Abstract
Aurora kinase A (AURKA) belongs to the family of serine/threonine kinases, whose activation is necessary for cell division processes via regulation of mitosis. AURKA shows significantly higher expression in cancer tissues than in normal control tissues for multiple tumor types according to the TCGA database. Activation of AURKA has been demonstrated to play an important role in a wide range of cancers, and numerous AURKA substrates have been identified. AURKA-mediated phosphorylation can regulate the functions of AURKA substrates, some of which are mitosis regulators, tumor suppressors or oncogenes. In addition, enrichment of AURKA-interacting proteins with KEGG pathway and GO analysis have demonstrated that these proteins are involved in classic oncogenic pathways. All of this evidence favors the idea of AURKA as a target for cancer therapy, and some small molecules targeting AURKA have been discovered. These AURKA inhibitors (AKIs) have been tested in preclinical studies, and some of them have been subjected to clinical trials as monotherapies or in combination with classic chemotherapy or other targeted therapies.
Similar content being viewed by others
Introduction
Aurora kinases belong to serine/threonine kinases which share a highly conserved catalytic domain containing auto-phosphorylating sites. This family contains three members: Aurora A (AURKA), Aurora B (AURKB), and Aurora C (AURKC). Both AURKA and AURKB play essential roles in regulating cell division during mitosis while AURKC has a unique physiological role in spermatogenesis. Relatively less information is available for the roles of AURKC in cancer. AURKA and AURKB have been found to function as oncogenes to promote tumorigenesis in multiple types of cancer including solid tumors and hematological malignancies. Even though, AURKA has attracted researchers’ attentions and has been a more popular target than AURKB for cancer therapy with nearly fifty clinical trials using specific AKIs. However, only about ten clinical trials using inhibitors specifically targeting AURKB and most of them are still in phase I stage. In comparison, the most popular AKI alisertib has finished phase III clinical assessment. In this review, we will focus on research progress associated with AURKA in cancer. Apart from playing a role in mitosis, an increasing number of studies have suggested that AURKA, when abnormally expressed, could be an oncogene involved in tumorigenesis. Gene amplification, transcriptional activation and inhibition of protein degradation could contribute to the elevated levels of AURKA expression in cancer tissues. AURKA promotes tumorigenesis by participating in the cancer cell proliferation, epithelial-mesenchymal transition (EMT), metastasis, apoptosis, and self-renewal of cancer stem cells. Given that overexpression and gene amplification of AURKA have been identified in diverse cancers, small molecule kinase inhibitors of AURKA have attracted considerable interest. A series of AURKA kinase inhibitors (AKIs) have been produced over the past decades; inhibition of the expression or activity of AURKA by AKIs suppresses cancer cell proliferation, migration and invasion. Excitingly, some AKIs have already been used in clinical trials. In this review, we highlight the importance of AURKA in cancer cell signal transduction. Moreover, we provide a summary of the selective inhibitors and pan-inhibitors of AURKA tested in various preclinical and clinical studies for the treatment of cancer.
Expression of Aurora kinases in cancer
Aurora kinases are expressed in a wide range of cancers according to The Cancer Genome Atlas (TCGA) UALCAN database. As shown in Fig. 1a, AURKA expression is lowest in the thyroid carcinoma (THCA) dataset (median value 1.384) and highest in the rectum adenocarcinoma (READ) dataset (median value 5.329). AURKB has the lowest expression in the kidney chromophobe carcinoma (KICH) dataset (median value 0.576) and the highest expression in the diffuse large B-cell lymphoma (DLBC) dataset (median value 6.525). Four out of 33 (12.1%) cancer types show expression of AURKA with log2 (transcripts per million [TPM] + 1) values < 2, including brain lower-grade glioma (LGG), prostate adenocarcinoma (PRAD), pheochromocytoma and paraganglioma (PCPG) and THCA versus other tumors. In contrast, as many as 7 out of 33 (21.2%) cancer types show AURKB expression with a log2 (TPM + 1) value < 2. Moreover, all cancers exhibit AURKC expression with a log2 (TPM + 1) value < 2.
As shown in Fig. 1b, compared with normal tissues, most tumor types show significantly higher expression of AURKA, except for pancreatic adenocarcinoma (PAAD), PCPG, skin cutaneous melanoma (SKCM), and thymoma (THYM). Notably, AURKA expression is reduced in tumor tissues versus normal tissues in the THCA dataset. In samples from patients with 27 out of 33 tumor types, excluding KICH, PAAD, sarcoma (SARC), SKCM, THCA and THYM, AURKB has markedly higher expression in tumor tissues than in normal tissues. In contrast, AURKC expression is higher in tumor tissues than in normal tissues only in samples from patients with 9 out of 33 tumor types, including bladder urothelial carcinoma (BLCA), cholangiocarcinoma (CHOL), esophageal carcinoma (ESCA), head and neck squamous cell carcinoma (HNSC), lung squamous cell carcinoma (LUSC), READ, THCA and stomach adenocarcinoma (STAD). These data suggest that AURKA and AURKB are better potential targets than AURKC for cancer treatment.
Significance of Aurora kinases expression
According to the TCGA UALCAN database, high expression of AURKA may be a sensitive prognostic marker in adrenocortical carcinoma (ACC), LGG, KICH, kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), mesothelioma (MESO), PAAD, SARC and uveal melanoma (UVM). High AURKB expression was more closely related to worse overall survival in ACC, LGG, cervical squamous cell carcinoma (CESC), KICH, KIRC, KIRP, LIHC, LUAD, MESO, SARC, SKCM and UVM. Interestingly, AURKA and AURKB show similar patterns of survival correlation in ACC, LGG, KICH, KIRC, KIRP, LIHC, LUAD, MESO, SARC and UVM. Targeting both AURKA and AURKB in tumors of these cancer types may exert considerable antitumor effects. However, the expression of AURKC can predict patient survival only in LGG, KIRC and SKCM. These survival data are summarized in Fig. 2.
Correlation between Aurora kinases expression and patient overall survival. Red text: gene expression had significant relation with survival; black text: gene expression had no significant relation with survival. The survival data are derived from ULCAN database. Samples were categorized into two groups for analysis: High AURKA expression (with TPM values above upper quartile); Low/Medium AURKA expression (with TPM values below upper quartile). * P < 0.05, ** P < 0.01, *** P < 0.001
Upstream molecular regulation of AURKA
There is overwhelming evidence of overexpression and gene amplification of AURKA in a wide range of cancers. The underlying mechanisms for AURKA upregulation in cancer include gene amplification, gene mutation, microRNA regulation, transcriptional or posttranscriptional modification, and others. Here, we summarize the molecules that positively or negatively regulate AURKA through interactions (Table 1).
