FBXW7 dimerization, phosphorylation, and auto-ubiquitination. LSD1 inhibits FBXW7 dimerization, ERK1/2 promotes FBXW7 phosphorylation, and USP28 inhibits FBXW7 auto- and substrate ubiquitination. FBXW7, F-box and WD repeat domain-containing 7. HES5, HES family bHLH transcription factor 5. C/EBPδ, CCAAT/enhancer-binding protein-delta. LncRNA, long non-coding RNA. CircRNA, circular RNA. MiRNA, microRNA. RISC, RNA-induced silencing complex, USP28, ubiquitin-specific proteases 28. LSD1, lysine-specific demethylase 1. ERK1/2, extracellular signal-regulated kinases 1 and 2
Non-coding RNAs regulate FBXW7 protein
MicroRNAs
MicroRNAs bind to the 3' untranslated region (3' UTR) of the mRNA to control FBXW7 protein translation and mRNA degradation (Fig. 3). Chen et al. revealed that miR-194 suppresses protein translation by specifically combining with the 3' UTR of FBXW7, leading to a rise in the synthesis of cyclin D and cyclin E and the promotion of breast cancer cell proliferation [30]. Additionally, microRNAs' control over FBXW7 has a role in breast cancer metastasis caused by the epithelial-mesenchymal transition (EMT). Jiang et al. revealed that miR-27a, an upriver controller gene of FBXW7, restricts its expression at the protein expression level, resulting in EMT occurrence and breast cancer metastasis [31]. Related studies on FBXW7 regulation by miRNAs are summarized in Table 1.
CircRNAs
A unique family of single-stranded non-coding RNA molecules called circular RNAs (circRNAs) lacks polyadenylate tails, 5′ to 3′ polarities, and have closed-loop topologies [38]. Akin to long non-coding RNAs (lncRNAs), the majority of circRNAs serve as competing endogenous RNAs (ceRNAs) for miRNAs that modulate FBXW7 (Fig. 3). Liu et al. demonstrated that hsa_circ_0022742 reverses the level of repressed FBXW7 expression by sponging miR-503-5p [39]. CircRNAs are typically regarded as incapable of encoding proteins. Recently, however, Yang et al. uncovered that circFBXW7 can bind FBXW7α competitively with ubiquitin-specific proteases 28 (USP28) by encoding the FBXW7-185aa protein, thereby inhibiting the deubiquitination of FBXW7α by USP28 [40]. Moreover, Ye et al. demonstrated that circFBXW7 may destabilize c-Myc by competing for sponging miR-197-3p, boosting the protein level of FBXW7, in addition to encoding protein. This prevents the development and migration of triple-negative breast cancer (TNBC) cells [37]. Related studies on FBXW7 regulation by circRNAs are summarized in Table 1.
FBXW7 regulation at post-translational level
The regulation of post-translational modifications of FBXW7 consists of several key processes, including dimerization, phosphorylation, auto-ubiquitination, and deubiquitination.
Dimerization
In essence, FBXW7 dimerization is a superhelical structure formed by D domain precursors with spatial variability that enhances the FBXW7's capacity to bind to a diversity of inferior degraders and encourages the ubiquitination and destruction of substrates [41, 42]. Notably, some oncogenic factors could promote cancer development by regulating the dimerization of FBXW7. For example, lysine-specific demethylase 1 (LSD1) blocks FBXW7 dimerization by acting as a pseudosubstrate rather than a demethylase to bind to FBXW7, leading to a reduction in oncoprotein turnover by promoting FBXW7 autoubiquitination [43] (Fig. 3). Despite the current lack of clarity regarding the role played by FBXW7 dimers in breast cancer progression, making clear the crosstalk between dimerization in FBXW7 and substrate ubiquitination could provide a potential molecular mechanism for cellular regulation.
Phosphorylation
Post-translational phosphorylation of FBXW7 is catalyzed by multiple kinases, including extracellular signal-regulated kinases 1 and 2 (ERK1/2), polo-like kinase 1 and 2 (PLK1/2), phosphoinositide 3-kinase (PI3K), and protein kinase C (PKC) [44,45,46,47] (Fig. 3). It was reported that the regulation of FBXW7 phosphorylation by associated enzymes has facilitated the turnover of FBXW7 itself [44]. Neuregulin-1 (NRG1)-activated ERK1/2 stabilizes downstream c-Myc recruitment to the FOS-like 1 and AP-1 transcription factor subunit (Fra-1) promoters by promoting FBXW7 phosphorylation, in turn facilitating TNBC metastasis [47]. Meanwhile, phosphorylation of FBXW7 by PI3K at S227 inhibits the self-ubiquitination, which consequently promotes the turnover of downstream targets c-Myc and cyclin E [46].
Auto-ubiquitination and deubiquitination
At the post-translational level, auto-ubiquitination and deubiquitinating enzymes (DUBs) modulate the degradation of the FBXW7 protein. According to reports, F-box proteins catalyze auto-ubiquitination in the SCF complex in a manner that is SCF-dependent [48]. Chen et al. displayed that COP9 signalosome 6 (CSN6) promotes auto-ubiquitination of FBXW7, causing an increase in c-Myc expression [49]. Moreover, FBXW7 dimerization also facilitates its auto-ubiquitination [42]. Intriguingly, the regulation of FBXW7 by USP28 deletion is complicated due to the dual regulation of the ubiquitination of FBXW7 itself and its substrates (Fig. 3). Complete knockdown of USP28 in mice triggers the auto-ubiquitination of FBXW7 and leads to substrate accumulation, while maintaining the single allele deletion of USP28 partially restores FBXW7 expression, and substrate protein levels are reduced in all mouse tissues [50]. USP28 deletion is associated with 53.2%-9.7% of breast cancer cases, which reveals that USP28 gene deletion-mediated disruption of FBXW7 auto-ubiquitination might correlate with breast cancer development in certain way, but this remains to be further investigated [51].
Overall, the regulation of FBXW7 is intertwined with a variety of positive and negative regulators and complex molecular pathways at the transcriptional, translational, and post-translational levels. An exploration of whether additional mechanisms regulate the intracellular expression of FBXW7 will help identify promising new breast cancer therapeutic targets.
FBXW7 regulates breast cancer-related signaling pathways
FBXW7 regulates NOTCH/NICD pathway
The NOTCH gene encodes conserved cell membrane protein receptors. The binding of NOTCH ligands to the receptors triggers NOTCH activation and releases NOTCH protein fragments (NICD/ICN) via triple protein hydrolysis into the nucleus to activate transcription of downstream target genes, causing cancer cell processes [52]. It has been shown that FBXW7 is responsible for ubiquitin binding to NICD and promotes its proteasomal degradation, inhibiting NOTCH pathway activation [53]. Zhao et al. found that FBXW7 enhances the binding to NICD1 mediated by the melanoma-associated antigen A1 (MAGEA1) gene, which promotes the ubiquitination of FBXW7SCF-targeted NICD1, further reducing its binding to the transcriptional repressor CBFl/Suppressor of Hairless/Lag1 (CSL) in the nucleus, thus hindering MFC-7 and MDA-MB-231 cells proliferation [15]. FBXW7α specifically recognizes residues T2512/P2513 in the cdc4-phosphodegron of the nuclear molecules NICD1 and NICD4, while highly expressed prolyl-isomerase1 (Pin1) competitively binds to the site where NICD1/NICD4 docks with FBXW7α, disrupting the feed-forward molecular circuit between the three, inducing breast carcinogenesis and promoting breast cancer stem cell (BCSC) self-renewal [17]. These observations revealed that the relationship between FBXW7 and the NOTCH/NICD pathway is not a simple unidirectional one, but rather a cross-talk between upstream and downstream regulators of FBXW7 and NOTCH effector molecules (Fig. 4).
FBXW7 Regulates Breast Cancer-Related Signaling Pathways. Red arrows indicate inhibition, black arrows facilitation, and green arrows indicate interaction facilitation or crosstalk. Delta or Jagged binds to the NOTCH receptor to activate the NOTCH/NICD pathway. FBXW7 inhibits NICD1/NICD4 expression by promoting NICD1/NICD4 ubiquitination, which inhibits CSL DNA binding proteins downstream of the NOTCH pathway and target genes HES1, SLUG, and Hey1. Akt inhibits GSK-3-mediated upregulation of FBXW7, while FBXW7 in turn inhibits the PI3/Akt/mTOR pathway by promoting proteasomal degradation of Akt/mTOR/HIF-1α. C/EBPδ-mediated PI3K/Akt/mTOR-NF-κB crosstalk involves FBXW7. C/EBPδ promotes mTOR accumulation by inhibiting FBXW7 expression. IKKα is activated by Akt and C/EBPδ and inhibited as a substrate for FBXW7. CDK and cyclin create a complex that activates Rb when phosphorylated, while FBXW7 ubiquitinates and degrades CDK and cyclin to sustain the cell cycle. Uncontrolled transcription of downstream target genes by FBXW7 will cause anomalies in cell proliferation, cell cycle processes, metastasis, invasion, and immunosuppression. FBXW7, F-box and WD repeat domain-containing 7. HES1, HES family bHLH transcription factor 1. C/EBPδ, CCAAT/enhancer-binding protein-delta. CSL, CBFl/Suppressor of Hairless/Lag1. NICD1, NOTCH intracellular domain 1. mTOR, mechanistic target of rapamycin. NF-κB, nuclear factor-kappa B. HIF-1α, hypoxia inducible factor 1 alpha
FBXW7 regulates PI3K/Akt/mTOR pathway
High-frequency activation of the PI3K/Akt/mTOR pathway in BC greatly contributes to the transcription of downstream pro-oncogenic target genes such as HIF-1α, c-Myc and forkhead box O (FOXO), promoting cell proliferation, metastasis and drug resistance [54,55,56]. PI3K, the upstream signaling molecule of mTOR, regulates FBXW7 expression post-translationally through specific phosphorylation of the CPD motif. Aberrant stimulation of the signaling pathways PI3K/Akt and Wnt dramatically suppresses GSK-3β-mediated ubiquitination of FBXW7 [57]. C/EBPδ knock out (C/EBPδ-KO) breast tumor cells exhibit reduced Akt activity and decreased Ser9 phosphorylation of GSK-3β, which causes increased expression of FBXW7 downstream of GSK-3β. FBXW7 liberation from C/EBPδ transcriptional repression promotes polyubiquitination of endogenous mTOR and HIF-1α protein binding and inhibits intracellular accumulation, reducing lung metastasis in breast cancer model mice under hypoxic adaptation [16]. Furthermore, knocking down p50 and RelA, subunits of nuclear factor-kappa B (NF-κB), significantly attenuates IL-1-induced C/EBPδ expression [58]. Interestingly, while NF-κB interacts with and amplifies C/EBPδ in cancer cells, FBXW7, which is controlled by C/EBPδ, in turn triggers ubiquitination and destruction of NF-κB via GSK-3 phosphorylation, inhibiting the NF-κB cascade response in breast cancer [59]. Thus, the FBXW7 gene is a key hub of the C/EBPδ-mediated crosstalk between the Akt/mTOR/HIF-1α pathway and the NF-κB pathway, and its ubiquitination activity on different substrates deepens the linkage with the upstream and downstream signaling pathways (Fig. 4).
FBXW7 regulates cyclin/CDK pathway
Cyclin/CDK forms a functional kinase complex at the GI/S to control the cell cycle into the S phase and aberrant activation of cyclin/CDK is linked to breast cancer carcinogenesis [60]. FBXW7 has been shown to interact with cyclin E, exploring the potential link between FBXW7 and the cyclin/CDK pathway would be useful in elucidating the mechanism of breast carcinogenesis [61]. Chromosomal instability (CIN) is a hallmark of cancer leading to cancer progression, tumor heterogeneity and drug resistance, and the deletion and mislocalization of the mitophagy-enriched envelope protein A (CENP-A) in breast cancer can cause abnormal chromosome division and rupture, promoting tumor progression and heterogeneity [62, 63]. Takada et al. detected less chromatin fraction CENP-A levels in FBXW7−/− cells compared to FBXW7+/+ cells, rather than CENP-A for all cleavage products, and that ectopic introduction of FBXW7 into FBXW7−/− cells significantly reduced cyclin E1 and CDK2 protein concentrations and rescued CENP-A expression [64]. The FBXW7-cyclin E/CDK2 targeting connection was also linked to cellular senescence phenotype [65]. Breast cancer development also involves FBXW7 control of cyclin D/CDK4/6. Sterol-regulatory-element binding protein 1 (SREBP1) is repressed by FBXW7 in a transcriptionally active version, and FBXW7 in turn indirectly represses SREBP1-dependent activation of cyclin D1 and downstream cyclin D1/CDK4/6 phosphorylation of Rb, blocking MCF-7 cell proliferation and causing partial G1 phase cell cycle arrest [66] (Fig. 4).
FBXW7 regulates NF-κB pathway
NF-κB family proteins include p65 (RelA), RelB, c-Rel, p105/p50 (NF-κB1), and P100/52 (NF-κB2). High levels of NF-κB pathway accumulation are associated with an inflammatory, infiltrative clinicopathological breast cancer phenotype, and NF-κB nuclear accumulation is negatively correlated with ERα + expression in breast cancer [67, 68]. In TNBC, overexpression of the inhibitor of growth 5 (ING5) represses FBXW7, increasing p-NF-κB and activating the PI3K/Akt and NF-κB pathways, causing apoptosis, autophagy, migration, and invasion [69]. This indicates that in FBXW7-deficient breast cancers, the NF-κB signaling pathway that should be subject to normal E3 ubiquitin ligase binding and degradation is blocked, leading to increased NF-κB DNA binding activity and promoting tumor growth and metastasis. NF-κB, as a pro-inflammatory factor, also mediates the regulation of intrinsic and adaptive immune functions [70], and FBXW7 can regulate breast cancer through interaction with the pathway. Wu et al. found that silencing miR-182-5p in BT-549 and MDA-MB-231 cells significantly downregulates the expression of factors like p-p65, p-I-κB, tumor necrosis factor alpha (TNF-α), and IL-1β, while inhibiting FBXW7 restores NF-κB pathway-related complexes and promotes breast cancer apoptosis, proliferation, and decreased immune resistance [32] (Fig. 4).
Overall, the pleiotropic nature of FBXW7 as an E3 ubiquitin ligase receptor to recognize and bind substrates determines its fate in regulating multiple signaling pathways in breast cancer, and this process changes dynamically depending on the location of the FBXW7 substrate in the pathway and the biological function performed by the substrate.
Connection between FBXW7 and breast cancer progression
Chromosome deletions, mutations, and methylation can cause FBXW7 to lose tumor suppressor function in breast cancer [71,72,73]. More importantly, by analyzing FBXW7 mRNA levels in 23 patients with recurrent breast cancer, the time from recurrence to death is significantly shorter in the FBXW7-Low group than in the FBXW7-High group, and the Ki67 labeling index (50.6%) and cyclin E staining positivity (24%) are significantly higher than in the FBXW7-High group (30.7%, 8.3%) [74]. Breast cancer patients with FBXW7 mutant somatic cells display a kinase signaling mutation profile and a negative prognosis for patients [75]. Furthermore, in 1900 breast cancer cases from publicly available datasets, FBXW7 mRNA levels do not segregate with ER status, with ER- patients exhibiting lower FBXW7 expression, disease-free survival (DFS) and overall survival (OS), and that in basal-like subtypes group with higher FBXW7 expression has higher DFS [76]. FBXW7 expression varies significantly between tumors, and low FBXW7 expression is associated with a poorer prognosis in breast cancer patients and could be a potential prognostic indicator for different breast cancer subtypes [72]. In summary, FBXW7 expression correlates with breast cancer development.
Crosstalk between FBXW7 and binding proteins in BC progression
Cell cycle process
Through facilitating the ubiquitination and destruction of cell cycle-relevant molecules, FBXW7 has been implicated in the modulation of the cell cycle process in breast cancer [77] (Fig. 5E). Cyclin E and cyclin D1 are often amplified in breast cancer, leading to cell cycle dysregulation and uncontrolled cell proliferation [78]. Low FBXW7 expression in breast cancer deregulates cyclin E and D1, promoting aberrant cell division and G1/S phase transition [64, 66]. c-Myc is one of the classical targets of FBXW7, and genetic changes and accumulation of c-Myc in breast cancer severely affect disease recovery [79]. Eyes absent 1 (EYA1) is a class of oncogenes and is upregulated in breast cancer. Ubiquitination of FBXW7 targeting c-Myc can be rescued by EYA1 knockdown-mediated elevation of c-Myc pT58 and reduced endogenous c-Myc deposition, resulting in increased FBXW7-c-Myc binding, reduced c-Myc half-life and number of cycling cells [80]. Aside from the effects indicated above, FBXW7 downregulation also modulates the ubiquitination of related targets including cell cycle proteins G-associated kinase (GAK), p53, Aurora-B, γ-catenin, and CENP-A, which increases breast cancer cell cycle progression [21, 80,81,82,83]. Related studies on the regulation of the cell cycle by FBXW7 in breast cancer are summarized in Table 2.