Positive regulators of AURKA
Transcriptional regulation of AURKA
Initially, AURKA function is regulated at the transcriptional level. In breast cancer stem cells, nuclear AURKA is recruited by FOXM1 and binds to the FOXM1 promoter to transactivate its expression, while FOXM1 activates AURKA expression at the transcriptional level in a similar manner [1]. The positive feedback signaling loop between AURKA and FOXM1 is crucial for breast cancer stem cell self-renewal. One study has reported that the transcription of AURKA is positively regulated by E4TF1, which is a ubiquitously expressed ETS family protein [4]. Another study has indicated that EGF-induced AURKA expression depends on the interaction of nuclear EGFR and STAT5 [6]. EGFR associated with STAT5 binds to the AT-rich sequence of AURKA and subsequently increases AURKA transcriptional activity [6]. ARID3A (AT-rich interaction domain 3A) is a transcriptional factor. In colorectal cancer cells, ARID3A can bind with the AURKA promoter region and promote AURKA expression [2]. As a nucleic acid-binding protein, PUF60 contributes to malignant phenotypes of bladder cancer through binding to AURKA promoter and activating AURKA transcription [3]. The TRAP220/MED1 complex [5] and β-catenin/TCF4 complex [7] also directly bind to the AURKA promoter to enhance AURKA transcriptional activity.
Translational regulation of AURKA
AURKA is identified as a target protein of HnRNP Q1 by RNA-immunoprecipitation assay following next-generation sequencing. HnRNP Q1 enhances the translational efficiency of AURKA mRNA by interacting with the 5′-UTR of AURKA mRNA through its RNA-binding domains [8]. More importantly, this regulation mechanism is vital for the pro-proliferative properties of HnRNP Q1in colorectal cancer.
Regulators promoting AURKA activity
Posttranslational regulation of AURKA is vital for AURKA autophosphorylation and kinase activity. Among the proteins that interact with and activate AURKA, some are well-established activators, such as Ajuba, TPX2, NEDD9 and PUM2. The LIM protein Ajuba efficiently stimulates AURKA autophosphorylation at Thr288 and increases kinase activity toward histone H3 in the late G2 phase [25]. Both the LIM-2 and LIM-3 domains of Ajuba mediate the interaction with the N-terminus of AURKA, and the Ajuba-AURKA complex induces mitotic entry and progression of cell division [25]. Furthermore, activation of AURKA is also stimulated by TPX2. The N-terminal domain of TPX2 binds to AURKA and protects AURKA from dephosphorylation according to experimental and structural analyses [10, 11]. TPX2 primarily exists in an inhibitory complex along with importin α/β at the onset of mitosis, and it is immediately released by Ran-GTP after nuclear envelope breakdown to bind to AURKA and stimulate AURKA autophosphorylation at Thr288. Two other kinases, PAK1 and PKC, directly phosphorylate AURKA and then increase AURKA activity [19, 24]. Other molecules also modulate AURKA activity, such as PNUTS [20], BUGZ [21], RASSF1A [22], IPP2 [23] and KCTD12 [26].
Regulators stabilizing AURKA protein expression
Abnormally upregulated AURKA in cancers is always stabilized by other molecules. Protein kinases such as LIMK2 are associated with AURKA through LIM domains, and this interaction is responsible for AURKA stabilization [14]. TPX2 protects AURKA from degradation both in interphase and in mitosis in a cdh1-dependent manner [12]. Likewise, NEDD9 [9] and PUM2 [13] not only stimulate autophosphorylation and autoactivation of AURKA but also stabilize AURKA protein expression through disassociation from cdh. AURKA protein stability is also maintained by Twist [15], ALDH1A1 [16], YBX1 [17] and the deubiquitinase USP2a [18] through ubiquitin-proteosomal degradation pathway.
Negative regulators of AURKA
Although many proteins determine the active state of AURKA to a great extent, negative AURKA regulators that tightly control AURKA expression or activity exist. These regulators are usually tumor suppressors, and inhibition of AURKA is one of the mechanisms explaining their tumor-suppressive functions.
Transcriptional regulation of AURKA
INI1/hSNF5 is a core component of the mammalian chromatin-remodeling SWI/SNF complex, which regulates the transcription of target genes. In rhabdoid tumor (RT) cells and normal fibroblast cells, INI1/hSNF5 complex associates with the AURKA promoter and represses AURKA transcription. This regulation is dependent on cell type because in non-RT cells such as Jurkats, CEMX-174, HeLa and SF268, downregulation of INI1/hSNF5 had either no effect or a slight decrease in AURKA [27]. ARID1A, a component of the SWI/SNF chromatin remodeling complex, occupies the AURKA gene promoter to negatively regulate its transcription [28]. SIX3, a member of the sine oculis homeobox transcription factor family, suppresses the transcription of both AURKA and AURKB by directly binding with their promoters in astrocytoma [29]. Apart from the regulation of AURKA transcription through interaction with AURKA promoter, it was reported that ribonuclease MCPIP1 destabilized AURKA mRNA [30]. A highly conserved 95-base region in AURKA 3′-UTR was required for MCPIP1-dependent cleavage of the AURKA transcript [30].
Regulators reducing AURKA activity
GSK-3β interacts with AURKA and phosphorylates AURKA at Ser290/291 in vitro, after which autophosphorylation occurs at Ser349, which is an AURKA activity-inhibiting phosphorylation site [44]. Gadd45a is a stress gene that is highly induced by a variety of genotoxic agents. Interaction between Gadd45a and AURKA has been shown to strongly inhibit AURKA kinase activity and antagonize AURKA-induced centrosome amplification [42]. PTTG1 is a transforming gene highly expressed in several cancers. One study has indicated that PTTG1 represses AURKA autophosphorylation, inhibits phosphorylation of histone H3 and results in abnormally condensed chromatin [41]. Another study has shown that the phosphatase PP1, but not PP2, dephosphorylates AURKA and abolishes AURKA kinase activity [43].
Regulators promoting AURKA protein degradation
Apart from AURKA activity, AURKA protein expression is tightly controlled as well. During the process of mitosis, IKK2 acts as an antagonist of AURKA. Phosphorylation of AURKA by IKK2 targets it for β-TRCP-mediated degradation and serves to maintain appropriate levels of AURKA to assure proper bipolar spindle assembly and mitotic progression [35]. AURKAIP1, an AURKA-interacting protein, is involved in the degradation of AURKA through a proteasome-dependent pathway [36]. A mechanistic study has revealed that AURKAIP1-mediated AURKA degradation is dependent on antizyme1 (Az1). AURKAIP1 enhances the ability of Az1 to bind to AURKA in order to promote proteasomal localization and subsequent degradation [37]. Cdh1 is a WD40 repeat protein serving as an anaphase-promoting complex/cyclosome (APC/C) coactivator. AURKA degradation is dependent on Cdh1 in vivo, and AURKA is targeted for proteolysis through distinct structural features of its destruction box, its KEN box motifs and its kinase activity [31]. VHL is an E3 ligase that multi-monoubiquitinates AURKA in quiescent cells and targets it for proteasome-mediated degradation under both normoxic and hypoxic conditions [38]. Phosphatase PRL-3 enhances AURKA ubiquitination and degradation in colorectal cancer [34]. Destabilization of AURKA by PRL-3 requires PRL-3-mediated dephosphorylation of FZR1 and assembly of the APC/CFZR1 complex [34]. PTPRD is a protein tyrosine phosphatase and a tumor suppressor. It destabilizes the AURKA protein by dephosphorylating tyrosine residues in AURKA, leading to downstream destabilization of the MYCN protein [39]. NQO1 [32], SMAD4 [33] and PHLDA1 [40] are also tumor suppressors mediating AURKA protein degradation. NQO1 competes with TPX2 for binding to AURKA and inhibits excessive increases in AURKA protein levels, thereby suppressing the generation of aneuploidy in irradiated cells [32]. The tumor suppressor SMAD4 interacts with AURKA and inhibits the expression of AURKA via proteasomal degradation which is independent of TGFβ signaling [33].
Downstream targets of AURKA
Based on the high expression and significance of AURKA in multiple types of tumors, it is crucial to discover the mechanism of action for AURKA in cancer. As a serine/threonine protein kinase, AURKA is reported to interact with numerous proteins, including tumor suppressors and oncogenes, to promote carcinogenesis, as shown in Table 2.
AURKA substrates regulating mitosis
AURKA is involved in the regulation of spindle-associated events during early mitosis. Many of the substrates regulated by AURKA coordinate with AURKA to control mitotic progression, and aberrant expression of AURKA in a variety of human cancers has been linked with mitotic defects. Phosphorylation of histone H3 is a crucial event for the onset of mitosis. AURKA physically interacts with the histone H3 tail and efficiently phosphorylates Ser10 both in vitro and in vivo [61]. NDEL1 phosphorylation by AURKA at the Ser251 site is essential for centrosomal separation and centrosomal maturation. After phosphorylation, NDEL1 displays high affinity for the mitotic protein TACC3, mediating TACC3 recruitment to the centrosome [68]. TACC3 is another substrate of AURKA that is localized to mitotic spindles and proximal mitotic spindles after phosphorylation at Ser558 [52]. The NDEL1-TACC3 protein complex activated and initiated by AURKA plays a significant role in centrosome maturation and separation during mitosis. Another centrosome-associated protein, CPAP, directly interacts with AURKA and is phosphorylated by AURKA at Ser467 to maintain the integrity of the spindle pole [65]. ASAP is also a spindle-associated protein, deregulation of which induces severe mitotic defects. After phosphorylation at Ser625 by AURKA, ASAP localizes to centrosomes from late G2 to telophase and around the midbody during cytokinesis [69]. The AURKA activator TPX2 is an AURKA substrate with phosphorylation sites at Ser121 and Ser125. Phosphorylation of TPX2 by AURKA is required for establishment of normal spindle length and interaction with cytoplasmic linker-associated protein 1 [77]. PLK1 is an essential mitotic kinase regulating multiple aspects of the cell division process, and activation of PLK1 requires phosphorylation at Thr210 in the T-loop of the PLK1 kinase domain. It has been reported that AURKA is responsible for the Thr210 phosphorylation of PLK1, which is required for checkpoint recovery [58]. Another study has demonstrated that the phosphatase CDC25B is phosphorylated by AURKA at the Ser353 site to contribute to the G2-M transition [67]. CENP-A, a well-conserved variant of histone H3, is phosphorylated by AURKA at Ser7, which is required for the concentration of AURKB at inner centromeres and for kinetochore function [48].
AURKA substrates acting as functional oncogenes
Some AURKA substrates, such as GSK-3β, β-catenin, Twist, ERα, IκBα, and YAP, participate in crucial oncogenic signaling. AURKA and GSK-3β exist in a complex, and a significant increase in the phosphorylation of GSK-3β at Ser9 has been observed following overexpression of AURKA [57]. Furthermore, AURKA inhibits the degradation of β-catenin, a known substrate of GSK-3β, by phosphorylating β-catenin at the Ser552 and Ser675 sites [54]. This phosphorylation also regulates β-catenin nuclear localization and transcriptional activity toward its target genes [54]. Research has shown that AURKA phosphorylation of Twist at Ser123, Thr148 and Ser184 facilitates Twist-mediated promotion of EMT and chemoresistance in pancreatic cancer cells [15]. In addition, AURKA interacts with ERα and phosphorylates it at Ser167 and Ser305, leading to an increase in the DNA-binding ability of ERα and the transcriptional activity of ERα toward its target cyclin D1 [55]. More interestingly, elevated expression of AURKA predicts poor survival in ERα-positive but not in ERα-negative breast cancers [55]. Regarding the pathway by which AURKA regulates NF-κB signaling, a mechanistic study has revealed that IκBα phosphorylation by AURKA promotes its degradation, thus activating the NF-κB pathway [59]. YAP is the main downstream effector of the Hippo pathway. AURKA-mediated phosphorylation of YAP at Ser397 is crucial for YAP-mediated transcriptional activity and transformation in triple-negative breast cancer cells [51].
SOX2 and YBX1 are oncogenic transcription factors phosphorylated by AURKA. After phosphorylation, SOX2 is able to maintain the ratio of stem cell-like cells, while YBX1 is stabilized and enhances EMT, stem cell formation and chemoresistance [17, 64]. LDHB is a subunit of the tetrameric enzyme LDH that catalyzes the interconversion between pyruvate and lactate. Phosphorylation of LDHB by AURKA at Ser162 amplifies its activity in reducing pyruvate to lactate, thus promoting glycolysis and biosynthesis and promoting tumor growth [45]. Recently, our research has indicated that phosphorylation of the scaffold and oncogenic protein SDCBP by AURKA maintains its protein stability and pro-proliferative functions [63]. Furthermore, the ability of SDCBP to bind to its partners, including EGFR, SRC and FAK, is attenuated when the phosphorylation sites are inactivated [63]. Another unique substrate of AURKA is HURP, which is phosphorylated at four serine positions [75]. HURP protein stability and serum-independent growth are enhanced after phosphorylation [75].