Loss of FBXW7 in vivo plays a role in breast cancer progression. Blue upward arrows indicate upregulation of the relevant target in breast cancer, and green downward arrows indicate downregulation. A Loss of FBXW7 in vivo. B FBXW7 deletion-mediated upregulation of GATA3, c-Myc, AIB1, and EYA1 and downregulation of PTEN and GSK3 promote breast cancer cell proliferation. C BXW7 deletion-mediated upregulation of c-Myc, NICD1, Fra-1, IFNGR1, mTOR, and CCL2 promotes breast cancer cell metastasis. D FBXW7 deletion-mediated upregulation of MCL-1, PLK1, ERK1/2, NOTCH, mTOR, and HSF-1 and downregulation of HIPK2 promote drug resistance in breast cancer. E FBXW7 deletion-mediated upregulation of c-Myc, PLK2, cyclin E, cyclin D1, cyclin B1, and Aurora-B promotes breast cancer cell cycle progression. F FBXW7 deletion-mediated upregulation of TAM, IFN-γ, PD-L1, TLR4, NF-κB, and CCL2 promotes the immune escape of breast cancer cells. G FBXW7 deletion-mediated upregulation of HIF-1α, VEGF, VEGFR1, and β-catenin promotes angiogenesis in breast cancer. H A series of malignant behaviors induced by the FBXW7 deletion promotes breast cancer progression. FBXW7, F-box and WD repeat domain-containing 7. GATA3, GATA-binding protein 3. AIB1, amplified in breast cancer 1. EYA1, eyes absent 1. PTEN, phosphatase and tensin homolog. GSK3, glycogen synthase kinase 3. NICD1, NOTCH intracellular domain 1. Fra-1, FOS-like 1 and AP-1 transcription factor subunit. IFNGR1, interferon gamma receptor 1. mTOR, mechanistic target of rapamycin. CCL2, C–C motif chemokine 2. MCL-1, myeloid cell leukemia-1. PLK1, polo-like kinase 1. ERK1/2, extracellular signal-regulated kinases 1 and 2. TAM, tumor associated macrophage. IFN-γ, interferon-gamma. PD-L1, programmed death ligand 1. TLR4, Toll-like receptor 4. NF-κB, nuclear factor-kappa B. HIF-1α, hypoxia inducible factor 1 alpha. VEGF, vascular endothelial growth factor. VEGFR1, vascular endothelial growth factor receptor 1
Proliferation
FBXW7 strictly controls the ubiquitination and degradation of proliferation-associated binding proteins, and its expression profile with substrates governs breast cancer cell proliferation and apoptosis [15, 87, 88] (Fig. 5B). In fact, FBXW7 binding to phosphatase and tensin homolog (PTEN) affects substrate ubiquitination. Amplified in breast cancer 1 (AIB1) is abundant in high-grade invasive ductal carcinoma, research has suggested that FBXW7α can bind to the C2 domain of PTEN and promote the ubiquitination of AIB1 bound to the PTEN phosphatase domain, reducing AIB1 transcriptional activity and inhibiting MCF-7 cell proliferation [89, 99]. Moreover, GSK3β-mediated CPD phosphorylation of FBXW7 is critical for breast cancer proliferation-associated substrate recruitment. NONO (one of the nuclear proteins) is an RNA-binding protein and silencing NONO significantly inhibits TNBC cell proliferation in vitro/vivo [100]. In the presence of GSK3β kinase initiation, FBXW7α recognizes the T428A/T432A phosphorylation site of NONO and promotes degradation of NONO-WT proteins, but not polyubiquitination of NONO mutant proteins [90]. Crosstalk between FBXW7 and substrates is regulated by various factors, but aberrant post-translational substrate changes can also change its binding status. Emerging evidence has suggested that breast cancer progression may be connected to SUMOylation's antagonistic effect on FBXW7-mediated monocarboxylate transporter 4 (MCT4) ubiquitination [91]. Related studies on the inhibition of proliferation by FBXW7 in breast cancer are summarized in Table 2.
Metastasis and invasion
Metastatic breast cancer correlates with poor clinicopathological phenotype of invasion and drug resistance, downregulation of FBXW7 expression attenuates the ubiquitination and destruction of associated substrates, hence boosting breast cancer invasion and metastasis [18, 25, 95, 96] (Fig. 5C). HIF-1α and IL-6Rα overexpression in breast cancer patient sera creates a hypoxic environment and abnormal inflammation in tumor cells, promoting metastasis [101, 102]. C/EBPδ inhibits FBXW7 in ER + cells, releasing HIF-1α and IL-6Rα expression and activating downstream STAT3 phosphorylation, promoting in vitro calmodulin transformation, sphere formation, and patient-derived xenograft (PDX) tumor metastasis [24]. Breast cancer spread can also be driven by the EMT process, which makes epithelial cancer cells more metastatic and aggressive [103]. Anti-miR-223-3p upregulates FBXW7 translationally, which suppresses EMT, breast cancer cell migration, and invasiveness [33], but information on the specific targets of FBXW7 remains to be investigated. FBXW7 has a wide range of breast cancer targets, and imbalance in its binding to oncogenic substrates may drive tumor spread. For instance, cophosphorylation of Serum/glucocorticoid-regulated kinase 3 (SGK3) with GSK-3β enhances the binding of FBXW7 to metastasis suppressor N-Myc downstream regulated gene 1 (NDRG1) and promotes NDRG1's ubiquitination, increasing breast cancer invasion and metastasis [97]. This offers fresh perspectives on the application of FBXW7 as a therapeutic target for breast cancer. Related studies on the regulation of metastasis by FBXW7 in breast cancer are summarized in Table 2.
Taken together, various signaling pathways and gene crossing are involved in the process FBXW7 controls breast cancer proliferation, cell cycle, and metastasis. However, additional research into the mechanisms through which FBXW7 influences the biological behavior of breast cancer is still required, and exploring the suppressive effects of FBXW7 in depth might provide new targets for anti-breast cancer therapy.
Angiogenesis
Advanced tumor staging, vascular invasion, and lymphatic metastasis have been linked to mutations in the FBWX7 gene [104] (Fig. 5G). The primary endothelial response that initiates angiogenic hypoxia signaling in breast cancer and particularly activates tumor-associated macrophages (TAMs) to produce pro-angiogenic cytokines and growth factors involves stable HIF-1α expression [105, 106]. FBXW7 indirectly regulates breast cancer angiogenesis through its interaction with HIF-1α, and high HIF-1α expression is frequently observed in FBXW7-deficient breast cancers along with angiogenesis and migration, suggesting that normal FBXW7-HIF-1α binding is essential for remodeling normal vascular structure and tumor microenvironment (TME) [107]. Similarly, modification of FBXW7-HIF-1α crosstalk by oncoproteins can disrupt FBXW7 to inhibit breast cancer angiogenesis, for example, TAR (HIV-1) RNA binding protein 2 (TARBP2) reduces the ubiquitination level and proteasomal degradation of HIF-1α by downregulating FBXW7-E3 ligase, which induces the formation of a hypoxic microenvironment and angiogenesis in breast cancer [108]. Furthermore, FBXW7 also regulates angiogenesis through direct or indirect control of vascular endothelial growth factor (VEGF) expression in tumors. VEGF-A promotes the release of VWF and osteoprotegerin from MDA-MB-231 and MCF-7 cell-induced endothelial cells (EC) and is pro-angiogenic [109]. Chiang et al. displayed that targeted binding of FBXW7 to miR-182 attenuates the proteasomal degradation of VEGF and HIF-1α, while reduced VEGF turnover promotes breast cancer angiogenesis and invasion [34].
It is meaningful to take into account the potential therapeutic strategies of reducing breast cancer angiogenesis by targeting FBXW7 and its downstream proteins in light of the critical function that FBXW7 plays in modulating angiogenesis.
Immunosuppression
A major cause of recurrence, metastasis and drug resistance in breast cancers lacking effective anti-hormonal targets is the formation of immunosuppression in the tumor microenvironment, and clinical breast cancer treatment strategies have shown great interest in single agent co-immunosuppressive therapy [110]. Crosstalk between FBXW7 and related binding proteins regulates immune cell numbers, metastasis, and the premetastatic niche, playing a role in breast cancer immunosuppression [111, 112] (Fig. 5F). Interferon-gamma (IFN-γ) activates immune cells and induces differentiation and maturation, controlling a key aspect of the immunotherapeutic response. Breast cancer hijacks IFN-γ signaling and activates IFNGR and downstream cascade pathways, promoting inflammation and tumor spread [113]. In TNBC, ELF5 cannot bind to the enhancer region of FBXW7, which downregulates FBXW7 expression, reduces IFNGR1 ubiquitination, enhances IFN-γ signaling, increases immunosuppressed neutrophils, and promotes programmed death ligand 1 (PD-L1) expression [96]. This demonstrates that FBXW7 slows the advancement of breast cancer by abating the number of immune cells involved in immune evasion and present in TME. C–C motif chemokine 2 (CCL2) induces infiltration and accumulation of TAMs in breast cancer and is positively associated with cancer progression [114]. Yumimoto et al. uncovered that in breast cancer, FBXW7 deletion activates CCL2 promoter by reducing NOTCH turnover, which recruits monocytic myeloid-derived suppressor cells (Mo-MDSCs) and TAMs to the premetastatic niche and accelerates tumor metastasis and immunosuppression [115, 116]. In addition, FBXW7 has been shown to regulate solid tumor tolerance to immune checkpoint inhibitors (ICIs) and anti-PD-L1 therapy by blocking the nuclear factor of activated T cell 1 (NFAT1)/PD-L1 axis [117], which also offers new insights into the improvement of immunotherapeutic agents for breast cancer from the perspective of targeting FBXW7.