RPS6KB1, a mitogen-activated serine/threonine protein kinase, is activated in human malignancies. Activation of RPS6KB1 occurs through phosphorylation by AURKA at the Thr389 position, which is important for promoting cell proliferation and survival [47]. LIMK2 is a crucial oncogenic regulator with serine/threonine protein kinase activity. AURKA regulates LIMK2 kinase activity, subcellular localization and protein levels by directly phosphorylating LIMK2 at Ser283, Thr494 and Thr505 [14]. The small GTPase RalA is also a target of AURKA; phosphorylation of RalA at Ser194 enhances cell migration and anchorage-independent growth [66]. ALDH1A1 is an AURKA substrate enzyme whose phosphorylation by AURKA at Thr267, Thr442 and Thr493 regulates ALDH1A1 protein stability, enhancing the role of this protein in the process of EMT [16].
AURKA substrates acting as tumor suppressors
P53 is one of the most important substrates of AURKA. It has been reported that AURKA phosphorylates p53 at Ser315, after which p53 is destabilized and the G2-M transition is enhanced [72]. However, Ser106 residue phosphorylation by AURKA has the opposite effect. After phosphorylation, the interaction of p53 with MDM2 is inhibited and the p53 protein expression is stabilized [73]. Another study has revealed that the p53 Ser215 site is phosphorylated by AURKA. P53 DNA binding ability and transactivation activity are inhibited after phosphorylation and p53 tumor suppressor activity is inhibited by AURKA [74]. RASSF1A, initially identified as a microtubule- and centrosome-associated protein, is a scaffold protein with tumor-suppressive function. Phosphorylation of RASSF1A by AURKA at Ser203 and Thr202 removes the ability of RASSF1A to interact with microtubules and induce M-phase cell cycle arrest [71]. PHLDA1, a novel p53 target, can repress the Akt signaling pathway. AURKA directly phosphorylates PHLDA1 at Ser89, which results in degradation of PHLDA1 [40]. Another novel substrate of AURKA with tumor-suppressive function is LKB1. Phosphorylation of LKB1 at Ser299 causes LKB1 to dissociate from AMPK, resulting in impairment of the AMPK signaling pathway and facilitating non-small-cell lung cancer (NSCLC) growth and migration [49]. Merlin suppresses tumor development through distinct mechanisms and is a substrate of AURKA that is phosphorylated at its main regulatory site, Ser518, during mitosis [46]. Another AURKA substrate acting as a tumor suppressor is Lats2. Phosphorylation of Lats2 by AURKA at the Ser83 site regulates its centrosomal localization [78]. This process may be important for Lats2 to suppress tumorigenicity and to inhibit cell proliferation via centrosomal regulation.
Other AURKA substrates
Several AURKA substrates exhibit multiple and counteracting functions in cancer development. YY1 and P73, as transcription factors, have been shown to bind hundreds of DNA sites and to regulate a very large number of target genes with a wide range of functionalities. Once YY1 is phosphorylated by AURKA at Ser365, its DNA-binding activity and transcriptional activity are abolished [62]. Furthermore, AURKA phosphorylation of p73 at Ser235 eliminates the p73 transactivation function in both DNA damage-induced cell death and mitotic spindle assembly checkpoint pathways [76]. Another multifunctional protein is the ubiquitin ligase CHIP, which has been shown to be a regulator of oncogenic pathways or tumor-suppressive pathways. AURKA-mediated phosphorylation of CHIP at Ser273 promotes androgen degradation in castration-resistant prostate cancer [50]. KCTD12 also exhibits dual and opposite functions in cancer. Phosphorylation by AURKA at Ser243 may account for the cancer-promoting effects of KCTD12 [26].
MBD3 [70], HDM2 [53], PP1 [43], VHL [60] and BimEL [56] are also phosphorylated by AURKA, but their subsequent specific functions remain to be revealed. Notably, some proteins, including ALDH1A1, Twist, YBX1, KCTD12, RASS1A, PHLDA1, PP1, TPX2, LIMK2 and VHL, usually form negative or positive feedback loops with AURKA.
Signaling pathways involving AURKA-interacting proteins
AURKA has been identified to regulate many signaling pathways, such as the PI3K/Akt, mTOR, β-catenin/Wnt and NF-κB pathways, and tumorigenesis requires interactions among multiple signaling pathways. We obtained an interactome network using the STRING database based on the AURKA-interacting proteins mentioned in the previous section (Fig. 3). Then, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Ontology (GO) enrichment analyses of the signaling pathways. The results indicated that AURKA-related proteins are involved in the processes of mitosis, cell cycle progression and apoptosis. Furthermore, these proteins are directly or indirectly associated with key molecules in crucial signaling pathways such as the Hippo pathway, the p53 pathway, the PI3K-Akt pathway, the FOXO pathway and the Wnt pathway. Most importantly, AURKA is involved in all of these cancer-related pathways, suggesting the significance of AURKA in these processes and pathways.
AURKA interactome and related signaling pathways. The interactome in the center is obtained through STRING database based on AURKA-interacted proteins mentioned in Table 1 and Table 2. The interactome around are enriched pathway proteins. The left bottom literal statements are the alternative names of the molecular
Pharmacologic targeting of AURKA in cancer therapy
A series of molecules have been demonstrated to be able to inhibit AURKA activity. Although the majority also exerts effects on other members of the Aurora kinase family or even on other kinases, there is enough evidence to make some of them potent targets for cancer therapy both in vitro and in vivo in preclinical or clinical evaluations (Table 3and Table 4).
Specific AKIs
AKIs in preclinical studies
In recent years, several small molecules that selectively target AURKA have been identified with anticancer activity in preclinical studies including LY3295668 [86], BPR1K0609S1 [81, 82], LDD970 [83], MK-8745 [84, 85], AKI603 [80] and CYC3 [79]. The detailed information is shown in Table 3.
AKIs in clinical studies
Several inhibitors with high specificity for AURKA have been developed, and some of them have shown clinical efficacy in clinical trials. The common dose-limiting toxicity of specific AKIs, including MLN8237 and ENMD-2076, are neutropenia, somnolence and mucisitis.
MLN8237 is a highly selective small molecule inhibitor of AURKA with an IC50 of 1 nM. MLN8237 was developed as an enhancement of its predecessor, MLN8054, development of which was terminated after phase I studies due to central nervous system side effects, including dose-limiting somnolence [124, 125]. MLN8237 has been shown to inhibit cell proliferation by impairing mitosis, inducing cell cycle arrest and autophagy, and accelerating cancer cell apoptosis and senescence in multiple cancer types [132, 133]. The EMT process is also impeded by MLN8237 treatment in human epithelial ovarian and pancreatic cancer cells [134]. Importantly, MLN8237 significantly increases the sensitivity of tumor cells to chemotherapy drugs or radiation [135, 136]. Mechanistic studies have revealed that MLN8237 induces proteasomal degradation of N-myc in childhood neuroblastoma [137]. In THCA cells, MLN8237 disrupts c-Myc/AURKA complex formation, and c-Myc is a major determinant of MLN8237 responsiveness both in vitro and in vivo [138]. MLN8237 has demonstrated efficacy in cell-derived and patient-derived xenograft (PDX) models of numerous tumor types, including glioblastoma [139], bladder cancer [140], esophageal adenocarcinoma [136], multiple myeloma [132], neuroblastoma [141] and colon cancer [142].