Drug resistance
Multiple reports have confirmed that FBXW7 is closely linked to drug resistance in breast cancer [35, 118, 119] (Fig. 5D). Upregulation of FBXW7 has been found to feature prominently in promoting chemosensitization in breast cancer due to its part in the control of ubiquitination and proteasomal degradation of resistance-associated targets in tumors, such as Notch1-IC, myeloid cell leukemia-1 (MCL-1), and heat shock factor 1 (HSF-1). Increased Notch1-IC expression causes Adriamycin resistance in breast cancer cells, yet this process can be dramatically reversed by FBXW7 promoting proteasomal degradation of Notch1 [120]. HSF-1 is frequently overexpressed in drug-resistant cancer cells and promotes MDR formation through direct binding to the promoter of multidrug resistance 1 (MDR1). Activated ERK1/2 triggers FBXW7 down-regulation, which enhances HSF-1 binding to MDR1, leading to the emergence of paclitaxel-resistant breast cancer cells [121]. The anti-breast cancer mechanism of chemotherapeutic agents targeting microtubules, such as vincristine or paclitaxel, is to prolong or block mitosis, inducing apoptosis in mitotic cells [122]. Nevertheless, mitotic slippage (preventing premature cell exit from mitosis) promotes the production of mutant tetraploid cells, rendering tumor cells resistant to the drug. Compulsory expression of FBXW7 significantly downregulates the expression of MCL-1 and PLK1, and prevents cells from mitotic slippage and promoting apoptosis, hence restoring the sensitivity of MDA-MB-468 cells to paclitaxel [84].
It is possible that the role of FBXW7 is context-dependent and that its mediation of signaling cascade control extends beyond the reduction of tumor resistance. SRY-related HMG-box 4 (SOX4) binding to the FBXW7 promoter upregulates FBXW7 expression in ER + breast cancers, causing enhanced turnover of GATA-binding protein 3 (GATA3), and thus promoting tamoxifen tolerance [123]. This implies that FBXW7 mechanisms for controlling drug resistance in breast cancer are intricate. Therefore, a thorough investigation of the multiple mechanism underlying FBXW7 expression could benefit in the discovery of new chemotherapeutic approaches and possible indicators of drug resistance in breast cancer. Related studies on the regulation of drug resistance by FBXW7 in breast cancer are summarized in Table 3.
Targeting FBXW7 for breast cancer therapeutic strategies
As stated above, FBXW7 deletion reduces downstream target turnover and oncoprotein-dependent accumulation, which creates opportunities for targeted therapy of tumor cells. Here, we summarize the therapeutic strategy for targeting FBXW7 in breast cancer.
1) Targeting upstream miRNAs of FBXW7. The rapid spread of metastatic breast cancer and the lack of effective anti-hormonal targets necessitate the development of precisely targeted therapeutic techniques. mRNA regulators that target specific genes—miRNA mimics and antagonists—have shown positive effects in breast cancer treatment in combination with chemotherapy. Overexpression of miR-621 mimics downregulates F-box protein 11 (FBXO11), liberating p53 expression to promote apoptosis, and combining miR-621 mimics and paclitaxel plus carboplatin (PTX/CBP) increases cellular chemotherapy sensitivity and apoptosis [128]. The article's second point demonstrated that miRNAs exert an essential role in the advancement of breast cancer by binding to FBXW7's 3' UTR. MiR-27a antagonists upregulate FBXW7 and prevent invasion and metastasis in MDA-MB-231 and SKBR3 cells [31]. If the key issues of miRNA transport vectors and miRNA efficacy in cancer cells can be addressed in the future, mimics or antagonists targeting miRNAs upstream of FBXW7 may slow breast cancer progression. Natural items can block FBXW7 upstream miRNAs in addition to miRNA molecular agents. In invasive breast cancer, Honokiol downregulates miR-188-5p, which promotes the disruption of c-Myc by FBXW7 and reverses Adriamycin resistance [35] (Fig. 6A).
Targeting FBXW7 for breast cancer therapeutic strategies. Blue upward arrows indicate upregulation of the relevant target in breast cancer, and green downward arrows indicate downregulation. A Inhibition of miRNAs bound to FBXW7 mRNA restores FBXW7 expression in breast cancer. B FBXW7 gene methylation inhibition to restore FBXW7 expression in breast cancer. C Blocking breast cancer proliferation, metastasis, and drug resistance by inhibiting FBXW7 downstream substrates. D Promote the proliferation of dormant DTCs by inhibiting the expression of FBXW7 in DTCs, thereby suppressing drug resistance. BC, breast cancer. FBXW7, F-box and WD repeat domain-containing 7. MiRNA, microRNA. DNMT, DNA methyltransferase. DTCs, disseminated tumor cells
2) Restoration of FBXW7 tumor suppressor functions in breast cancer. Low FBXW7 expression is linked to breast cancer malignancy, therefore considering restoring its suppressive function and designing potential FBXW7 inducers may be a viable therapeutic strategy [72]. Promoter hypermethylation has been correlated to decreased FBXW7 gene transcription in primary breast cancer [73]. Recently, it has been explored that decitabine inhibition of DNA methyltransferase demethylates FBXW7, reducing MCL-1 expression and increasing cellular susceptibility to decitabine and Bcl-2 inhibitors [129]. Parallel to this, EZH2 inhibits FBXW7 and NUMB production by trimethylating histone H3 at Lys27 (H3K27me3), which activates anti-apoptotic genes and expresses T-cell multifunctional cytokines that attenuate immunosuppression in late tumor stages. FBXW7 demethylation reverses this [130]. This indicates that epigenetic targeted drugs may improve chemosensitivity in FBXW7-deficient breast tumors by acting as FBXW7 inducers. Low FBXW7 expression is also linked to overactive PKC and PLK1/2 signaling, which can be inhibited to slow its turnover [44, 45]. PKC inhibitor J-4 and PLK1/2 inhibitor onvansertib are in preclinical and clinical trials, respectively, and by inhibiting these molecules, endogenous FBXW7 expression may be restored and promote the turnover of breast cancer-associated oncogenic proteins [131, 132] (Fig. 6B).
3) Targeting downstream substrates of FBXW7. In FBXW7-negative breast tumors, due to the inability of FBXW7 to degrade typical drug-resistant proteins, drug sensitivity is often diminished. Inhibitors of downstream binding proteins may restore chemosensitivity. For instance, combining MCL-1 inhibitor sorafenib with nocodazole in MDA-MB-231 cells dramatically reverses drug resistance caused by low FBXW7 expression [119]. The mTOR inhibitor rapamycin significantly restores the poor FBXW7 expression caused by the family with sequence similarity 83, member D (FAM83D) and its pro-tumor effects [95]. Several AURKA inhibitors, an alternative target of FBXW7, have entered phase II/III clinical trials [133] (Fig. 6C). These findings demonstrate that FBXW7-negative breast cancer could be seen as an indicator of drug resistance and that inhibitors targeting substrates activated downstream of FBXW7 may relieve TNBC chemoresistance.
4) Inhibiting FBXW7 promotes sensitivity of BC latency DTCs. Tumor cells that have reached distant organs but have not yet formed clinically evident metastases are called disseminated tumor cells (DTCs). DTC in the bone marrow of breast cancer is in a quiescent single cell state and its low proliferation rate is a major cause of drug resistance [134]. FBXW7 degrades cyclin E to control cell cycle, yet this function precisely preserves DTCs in a quiescent state and high expression of the FBXW7 gene is detected in dormant cells [135]. FBXW7-ablated breast cancer cells break the stationary condition of DTCs and turn them into the drug-sensitive proliferative state. After treatment with FBXW7-ablation plus paclitaxel chemotherapy, DTCs become more sensitive to chemotherapy and fewer in number [136]. Thus, targeting FBXW7 ablation to stimulate DTCs from dormancy to proliferation may be a viable treatment to overcome breast cancer medication resistance (Fig. 6D).
Conclusion
In summary, through regulating multiple intricate signaling pathways and targets, FBXW7 has a significant impact on the development of breast cancer (Fig. 5, Tables 2 and 3). The wide range of oncogenic targets allows FBXW7 to influence breast cancer growth in various ways and is of high value for targeted therapy. Moreover, detection of FBXW7 mutational status has potential as a prognostic marker for breast cancer and to be instrumental in establishing the appropriate, tailored treatment [18, 36]. To develop novel breast cancer therapies, future research may target FBXW7 mutations in vivo and downstream targets. Nevertheless, due to the intricate network of FBXW7, substrates, and regulators, its expression and function across cells remain uncertain. Significant challenges of FBXW7-targeted breast cancer therapeutic strategies include: how miRNA mimics or anti-miRNAs are transmitted is unknown; demethylation to restore FBXW7 expression may harm normally expressed genes; drugs cannot be targeted precisely due to the functional heterogeneity of FBXW7 isoforms; and the dual role of FBXW7 in breast cancer chemoresistance limits the action of chemotherapeutic agents. As a result, further technological advancements in the field of genomics and increased research on the complex role of FBXW7 in chemoresistance are required to target FBXW7 in breast cancer therapy. Further research on FBXW7's biological and molecular mechanisms in breast cancer will improve present therapy options and benefit more advanced breast cancer patients.