Due to its potent efficiency in preclinical studies, MLN8237 has been tested in clinical trials for multiple cancers and is the only AURKA inhibitor that has proceeded to phase III evaluation. Many phase I and II studies have described the pharmacokinetic and pharmacodynamic properties of MLN8237 in patients with advanced tumors and hematologic malignancies [143,144,145,146]. Based on the results of these studies, the recommended phase II dose of MLN8237 is 50 mg twice daily orally for 7 days in 21-day cycles. However, because children experience greater frequencies of myelosuppression and hand-foot-skin syndrome on this schedule, the recommended pediatric phase II dose is 80 mg once daily for 7 days [147]. One phase II trial of MLN8237 in patients with ovarian cancer, fallopian tube cancer, peritoneal carcinoma, acute myelogenous leukemia and high-grade myelodysplastic syndrome showed that MLN8237 has modest single-agent antitumor activity [148]. In a multicenter phase II study, MLN8237 treatment obtained an objective response in 18% of 49 women with breast cancer and 21% of 48 participants with small-cell lung cancer [149]. In another phase II study of MLN8237 in advanced/metastatic sarcoma, occasional responses and prolonged stable disease were observed, and progression-free survival (PFS) was promising [150]. In castration-resistant neuroendocrine prostate cancer patients, those with AURKA and N-myc activation achieve significant clinical benefit from single-agent MLN8237 treatment [151]. Another phase II study has shown that in relapsed or refractory peripheral T-cell NHL (PTCL) patients, MLN8237 has antitumor activity with an overall response rate of 30% [152]. In a recently reported phase III study conducted in patients with PTCL, although MLN8237 did not demonstrate superior efficacy over comparators, it did show antitumor activity and acceptable tolerability and safety [153]. All these encouraging outcomes make MLN8237 a promising agent for cancer treatment.
ENMD-2076, a novel, orally bioavailable multitarget inhibitor whose main targets are FLT3 (IC50 = 3 nM) and AURKA (IC50 = 14 nM), exhibits much greater potency against AURKA than against AURKB (IC50 = 350 nM) [154]. Because of its multitarget properties, ENMD-2076 inhibits the growth of a wide range of human solid tumor and hematopoietic cancer cell lines, with IC50 values ranging from 0.025 to 0.7 μM [155]. ENMD-2076 shows antitumor activity in colorectal cancer [154], multiple myeloma [156] and triple-negative breast cancer [157, 158] both in vitro and in vivo. Due to the potent inhibitory effects of ENMD-2076 on cancer cells and xenografts, several phase I/II clinical trials on this compound have been conducted in solid tumors and hematologic malignancies [113,114,115,116,117,118,119] (Table 4).
MK-5108 is a novel small molecule that shows robust selectivity for AURKA over AURKB (220-fold greater selectivity) and AURKC (190-fold greater selectivity) [159]. It inhibits the growth of 14 tumor cell lines with IC50 values between 0.16 and 6.4 μM and shows antitumor effects alone or in combination with docetaxel in xenografts [159]. In EOC stem cells, MK-5108 induces cell cycle arrest by affecting the NF-ĸB pathway [160]. MK-5108 also decreases the rate of proliferation and increases intratumoral apoptosis in uterine leiomyosarcoma xenografts [161]. MK-5108 effect has been evaluated in a phase I clinical study as shown in Table 4 [123].
Pan Aurora kinase inhibitors
Clinically significant side effects of pan Aurora kinase inhibitors include neutropenia, fatigue, diarrhea and hypertension. Even though the selective AURKA inhibitors might be less toxic than pan-inhibitors, it may also lead to drug resistance more easily, so it is necessary to develop broad Aurora kinase inhibitors to obtain drugs with greater potency for cancer treatment.
Inhibitors in preclinical studies
Recently, more than 10 pan-Aurora kinase inhibitors have been designed in preclinical studies. For example, AKI-001 [100], BPR1K871 [89], CCT137690 [97, 98], JNJ-7706621 [92], SAR156497 [93], SCH-1473759 [90] and VE-465 [95] have potent inhibitory effects on Aurora kinase activity with IC50 values< 50 nM. Other Aurora kinase inhibitors with IC50 values> 50 nM against kinase activity, such as BPR1K653 [87], CCT129202 [96], derrone [91], PHA-680632 [99], R1498 [94], reversine [101] and TY-011 [88], have also been tested in preclinical studies, and the preliminary data are shown in Table 3.
Inhibitors in clinical studies
AT9283 exhibits strong activity against several kinases [162]. The ability of AT9283 to inhibit the growth and survival of tumor cells as well as xenografts has been demonstrated in imatinib-resistant BCR-ABL-positive leukemic cells [163], aggressive B-cell lymphoma [164] and medulloblastoma [165]. Several clinical studies have been completed on AT9283 as shown in Table 4 [107,108,109,110,111]. However, there have been no clinical or objective responses in patients in these trials because of the small numbers of patients.
MK-0457, a pyrazoloquinazoline compound, inhibits all three Aurora kinases [166] and inhibits FLT-3 and Abl kinases [167]. This compound increases the Bax/Bcl-2 ratio and induces apoptosis in AML cases with high AURKA expression [168]. MK-0457 has been confirmed to show efficiency in anaplastic THCA cells [169], chemoresistant ovarian cancer models [170], myeloma cell lines and primary myeloma cell samples [171], and imatinib-resistant chronic myelogenous leukemia [172]. MK-0457 induces accumulation of cells with ≥4 N DNA content, inhibits cell cycle progression and induces apoptosis of anaplastic THCA cells [169]. In a phase I study conducted in patients with advanced solid tumors, almost half of them attained stable disease, including one patient with advanced ovarian cancer who attained prolonged stable disease for 11 months after receiving 15 cycles of MK-0457 [120]. The activity of MK-0457 was also assessed in two other phase I/II studies, both of which showed promising outcomes in patients with BCR-ABL T315I leukemia [121, 122].