Availability of data and materials
Not applicable.
Abbreviations
- BC:
-
Breast cancer
- FBXW7:
-
F-box and WD repeat domain-containing 7
- mTOR:
-
Mechanistic target of rapamycin
- SCF:
-
Skp1-Cullin1-F-box
- RBX1:
-
RING-box protein 1
- CUL1:
-
Cullin1
- Skp1:
-
S-phase kinase-associated protein 1
- E1:
-
Ubiquitin-activating enzymes
- E2:
-
Ubiquitin-conjugating enzymes
- E3:
-
Ubiquitin ligase
- CPD:
-
Cdc4 phospho-degron
- GSK3β:
-
Glycogen Synthase Kinase 3 Beta
- KLF5:
-
Kruppel-like factor 5
- dsRNA:
-
Double-stranded RNA
- RANBP10:
-
RAN binding protein 10
- HES5:
-
HES family bHLH transcription factor 5
- C/EBPδ:
-
CCAAT/enhancer-binding protein-delta
- 27-HC:
-
27-Hydroxycholesterol
- p53BS:
-
P53 binding site
- TGIF1:
-
TGF-β-induced factor 1
- TGF-β:
-
Transforming growth factor beta
- NICD1:
-
NOTCH intracellular domain 1
- LncRNAs:
-
Long non-coding RNAs
- ceRNA:
-
Competitive endogenous RNA
- MiRNA:
-
MicroRNA
- EMT:
-
Epithelial-mesenchymal transition
- CircRNAs:
-
Circular RNAs
- USP28:
-
Ubiquitin-specific proteases 28
- TNBC:
-
Triple-negative breast cancer
- LSD1:
-
Lysine-specific demethylase 1
- ERK1/2:
-
Extracellular signal-regulated kinases 1 and 2
- PLK1/2:
-
Polo-like kinase 1 and 2
- PI3K:
-
Phosphoinositide 3-kinase
- SGK1:
-
Serum- and glucocorticoid-inducible protein kinase 1
- NRG1:
-
Neuregulin-1
- Fra-1:
-
FOS-like 1 and AP-1 transcription factor subunit
- CSN6:
-
COP9 signalosome 6
- SREBP1:
-
Sterol-regulatory-element binding protein 1
- Rb:
-
Retinoblastoma
- CENP-A:
-
Centromere protein A
- CIN:
-
Chromosomal instability
- GAK:
-
G-associated kinase
- PTEN:
-
Phosphatase and tensin homolog
- AIB1:
-
Amplified in breast cancer 1
- MCT4:
-
Monocarboxylate transporter 4
- ELF5:
-
E74-like factor 5
- HIF-1α:
-
Hypoxia-inducible factor 1 alpha
- BCSCs:
-
Breast cancer stem cells
- SGK3:
-
Serum/glucocorticoid-regulated kinase 3
- NDRG1:
-
N-Myc downstream regulated gene 1
- TAMs:
-
Tumor-associated macrophages
- VEGF:
-
Vascular endothelial growth factor
- TLR4:
-
Toll-like receptor 4
- NF-κB:
-
Nuclear factor-kappa B
- IFN-γ:
-
Interferon-gamma
- PD-L1:
-
Programmed death ligand 1
- TME:
-
Tumor microenvironment
- CCL2:
-
C–C motif chemokine 2
- Mo-MDSCs:
-
Monocytic myeloid-derived suppressor cells
- ICIs:
-
Immune checkpoint inhibitors
- NFAT1:
-
Nuclear factor of activated T cell 1
- MCL-1:
-
Myeloid cell leukemia-1
- HSF-1:
-
Heat shock factor 1
- MDR1:
-
Multidrug resistance 1
- GATA3:
-
GATA-binding protein 3
- FAM83D:
-
Family with sequence similarity 83, member D
- DTCs:
-
Disseminated tumor cells
References
Fahad UM. Breast cancer: current perspectives on the disease status. Adv Exp Med Biol. 2019;1152:51–64.
Koual M, Tomkiewicz C, Cano-Sancho G, Antignac JP, Bats AS, Coumoul X. Environmental chemicals, breast cancer progression and drug resistance. Environ Health. 2020;19(1):117.
Díaz VM, de Herreros AG. F-box proteins: Keeping the epithelial-to-mesenchymal transition (EMT) in check. Semin Cancer Biol. 2016;36:71–9.
Yeh CH, Bellon M, Nicot C. FBXW7: a critical tumor suppressor of human cancers. Mol Cancer. 2018;17(1):115.
Welcker M, Orian A, Grim JE, Eisenman RN, Clurman BE. A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c-Myc and cell size. Curr Biol. 2004;14(20):1852–7.
Wei D, Sun Y. Small RING finger proteins RBX1 and RBX2 of SCF E3 ubiquitin ligases: the role in cancer and as cancer targets. Genes Cancer. 2010;1(7):700–7.
Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA. 2004;101(24):9085–90.
Thompson BJ, Buonamici S, Sulis ML, Palomero T, Vilimas T, Basso G, et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J Exp Med. 2007;204(8):1825–35.
Lin H, Ma N, Zhao L, Yang G, Cao B. KDM5c Promotes Colon Cancer Cell Proliferation Through the FBXW7-c-Jun Regulatory Axis. Front Oncol. 2020;10:535449.
Siu KT, Xu Y, Swartz KL, Bhattacharyya M, Gurbuxani S, Hua Y, et al. Chromosome instability underlies hematopoietic stem cell dysfunction and lymphoid neoplasia associated with impaired Fbw7-mediated cyclin E regulation. Mol Cell Biol. 2014;34(17):3244–58.
Mori A, Masuda K, Ohtsuka H, Shijo M, Ariake K, Fukase K, et al. FBXW7 modulates malignant potential and cisplatin-induced apoptosis in cholangiocarcinoma through NOTCH1 and MCL1. Cancer Sci. 2018;109(12):3883–95.
Finkin S, Aylon Y, Anzi S, Oren M, Shaulian E. Fbw7 regulates the activity of endoreduplication mediators and the p53 pathway to prevent drug-induced polyploidy. Oncogene. 2008;27(32):4411–21.
Davis H, Lewis A, Behrens A, Tomlinson I. Investigation of the atypical FBXW7 mutation spectrum in human tumours by conditional expression of a heterozygous propellor tip missense allele in the mouse intestines. Gut. 2014;63(5):792–9.
Zhang X, Howell GM, Guo L, Collage RD, Loughran PA, Zuckerbraun BS, et al. CaMKIV-dependent preservation of mTOR expression is required for autophagy during lipopolysaccharide-induced inflammation and acute kidney injury. J Immunol (Baltimore, Md : 1950). 2014;193(5):2405–15.
Zhao J, Wang Y, Mu C, Xu Y, Sang J. MAGEA1 interacts with FBXW7 and regulates ubiquitin ligase-mediated turnover of NICD1 in breast and ovarian cancer cells. Oncogene. 2017;36(35):5023–34.
Balamurugan K, Wang JM, Tsai HH, Sharan S, Anver M, Leighty R, et al. The tumour suppressor C/EBPδ inhibits FBXW7 expression and promotes mammary tumour metastasis. EMBO J. 2010;29(24):4106–17.
Rustighi A, Zannini A, Tiberi L, Sommaggio R, Piazza S, Sorrentino G, et al. Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast. EMBO Mol Med. 2014;6(1):99–119.
Yumimoto K, Akiyoshi S, Ueo H, Sagara Y, Onoyama I, Ueo H, et al. F-box protein FBXW7 inhibits cancer metastasis in a non-cell-autonomous manner. J Clin Investig. 2015;125(2):621–35.
Onoyama I, Nakayama S, Shimizu H, Nakayama KI. Loss of Fbxw7 Impairs Development of and Induces Heterogeneous Tumor Formation in the Mouse Mammary Gland. Can Res. 2020;80(24):5515–30.
Gstalder C, Liu D, Miao D, Lutterbach B, DeVine AL, Lin C, et al. Inactivation of Fbxw7 Impairs dsRNA Sensing and Confers Resistance to PD-1 Blockade. Cancer Discov. 2020;10(9):1296–311.
Mandal S, Freije WA, Guptan P, Banerjee U. Metabolic control of G1-S transition: cyclin E degradation by p53-induced activation of the ubiquitin-proteasome system. J Cell Biol. 2010;188(4):473–9.
Hou J, Liu Y, Huang P, Wang Y, Pei D, Tan R, et al. RANBP10 promotes glioblastoma progression by regulating the FBXW7/c-Myc pathway. Cell Death Dis. 2021;12(11):967.
Chen LJ, Hu B, Han ZQ, Liu W, Zhu JH, Chen XX, et al. Repression of FBXW7 by HES5 contributes to inactivation of the TGF-β signaling pathway and alleviation of endometriosis. FASEB J. 2021;35(2):e20938.
Balamurugan K, Mendoza-Villanueva D, Sharan S, Summers GH, Dobrolecki LE, Lewis MT, et al. C/EBPδ links IL-6 and HIF-1 signaling to promote breast cancer stem cell-associated phenotypes. Oncogene. 2019;38(20):3765–80.
Ma LM, Liang ZR, Zhou KR, Zhou H, Qu LH. 27-Hydroxycholesterol increases Myc protein stability via suppressing PP2A, SCP1 and FBW7 transcription in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2016;480(3):328–33.
Kimura T, Gotoh M, Nakamura Y, Arakawa H. hCDC4b, a regulator of cyclin E, as a direct transcriptional target of p53. Cancer Sci. 2003;94(5):431–6.
Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, et al. NUMB controls p53 tumour suppressor activity. Nature. 2008;451(7174):76–80.
He S, Nelson ER. 27-Hydroxycholesterol, an endogenous selective estrogen receptor modulator. Maturitas. 2017;104:29–35.
Tang D, Sivko GS, DeWille JW. Promoter methylation reduces C/EBPdelta (CEBPD) gene expression in the SUM-52PE human breast cancer cell line and in primary breast tumors. Breast Cancer Res Treat. 2006;95(2):161–70.
Chen Y, Wei H, Liu Y, Zheng S. Promotional effect of microRNA-194 on breast cancer cells via targeting F-box/WD repeat-containing protein 7. Oncol Lett. 2018;15(4):4439–44.
Jiang G, Shi W, Fang H, Zhang X. miR-27a promotes human breast cancer cell migration by inducing EMT in a FBXW7-dependent manner. Mol Med Rep. 2018;18(6):5417–26.
Wu X, Chen H, Wu M, Peng S, Zhang L. Downregulation of miR-182-5p inhibits the proliferation and invasion of triple-negative breast cancer cells through regulating TLR4/NF-κB pathway activity by targeting FBXW7. Ann Transl Med. 2020;8(16):995.
Wang Y, Shi S, Wang Y, Zhang X, Liu X, Li J, et al. miR-223-3p targets FBXW7 to promote epithelial-mesenchymal transition and metastasis in breast cancer. Thorac Cancer. 2022;13(3):474–82.
Chiang CH, Chu PY, Hou MF, Hung WC. MiR-182 promotes proliferation and invasion and elevates the HIF-1α-VEGF-A axis in breast cancer cells by targeting FBXW7. Am J Cancer Res. 2016;6(8):1785–98.
Yi X, Lou L, Wang J, Xiong J, Zhou S. Honokiol antagonizes doxorubicin resistance in human breast cancer via miR-188-5p/FBXW7/c-Myc pathway. Cancer Chemother Pharmacol. 2021;87(5):647–56.
Xia W, Zhou J, Luo H, Liu Y, Peng C, Zheng W, et al. MicroRNA-32 promotes cell proliferation, migration and suppresses apoptosis in breast cancer cells by targeting FBXW7. Cancer Cell Int. 2017;17:14.
Ye F, Gao G, Zou Y, Zheng S, Zhang L, Ou X, et al. circFBXW7 Inhibits Malignant Progression by Sponging miR-197-3p and Encoding a 185-aa Protein in Triple-Negative Breast Cancer. Mol Ther Nucleic Acids. 2019;18:88–98.
Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–8.
Liu S, Wang L, Wu X, Wu J, Liu D, Yu H. Overexpression of hsa_circ_0022742 suppressed hyperglycemia-induced endothelial dysfunction by targeting the miR-503-5p/FBXW7 axis. Microvasc Res. 2022;139:104249.
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J Natl Cancer Inst. 2018;110(3):304–15.
Tang X, Orlicky S, Lin Z, Willems A, Neculai D, Ceccarelli D, et al. Suprafacial orientation of the SCFCdc4 dimer accommodates multiple geometries for substrate ubiquitination. Cell. 2007;129(6):1165–76.
Welcker M, Larimore EA, Swanger J, Bengoechea-Alonso MT, Grim JE, Ericsson J, et al. Fbw7 dimerization determines the specificity and robustness of substrate degradation. Genes Dev. 2013;27(23):2531–6.
Lan H, Tan M, Zhang Q, Yang F, Wang S, Li H, et al. LSD1 destabilizes FBXW7 and abrogates FBXW7 functions independent of its demethylase activity. Proc Natl Acad Sci USA. 2019;116(25):12311–20.
Cizmecioglu O, Krause A, Bahtz R, Ehret L, Malek N, Hoffmann I. Plk2 regulates centriole duplication through phosphorylation-mediated degradation of Fbxw7 (human Cdc4). J Cell Sci. 2012;125(Pt 4):981–92.
Durgan J, Parker PJ. Regulation of the tumour suppressor Fbw7α by PKC-dependent phosphorylation and cancer-associated mutations. Biochem J. 2010;432(1):77–87.
Schülein C, Eilers M, Popov N. PI3K-dependent phosphorylation of Fbw7 modulates substrate degradation and activity. FEBS Lett. 2011;585(14):2151–7.
Shu L, Chen A, Li L, Yao L, He Y, Xu J, et al. NRG1 regulates Fra-1 transcription and metastasis of triple-negative breast cancer cells via the c-Myc ubiquitination as manipulated by ERK1/2-mediated Fbxw7 phosphorylation. Oncogene. 2022;41(6):907–19.
Kus BM, Caldon CE, Andorn-Broza R, Edwards AM. Functional interaction of 13 yeast SCF complexes with a set of yeast E2 enzymes in vitro. Proteins. 2004;54(3):455–67.
Chen J, Shin JH, Zhao R, Phan L, Wang H, Xue Y, et al. CSN6 drives carcinogenesis by positively regulating Myc stability. Nat Commun. 2014;5:5384.
Schülein-Völk C, Wolf E, Zhu J, Xu W, Taranets L, Hellmann A, et al. Dual regulation of Fbw7 function and oncogenic transformation by Usp28. Cell Rep. 2014;9(3):1099–109.
Fito-Lopez B, Salvadores M, Alvarez MM, Supek F. Prevalence, causes and impact of TP53-loss phenocopying events in human tumors. BMC Biol. 2023;21(1):92.
Kumar V, Vashishta M, Kong L, Wu X, Lu JJ, Guha C, et al. The Role of Notch, Hedgehog, and Wnt Signaling Pathways in the Resistance of Tumors to Anticancer Therapies. Front Cell Dev Biol. 2021;9:650772.
Carrieri FA, Dale JK. Turn it down a notch. Front Cell Dev Biol. 2016;4:151.
Xie X, Zhu Y, Cheng H, Li H, Zhang Y, Wang R, et al. BPA exposure enhances the metastatic aggression of ovarian cancer through the ERα/AKT/mTOR/HIF-1α signaling axis. Food Chem Toxicol. 2023;176:113792.
Liao WL, Liu YF, Ying TH, Shieh JC, Hung YT, Lee HJ, et al. Inhibitory Effects of Ursolic Acid on the Stemness and Progression of Human Breast Cancer Cells by Modulating Argonaute-2. Int J Mol Sci. 2022;24(1):366.
Huo Y, Shao S, Liu E, Li J, Tian Z, Wu X, et al. Subpathway Analysis of Transcriptome Profiles Reveals New Molecular Mechanisms of Acquired Chemotherapy Resistance in Breast Cancer. Cancers. 2022;14(19):4878.
Sun Y, Li X. The canonical wnt signal restricts the glycogen synthase kinase 3/fbw7-dependent ubiquitination and degradation of eya1 phosphatase. Mol Cell Biol. 2014;34(13):2409–17.
Ali S, Singh NN, Yildirim H, Ramji DP. Requirement for nuclear factor kappa B signalling in the interleukin-1-induced expression of the CCAAT/enhancer binding protein-delta gene in hepatocytes. Int J Biochem Cell Biol. 2010;42(1):113–9.
Fukushima H, Matsumoto A, Inuzuka H, Zhai B, Lau AW, Wan L, et al. SCF(Fbw7) modulates the NFkB signaling pathway by targeting NFkB2 for ubiquitination and destruction. Cell Rep. 2012;1(5):434–43.
Di Sante G, Pagé J, Jiao X, Nawab O, Cristofanilli M, Skordalakes E, et al. Recent advances with cyclin-dependent kinase inhibitors: therapeutic agents for breast cancer and their role in immuno-oncology. Expert Rev Anticancer Ther. 2019;19(7):569–87.
Lerner M, Lundgren J, Akhoondi S, Jahn A, Ng HF, Akbari Moqadam F, et al. MiRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell cycle (Georgetown, Tex). 2011;10(13):2172–83.
Zhang W, Mao JH, Zhu W, Jain AK, Liu K, Brown JB, et al. Centromere and kinetochore gene misexpression predicts cancer patient survival and response to radiotherapy and chemotherapy. Nat Commun. 2016;7:12619.