PHA-739358 exerts strong activity against all three Aurora kinases and cross-reactivity with tyrosine kinases, including FGFR1 and Abl [173]. PHA-739358 exhibits strong antiproliferative activity in BCR-ABL-positive leukemia cells, including those harboring the T315I mutation [174]. The crystal structure of the Abl-T315I-PHA-739358 complex provides a possible structural explanation for the activity of PHA-739358 on the T315I mutation [175]. PHA-739358 also induces cell cycle arrest, apoptosis and autophagy and suppresses the EMT process [176, 177]. More importantly, PHA-739358 shows antimetastasis properties. In one study, PHA-739358 inhibited liver metastases from gastroenteropancreatic neuroendocrine tumors in an orthotopic xenograft model [178]. In another study, PHA-739358 inhibited early-stage bone metastases based on an ex vivo model named the ‘bone-in-culture array’ [179]. Several phase I/II clinical evaluations have been performed on PHA-739358 due to its encouraging antitumor effects [127,128,129,130,131].
Other drugs including AMG900 [102, 103], AS703569 [104,105,106], BI-847325 [112], CYC116, PF-03814735 [126], and SNS-314 are also in phase I clinical trials.
Combination therapy
Synergy between AKIs and chemotherapy or radiotherapy
AURKA inhibitors have been shown to have great potential for enhancing the efficacy of multiple established therapeutic agents in both preclinical and clinical studies. AURKA inhibitors combined with docetaxel can produce better therapeutic outcomes than docetaxel alone in mantle cell lymphoma and upper gastrointestinal adenocarcinomas [180,181,182]. This combination procedure was applied in a phase I clinical trial; in this trial, 20 mg of alisertib twice daily for days 1 to 7 with intravenous docetaxel at 75 mg/m2 on day 1 in a 21-day cycle was well tolerated, and the combination regimen demonstrated antitumor activity in various cancer types [183]. Combined treatment with alisertib and paclitaxel has been found to result in more potent inhibition of tumor growth and dissemination than single-agent treatment in an orthotopic xenograft model of EOC [184]. Moreover, AMG900 demonstrates potent inhibitory efficiency in paclitaxel-resistant tumor cell lines and xenografts [185]. A clinical trial in patients with recurrent ovarian cancer has shown that combined treatment with 40 mg of oral alisertib twice daily plus 60 mg/m2 paclitaxel weekly shows promising antitumor activity with an increased but generally manageable safety profile [186]. Gemcitabine has also been considered for combined treatment with AKIs. In patients with solid tumors, AS703569 in combination with the standard dose of gemcitabine produces preliminary signs of efficacy [106]. In AML, alisertib increases the efficacy of cytarabine in a FOXO-dependent manner [187]. Another two clinical trials have demonstrated that alisertib plus induction chemotherapy with cytarabine and idarubicin is effective and safe in patients with AML [188, 189].
In addition, MLN8237 has a synergistic effect in association with vincristine and rituximab in aggressive B-cell NHL [190]. Researchers have applied this strategy in clinical trials. A combination of 50 mg of alisertib b.i.d. plus 40 mg of rituximab or alisertib b.i.d. plus rituximab and vincristine is well tolerated and demonstrates activity against non-germinal center B-cell DLBC [191]. In a study on Myc-overexpressing lymphoma xenografts, a combination of cyclophosphamide and MLN8237 induced complete tumor regression in all mice, leading to improvements in survival [192].
The combination of alisertib and carboplatin is selectively effective in glioblastoma patients with high tumor O6-methylguanine DNA methyltransferase expression who are resistant to standard therapy [193]. Eribulin, a microtubule-targeting drug, is used in metastatic breast cancer patients in the clinic. Combined treatment with MLN8237 and eribulin leads to a synergistic increase in apoptosis in mammary tumors as well as cytotoxic autophagy in metastases through the LC3B/p62 axis and Akt pathway [194]. In multiple myeloma, studies on combined treatment with AT9283 and lenalidomide have shown significant synergistic antitumor effects of this regimen both in vitro and in vivo [195]. Recently, two clinical trials have revealed that 60 mg/m2 alisertib per dose for 7 days is tolerable with a standard irinotecan and temozolomide backbone and shows antitumor activity, particularly in neuroblastoma patients with MYCN-nonamplified tumors [196, 197].
In addition to clinical drugs, AURKA inhibitors also show synergistic effects when used in combination with radiotherapy. PHA680632 treatment prior to radiation treatment leads to an additive effect in cancer cells, especially in p53-deficient cells in vitro or in vivo [198]. Another AURKA inhibitor, MLN8054, sensitizes androgen-insensitive prostate cancer to radiation; this sensitization is associated with sustained DNA double-strand breaks [199]. Two other AURKA inhibitors, MLN8237 and ENMD-2076, also enhance radiation sensitivity in cancer cells [200, 201]. A phase I trial on alisertib with fractionated stereotactic reirradiation therapy for patients with recurrent high-grade glioma has been conducted and has revealed that 40 mg of alisertib twice daily in combination with irradiation is safe and well tolerated [202]. Further exploration in the phase II trial may provide a better sense of clinical outcomes moving forward.
Combination of AKIs with targeted therapies
Cancer is a multistep disease involving multiple genes, so targeting multiple oncogenes simultaneously may enhance the efficiency of AKIs. HDAC inhibitors have been shown to repress the expression of AURKA in various cancer cells, and AKIs can decrease the activity of HDAC proteins, suggesting that synergetic effects could be obtained by combining AKIs and HDAC inhibitors [203, 204]. Studies have shown that the HDAC inhibitor vorinostat synergistically potentiates MK-0457 lethality in leukemia cells and breast cancer cells [205,206,207]. In addition, vorinostat and MK-0457 or MK-5108 combination treatment enhances lymphoma cell killing with reductions in c-Myc, hTERT, and microRNA levels [208]. A study in T-cell lymphoma has suggested that the effects of alisertib in combination with the HDAC inhibitor romidepsin are highly synergistic through modulation of cytokinesis [209]. Combination treatment with vorinostat and AMG900 produces synergistic antiproliferative effects both in vitro and in vivo [210]. A phase I study on alisertib in combination with vorinostat in patients with relapsed/refractory lymphoid malignancies has shown encouraging clinical activity with a manageable safety profile [211].
EGFR inhibitors have been a major breakthrough for NSCLC treatment. However, resistance to EGFR inhibitors through multiple mechanisms has been identified, including activation of other oncogenic proteins. One recent study has demonstrated that EGFR-mutant LUAD cells that demonstrate acquired resistance to third-generation EGFR inhibitors are sensitive to AKIs [212]. Furthermore, combination treatment with AKIs and EGFR inhibitors has been found to robustly decrease tumor growth in an EGFR-mutant LUAD PDX model [212].
Both BRD4 and AURKA are regulators of the MYC gene at the translational and posttranslational levels, respectively, and targeting both of them simultaneously may produce synergistic antitumor effects. JQ1 treatment to repress BRD4 activity together with MLN8237 treatment synergistically promotes cell death in c-Myc expressing human glioblastoma cells [213]. Combined treatment with another BRD4 inhibitor, I-BET151, and alisertib is efficacious in exerting antitumor effects against neuroblastoma with or without MYCN amplification both in vitro and in vivo [214].