Zhang S, Xie Y, Tian T, Yang Q, Zhou Y, Qiu J, et al. High expression levels of centromere protein A plus upregulation of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway affect chemotherapy response and prognosis in patients with breast cancer. Oncol Lett. 2021;21(5):410.
Takada M, Zhang W, Suzuki A, Kuroda TS, Yu Z, Inuzuka H, et al. FBW7 Loss Promotes Chromosomal Instability and Tumorigenesis via Cyclin E1/CDK2-Mediated Phosphorylation of CENP-A. Can Res. 2017;77(18):4881–93.
Gong L, Cui D, Liu D, Shen X, Pan H, Xiong X, et al. FBXW7 inactivation induces cellular senescence via accumulation of p53. Cell Death Dis. 2022;13(9):788.
Aldaalis A, Bengoechea-Alonso MT, Ericsson J. The SREBP-dependent regulation of cyclin D1 coordinates cell proliferation and lipid synthesis. Front Oncol. 2022;12:942386.
Sun F, Qu Z, Xiao Y, Zhou J, Burns TF, Stabile LP, et al. NF-κB1 p105 suppresses lung tumorigenesis through the Tpl2 kinase but independently of its NF-κB function. Oncogene. 2016;35(18):2299–310.
Rojo F, González-Pérez A, Furriol J, Nicolau MJ, Ferrer J, Burgués O, et al. Non-canonical NF-κB pathway activation predicts outcome in borderline oestrogen receptor positive breast carcinoma. Br J Cancer. 2016;115(3):322–31.
Ding XQ, Zhao S, Yang L, Zhao X, Zhao GF, Zhao SP, et al. The nucleocytoplasmic translocation and up-regulation of ING5 protein in breast cancer: a potential target for gene therapy. Oncotarget. 2017;8(47):81953–66.
Mantsounga CS, Lee C, Neverson J, Sharma S, Healy A, Berus JM, et al. Macrophage IL-1β promotes arteriogenesis by autocrine STAT3- and NF-κB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022;38(5):110309.
Schwendel A, Richard F, Langreck H, Kaufmann O, Lage H, Winzer KJ, et al. Chromosome alterations in breast carcinomas: frequent involvement of DNA losses including chromosomes 4q and 21q. Br J Cancer. 1998;78(6):806–11.
Liu Y, Ren S, Castellanos-Martin A, Perez-Losada J, Kwon YW, Huang Y, et al. Multiple novel alternative splicing forms of FBXW7α have a translational modulatory function and show specific alteration in human cancer. PLoS One. 2012;7(11):e49453.
Akhoondi S, Lindström L, Widschwendter M, Corcoran M, Bergh J, Spruck C, et al. Inactivation of FBXW7/hCDC4-β expression by promoter hypermethylation is associated with favorable prognosis in primary breast cancer. Breast Cancer Res. 2010;12(6):R105.
Ibusuki M, Yamamoto Y, Shinriki S, Ando Y, Iwase H. Reduced expression of ubiquitin ligase FBXW7 mRNA is associated with poor prognosis in breast cancer patients. Cancer Sci. 2011;102(2):439–45.
Yang PS, Chao YT, Lung CF, Liu CL, Chang YC, Li KC, et al. Association of Pathway Mutations With Survival in Taiwanese Breast Cancers. Front Oncol. 2022;12:819555.
Wei G, Wang Y, Zhang P, Lu J, Mao JH. Evaluating the prognostic significance of FBXW7 expression level in human breast cancer by a meta-analysis of transcriptional profiles. J Cancer Sci Ther. 2012;4(9):299–305.
Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413(6853):316–22.
Susanti NMP, Tjahjono DH. Cyclin-Dependent Kinase 4 and 6 Inhibitors in Cell Cycle Dysregulation for Breast Cancer Treatment. Molecules (Basel, Switzerland). 2021;26(15):4462.
Meyer AE, Furumo Q, Stelloh C, Minella AC, Rao S. Loss of Fbxw7 triggers mammary tumorigenesis associated with E2F/c-Myc activation and Trp53 mutation. Neoplasia (New York, NY). 2020;22(11):644–58.
Li J, Rodriguez Y, Cheng C, Zeng L, Wong EYM, Xu CY, et al. EYA1's Conformation Specificity in Dephosphorylating Phosphothreonine in Myc and Its Activity on Myc Stabilization in Breast Cancer. Mol Cell Biol. 2017;37(1):e00499–16.
Li Y, Hu K, Xiao X, Wu W, Yan H, Chen H, et al. FBW7 suppresses cell proliferation and G2/M cell cycle transition via promoting γ-catenin K63-linked ubiquitylation. Biochem Biophys Res Commun. 2018;497(2):473–9.
Manzione MG, Rombouts J, Steklov M, Pasquali L, Sablina A, Gelens L, et al. Co-regulation of the antagonistic RepoMan:Aurora-B pair in proliferating cells. Mol Biol Cell. 2020;31(6):419–38.
Dolly SO, Gurden MD, Drosopoulos K, Clarke P, de Bono J, Kaye S, et al. RNAi screen reveals synthetic lethality between cyclin G-associated kinase and FBXW7 by inducing aberrant mitoses. Br J Cancer. 2017;117(7):954–64.
Gasca J, Flores ML, Giráldez S, Ruiz-Borrego M, Tortolero M, Romero F, et al. Loss of FBXW7 and accumulation of MCL1 and PLK1 promote paclitaxel resistance in breast cancer. Oncotarget. 2016;7(33):52751–65.
Salehi-Tabar R, Memari B, Wong H, Dimitrov V, Rochel N, White JH. The tumor suppressor FBW7 and the Vitamin D receptor are mutual cofactors in protein turnover and transcriptional regulation. Mol Cancer Res. 2019;17(3):709–19.
Richter KT, Kschonsak YT, Vodicska B, Hoffmann I. FBXO45-MYCBP2 regulates mitotic cell fate by targeting FBXW7 for degradation. Cell Death Differ. 2020;27(2):758–72.
Chen X, Li XY, Long M, Wang X, Gao ZW, Cui Y, et al. The FBXW7 tumor suppressor inhibits breast cancer proliferation and promotes apoptosis by targeting MTDH for degradation. Neoplasma. 2018;65(2):201–9.
Song N, Cao C, Tang Y, Bi L, Jiang Y, Zhou Y, et al. The ubiquitin ligase SCF(FBXW7α) promotes GATA3 degradation. J Cell Physiol. 2018;233(3):2366–77.
Yang C, Li S, Wang M, Chang AK, Liu Y, Zhao F, et al. PTEN suppresses the oncogenic function of AIB1 through decreasing its protein stability via mechanism involving Fbw7 alpha. Mol Cancer. 2013;12:21.
Alfano L, Caporaso A, Altieri A, Costa C, Forte IM, Iannuzzi CA, et al. NONO ubiquitination is mediated by FBW7 and GSK3 β via a degron lost upon chromosomal rearrangement in cancer. J Cell Physiol. 2018;233(5):4338–44.
Hu X, Liu Z, Duan X, Han X, Yuan M, Liu L, et al. Blocking MCT4 SUMOylation inhibits the growth of breast cancer cells. Mol Carcinog. 2021;60(10):702–14.
Parajuli P, Tiwari RV, Sylvester PW. Anti-proliferative effects of γ-tocotrienol are associated with suppression of c-Myc expression in mammary tumour cells. Cell Prolif. 2015;48(4):421–35.
Sun XX, He X, Yin L, Komada M, Sears RC, Dai MS. The nucleolar ubiquitin-specific protease USP36 deubiquitinates and stabilizes c-Myc. Proc Natl Acad Sci USA. 2015;112(12):3734–9.
Takada M, Zhuang M, Inuzuka H, Zhang J, Zurlo G, Zhang J, et al. EglN2 contributes to triple negative breast tumorigenesis by functioning as a substrate for the FBW7 tumor suppressor. Oncotarget. 2017;8(4):6787–95.
Wang Z, Liu Y, Zhang P, Zhang W, Wang W, Curr K, et al. FAM83D promotes cell proliferation and motility by downregulating tumor suppressor gene FBXW7. Oncotarget. 2013;4(12):2476–86.
Singh S, Kumar S, Srivastava RK, Nandi A, Thacker G, Murali H, et al. Loss of ELF5-FBXW7 stabilizes IFNGR1 to promote the growth and metastasis of triple-negative breast cancer through interferon-γ signalling. Nat Cell Biol. 2020;22(5):591–602.
Gasser JA, Inuzuka H, Lau AW, Wei W, Beroukhim R, Toker A. SGK3 mediates INPP4B-dependent PI3K signaling in breast cancer. Mol Cell. 2014;56(4):595–607.
Mendoza-Villanueva D, Balamurugan K, Ali HR, Kim SR, Sharan S, Johnson RC, et al. The C/EBPδ protein is stabilized by estrogen receptor α activity, inhibits SNAI2 expression and associates with good prognosis in breast cancer. Oncogene. 2016;35(48):6166–76.
Sharif GM, Campbell MJ, Nasir A, Sengupta S, Graham GT, Kushner MH, et al. An AIB1 isoform alters enhancer access and enables progression of early-stage triple-negative breast cancer. Can Res. 2021;81(16):4230–41.
Shen M, Zhang R, Jia W, Zhu Z, Zhao L, Huang G, et al. RNA-binding protein p54(nrb)/NONO potentiates nuclear EGFR-mediated tumorigenesis of triple-negative breast cancer. Cell Death Dis. 2022;13(1):42.
De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini M. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs). Breast Cancer Res. 2013;15(4):R64.
Gyamfi J, Lee YH, Eom M, Choi J. Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells. Sci Rep. 2018;8(1):8859.
Waryah C, Cursons J, Foroutan M, Pflueger C, Wang E, Molania R, et al. Synthetic Epigenetic Reprogramming of Mesenchymal to Epithelial States Using the CRISPR/dCas9 Platform in Triple Negative Breast Cancer. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2023;10(22):e2301802.
Watanabe T, Nanamiya H, Kojima M, Nomura S, Furukawa S, Soeda S, et al. Clinical relevance of oncogenic driver mutations identified in endometrial carcinoma. Transl Oncol. 2021;14(3):101010.
Liu Q, Guan C, Liu C, Li H, Wu J, Sun C. Targeting hypoxia-inducible factor-1alpha: A new strategy for triple-negative breast cancer therapy. Biomed Pharmacother = Biomedecine & pharmacotherapie. 2022;156:113861.
Hu H, Chen Y, Tan S, Wu S, Huang Y, Fu S, et al. The research progress of antiangiogenic therapy, immune therapy and tumor microenvironment. Front Immunol. 2022;13:802846.
Flügel D, Görlach A, Kietzmann T. GSK-3β regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1α. Blood. 2012;119(5):1292–301.
Li JN, Chen PS, Chiu CF, Lyu YJ, Lo C, Tsai LW, et al. TARBP2 Suppresses Ubiquitin-Proteasomal Degradation of HIF-1α in Breast Cancer. Int J Mol Sci. 2021;23(1):208.
Dhami SPS, Patmore S, Comerford C, Byrne CM, Cavanagh B, Castle J, et al. Breast cancer cells mediate endothelial cell activation, promoting von Willebrand factor release, tumor adhesion, and transendothelial migration. J Thrombosis and Haemostasis. 2022;20(10):2350–65.
Jiang G, Xiang Z, Fang Q. Engineering magnetotactic bacteria MVs to synergize chemotherapy, ferroptosis and immunotherapy for augmented antitumor therapy. Nanoscale horizons. 2023;8(8):1062–72.
Balamurugan K, Sharan S, Klarmann KD, Zhang Y, Coppola V, Summers GH, et al. FBXW7α attenuates inflammatory signalling by downregulating C/EBPδ and its target gene Tlr4. Nat Commun. 2013;4:1662.
Xing L, Xu L, Zhang Y, Che Y, Wang M, Shao Y, et al. Recent Insight on Regulations of FBXW7 and Its Role in Immunotherapy. Front Oncol. 2022;12:925041.
De Angelis C, Fu X, Cataldo ML, Nardone A, Pereira R, Veeraraghavan J, et al. Activation of the IFN Signaling Pathway is Associated with Resistance to CDK4/6 Inhibitors and Immune Checkpoint Activation in ER-Positive Breast Cancer. Clinical Cancer Res. 2021;27(17):4870–82.
Yoshimura T, Li C, Wang Y, Matsukawa A. The chemokine monocyte chemoattractant protein-1/CCL2 is a promoter of breast cancer metastasis. Cell Mol Immunol. 2023;20(7):714–38.
Yumimoto K, Nakayama KI. Fbxw7 suppresses cancer metastasis by inhibiting niche formation. Oncoimmunology. 2015;4(8):e1022308.
Masuda T, Noda M, Kogawa T, Kitagawa D, Hayashi N, Jomori T, et al. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020;111(3):924–31.
Liu W, Ren D, Xiong W, Jin X, Zhu L. A novel FBW7/NFAT1 axis regulates cancer immunity in sunitinib-resistant renal cancer by inducing PD-L1 expression. J Exp Clinical Cancer Res. 2022;41(1):38.
Yan L, Lin M, Pan S, Assaraf YG, Wang ZW, Zhu X. Emerging roles of F-box proteins in cancer drug resistance. Drug Resistance Updates. 2020;49:100673.
Yuan B, Hao J, Zhang Q, Wang Y, Zhu Y. Role of Bcl-2 on drug resistance in breast cancer polyploidy-induced spindle poisons. Oncol Lett. 2020;19(3):1701–10.
Ann EJ, Kim MY, Yoon JH, Ahn JS, Jo EH, Lee HJ, et al. Tumor Suppressor HIPK2 Regulates Malignant Growth via Phosphorylation of Notch1. Can Res. 2016;76(16):4728–40.
Mun GI, Choi E, Lee Y, Lee YS. Decreased expression of FBXW7 by ERK1/2 activation in drug-resistant cancer cells confers transcriptional activation of MDR1 by suppression of ubiquitin degradation of HSF1. Cell Death Dis. 2020;11(5):395.
Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7(5):637–51.
Sharma A, Thacker G, Mishra M, Singh AK, Upadhyay V, Sanyal S, et al. SOX4-mediated FBW7 transcriptional upregulation confers Tamoxifen resistance in ER+ breast cancers via GATA3 downregulation. Life Sci. 2022;303:120682.
Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science (New York, NY). 2008;321(5895):1499–502.
Wang LL, Wan XY, Liu CQ, Zheng FM. NDR1 increases NOTCH1 signaling activity by impairing Fbw7 mediated NICD degradation to enhance breast cancer stem cell properties. Mol Med (Cambridge, Mass). 2022;28(1):49.
Wang X, Wei X, Cao Y, Xing P. Mcl-1 inhibition overcomes BET inhibitor resistance induced by low FBW7 expression in breast cancer. J Cell Mol Med. 2022;26(5):1672–83.
Tian W, Hao S, Gao B, Jiang Y, Zhang X, Zhang S, et al. Lobaplatin inhibits breast cancer progression, cell proliferation while it induces cell apoptosis by downregulating MTDH expression. Drug Des Dev Ther. 2018;12:3563–71.
Xue J, Chi Y, Chen Y, Huang S, Ye X, Niu J, et al. MiRNA-621 sensitizes breast cancer to chemotherapy by suppressing FBXO11 and enhancing p53 activity. Oncogene. 2016;35(4):448–58.
Kim MJ, Chen G, Sica GL, Deng X. Epigenetic modulation of FBW7/Mcl-1 pathway for lung cancer therapy. Cancer Biol Ther. 2021;22(1):55–65.
Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol. 2016;17(1):95–103.
Li H, Wu J, Ying G, Chen L, Lai L, Liu Z, et al. J-4: a novel and typical preclinical anticancer drug targeting protein kinase C ζ. Anticancer Drugs. 2012;23(7):691–7.
Zeidan AM, Ridinger M, Lin TL, Becker PS, Schiller GJ, Patel PA, et al. A phase Ib study of Onvansertib, a novel oral PLK1 inhibitor, in combination therapy for patients with relapsed or refractory acute myeloid leukemia. Clinical Cancer Res. 2020;26(23):6132–40.
Du R, Huang C, Liu K, Li X, Dong Z. Targeting AURKA in Cancer: molecular mechanisms and opportunities for Cancer therapy. Mol Cancer. 2021;20(1):15.
Hartkopf AD, Wallwiener M, Fehm TN, Hahn M, Walter CB, Gruber I, et al. Disseminated tumor cells from the bone marrow of patients with nonmetastatic primary breast cancer are predictive of locoregional relapse. Ann Oncol. 2015;26(6):1155–60.
Takeishi S, Matsumoto A, Onoyama I, Naka K, Hirao A, Nakayama KI. Ablation of Fbxw7 eliminates leukemia-initiating cells by preventing quiescence. Cancer Cell. 2013;23(3):347–61.
Shimizu H, Takeishi S, Nakatsumi H, Nakayama KI. Prevention of cancer dormancy by Fbxw7 ablation eradicates disseminated tumor cells. JCI insight. 2019;4(4):e125138.
Acknowledgements
Not applicable.
Funding
Research reported in this publication was supported by the Chengdu University of Traditional Chinese Medicine (No. MPRC2022028).
Author information
Authors and Affiliations
Contributions
SYC and PL collected literature and wrote the manuscript; SYC drew the figures and tables; JLG and HZ conceived the topic area and supervised; SYC and HZ reviewed and edited the manuscript drafts. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests in this section.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, S., Leng, P., Guo, J. et al. FBXW7 in breast cancer: mechanism of action and therapeutic potential. J Exp Clin Cancer Res 42, 226 (2023). https://doi.org/10.1186/s13046-023-02767-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-023-02767-1