To investigate whether combined treatment with a p53-activating MDM2 antagonist and senescence-inducing AKIs can be useful for melanoma therapy, two studies have been performed. One study showed that AURKA and MDM2 antagonism with MLN8237 and Nutlin-3 halted melanoma growth by inducing growth arrest and senescence, limiting the lifespans of senescent cells, and enhancing tumor immune infiltration and clearance [215]. The other study showed that combined MK-0457 and Nutlin-3 treatment activated p53-dependent postmitotic checkpoints at pseudo-G1 phase and induced proapoptotic p53 signaling and mitochondrial apoptosis in AML [216]. Other molecules, such as SRC [217], CHEK1 [218], mTOR [219, 220], WEE1 [221], PDK1 [222, 223], and MEK [224], have also been chosen as targets together with AURKA in preclinical studies.
Combination of AKIs with immunotherapy
Immunotherapy and specific monoclonal antibodies targeting multiple molecules have been widely used for cancer therapy. Combining AKIs and these agonists may enhance therapeutic efficacy. In human neuroblastoma cells, MK-5108 increases the efficacy of an anti-ganglioside (GD2) 14G2a antibody, which is related to a reduction in N-Myc expression and an increase in PHLDA1 and p53 protein levels [225]. In addition, combined treatment with an anti-GD2 14G2a antibody and MK-5108 leads to enhancement of the autophagy process in IMR-32 neuroblastoma cells [226]. A death receptor 5 agonist antibody has been found to initiate significant apoptosis in tumor cells undergoing therapy-induced senescence induced by MLN8237 treatment [227]. In that study, the combination group achieved remarkable tumor growth inhibition in melanoma xenografts derived from cell lines and patient tissues [227]. Another study has indicated that alisertib facilitates an anticancer immune microenvironment with decreased numbers of myeloid-derived suppressor cells and increased numbers of active CD8+ and CD4+ T lymphocytes [228]. More importantly, combined administration of alisertib and a PD-L1 antibody has demonstrated synergistic efficacy for the treatment of breast cancer cell 4 T1 xenografts [228]. Combining AKI treatment with anti-PD-1/PD-L1 immune checkpoint therapy may be a promising strategy for cancer treatment.
Conclusions and outlooks
Activation of AURKA is responsible for the resistance of lung cancer to third-generation EGFR inhibitors [212]. Resistance initiated by AURKA may lead to tumor heterogeneity and promote the generation of distinct clones harboring different driving forces of drug resistance. AURKA attenuates the efficacy of inhibition of the PI3K-AKT-mTOR pathway, a downstream pathway of EGFR, in breast cancer [229]. These findings indicate that AKIs should be used together with oncogenic pathway inhibitors to treat drug resistance incrementally.
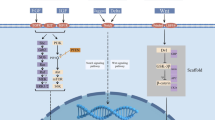
To obtain the desired clinical benefits of AKIs, it is essential to know the pathways and proteins involved in AURKA-mediated oncogenic function. In this review, we have summarized the interactome proteins regulating AURKA or regulated by AURKA and the inhibitors targeting AURKA (Fig. 4). Preclinical studies have shown that AKIs affect the regulation of various cellular processes, such as proliferation, invasion, metastasis, autophagy, EMT, chemoresistance and radioresistance. Furthermore, preclinical animal studies and clinical studies have illustrated the efficacy of AKIs and AKIs in combination with other standard chemotherapeutic drugs, such as paclitaxel, cisplatin and other targeted therapies.
An overview of AURKA-interacting proteins and AKIs. AURKA expression is regulated at transcriptional or post-transcriptional levels and AURKA activity is tightly controlled by numerous molecules. Once activated, AURKA interacts with and phosphorylates a wide variety of proteins serving as mitotic regulators, oncogenes or tumor suppressors. Selective AKIs and pan Aurora kinases inhibitors are developed and studied in preclinical or clinical evaluation
The high toxicity of AKIs should be considered given the crucial physiological function of AURKA in normal cells. Toxicities of AKIs mainly include reversible neutropenia together with mucositis and somnolence, among which neutropenia is the dose-limiting toxicity. The predominant toxicities of AKIs reflect the mechanism of action of AURKA in highly proliferating cells such as bone marrow cells and epithelial cells. The off-target adverse events in central nervous system including somnolence and dizziness reflect the binding of AKIs to the alpha-1 subunit of the GABA-A receptor [230]. Researchers can attempt to reduce the side effects of AKIs by combining low dose of AKIs with chemotherapeutics, targeted therapies or immunotherapy. To weaken the bone marrow suppression induced by AKIs, granulocyte colony-stimulating factor (G-CSF) is administrated in conjunction with PHA-739358. In this phase I study, escalating the PHA-739358 dose until 1000 mg/m2 do not cause any bone marrow related toxicities, particularly neutropenia [128]. Furthermore, development of nanoparticle therapeutic carriers that are passively targeted to tumors through the enhanced permeability and retention effect may be helpful [231]. This drug delivery technology has been applied to MLN8237 and the polysaccharide nanovesicle efficiently delivers low concentrations of MLN8237 to inhibit AURKA and disrupt the anchorage-independent growth of MCF-7 cell than free MLN8237 [232].
Several methods may be taken into consideration to overcome the side effects when developing new AKIs. Researchers can take advantage of the high-resolution 3D protein structures and computer docking tools to find natural compound or FDA approved drugs that target AURKA. For example, derrone, extracted from erythrina orientalis, is screened from 100 natural substances to inhibit AURKA kinase activity and cell growth [91]. Another case is bioactive tanshinone I which is from traditional Chinese herbal medicine Salvia miltiorrhiza. Although there is no direct evidence that tanshinone I can directly target AURKA, it exhibits potent effects on growth inhibition of colon cancer [233], lung cancer [234] and breast cancer cells [235] through downregulating AURKA expression. Another way is to attempt to develop inhibitors that disrupt the interaction between AURKA and its activators. AURKA can be activated by its protein partners, among which TPX2 is the best established one. Withanone is an herbal ligand isolated from ashwagandha. Withanone is reported to bind to the TPX2/AURKA complex which results in the dissociation of TPX2 from AURKA and disruption of mitotic spindle apparatus in cancer cells [236]. Furthermore, due to the fact that AURKA exerts its function through specific substrates in certain cancers, inhibition of AURKA substrates instead of targeting AURKA kinase activity may decrease the adverse effects.
The tumor types that most likely respond to AKIs should also be studied in order to obtain the desired clinical benefits. In one preclinical study, 29 breast cancer cell lines are evaluated for the sensitivity to AURKA inhibitor ENMD-2076 [157]. ENMD-2076 shows stronger activity in cell lines lacking estrogen receptor expression and HER2 expression [157]. Furthermore, in the triple-negative breast cancer cells, cell lines with a p53 mutation and increased p53 expression are more sensitive to ENMD-2076 than cell lines with decreased p53 expression [157]. Further studies are required to establish specific biomarkers predicting whether patients will respond well to AKIs.
Availability of data and materials
Not applicable.
Abbreviations
- AURKA:
-
Aurora kinase A
- AKIs:
-
AURKA inhibitors
- EMT:
-
Epithelial-mesenchymal transition
- TCGA:
-
The Cancer Genome Atlas
- ACC:
-
Adrenocortical carcinoma
- BLCA:
-
Bladder urothelial carcinoma
- BRCA:
-
Breast invasive carcinoma
- CESC:
-
Cervical squamous cell carcinoma
- CHOL:
-
Cholangiocarcinoma
- COAD:
-
Colon adenocarcinoma
- DLBC:
-
Diffuse large B-cell lymphoma
- ESCA:
-
Esophageal carcinoma
- GBM:
-
Glioblastoma multiforme
- HNSC:
-
Head and neck squamous cell carcinoma
- KICH:
-
Kidney chromophobe carcinoma
- KIRC:
-
Kidney renal clear cell carcinoma
- KIRP:
-
Kidney renal papillary cell carcinoma
- LGG:
-
Brain lower grade glioma
- OV:
-
Ovarian serous cystadenocarcinoma
- MESO:
-
Mesothelioma
- LIHC:
-
Liver hepatocellular carcinoma
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- PAAD:
-
Pancreatic adenocarcinoma
- PRAD:
-
Prostate adenocarcinoma
- PCPG:
-
Pheochromocytoma and paraganglioma
- READ:
-
Rectum adenocarcinoma
- SARC:
-
Sarcoma
- SKCM:
-
Skin cutaneous melanoma
- LAML:
-
Acute myeloid leukemia
- TGCT:
-
Testicular germ cell tumors
- THCA:
-
Thyroid carcinoma
- THYM:
-
Thymoma
- STAD:
-
Stomach adenocarcinoma
- UCEC:
-
Uterine corpus endometrial carcinoma
- UCS:
-
Uterine carcinosarcoma
- UVM:
-
Uveal melanoma
- RT:
-
Rhabdoid tumor
- Az1:
-
Antizyme1
- APC/C:
-
Anaphase-promoting complex/cyclosome
- NSCLC:
-
Non-small-cell lung cancer
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GO:
-
Gene Ontology
- 5-FU:
-
5-fluorouracil
- IC50:
-
Half-maximal inhibitory concentration
- NHL:
-
Non-hodgkin lymphoma
- CML:
-
Chronic myeloid leukemia
- PDX:
-
Patient-derived xenograft
- PFS:
-
Progression-free survival
- PTCL:
-
Peripheral T-cell NHL
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- AML:
-
Acute myeloid leukemia
- EOC:
-
Epithelial ovarian cancer
- MTD:
-
Maximum tolerated dose
References
Yang N, Wang C, Wang Z, Zona S, Lin SX, Wang X, et al. FOXM1 recruits nuclear Aurora kinase A to participate in a positive feedback loop essential for the self-renewal of breast cancer stem cells. Oncogene. 2017 Jun 15;36(24):3428–40.
Tang J, Yang L, Li Y, Ning X, Chaulagain A, Wang T, et al. ARID3A promotes the development of colorectal cancer by upregulating AURKA. Carcinogenesis. 2020, Nov 9.
Long Q, An X, Chen M, Wang N, Sui S, Li Y, et al. PUF60/AURKA Axis Contributes to Tumor Progression and Malignant Phenotypes in Bladder Cancer. Front Oncol. 2020;10:568015.
Tanaka M, Ueda A, Kanamori H, Ideguchi H, Yang J, Kitajima S, et al. Cell-cycle-dependent regulation of human aurora A transcription is mediated by periodic repression of E4TF1. J Biol Chem. 2002 Mar 22;277(12):10719–26.
Udayakumar TS, Belakavadi M, Choi KH, Pandey PK, Fondell JD. Regulation of Aurora-A kinase gene expression via GABP recruitment of TRAP220/MED1. J Biol Chem. 2006 May 26;281(21):14691–9.
Hung LY, Tseng JT, Lee YC, Xia W, Wang YN, Wu ML, et al. Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res. 2008 Aug;36(13):4337–51.
Chou CH, Yang NK, Liu TY, Tai SK, Hsu DS, Chen YW, et al. Chromosome instability modulated by BMI1-AURKA signaling drives progression in head and neck cancer. Cancer Res. 2013 Jan 15;73(2):953–66.
Lai CH, Huang YC, Lee JC, Tseng JT, Chang KC, Chen YJ, et al. Translational upregulation of Aurora-A by hnRNP Q1 contributes to cell proliferation and tumorigenesis in colorectal cancer. Cell Death Dis. 2017 Jan 12;8(1):e2555.
Ice RJ, McLaughlin SL, Livengood RH, Culp MV, Eddy ER, Ivanov AV, et al. NEDD9 depletion destabilizes Aurora A kinase and heightens the efficacy of Aurora A inhibitors: implications for treatment of metastatic solid tumors. Cancer Res. 2013 May 15;73(10):3168–80.
Eyers PA, Erikson E, Chen LG, Maller JL. A novel mechanism for activation of the protein kinase Aurora A. Curr Biol. 2003 Apr 15;13(8):691–7.
Bayliss R, Sardon T, Vernos I, Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell. 2003 Oct;12(4):851–62.
Giubettini M, Asteriti IA, Scrofani J, De Luca M, Lindon C, Lavia P, et al. Control of Aurora-A stability through interaction with TPX2. J Cell Sci. 2011 Jan 1;124(Pt 1):113–22.
Huang YH, Wu CC, Chou CK, Huang CY. A translational regulator, PUM2, promotes both protein stability and kinase activity of Aurora-A. PLoS One. 2011 May 11;6(5):e19718.
Johnson EO, Chang KH, Ghosh S, Venkatesh C, Giger K, Low PS, et al. LIMK2 is a crucial regulator and effector of Aurora-A-kinase-mediated malignancy. J Cell Sci. 2012 Mar 1;125(Pt 5):1204–1216.
Wang J, Nikhil K, Viccaro K, Chang L, Jacobsen M, Sandusky G, et al. The Aurora-A-Twist1 axis promotes highly aggressive phenotypes in pancreatic carcinoma. J Cell Sci. 2017 Mar 15;130(6):1078–93.
Wang J, Nikhil K, Viccaro K, Chang L, White J, Shah K. Phosphorylation-dependent regulation of ALDH1A1 by Aurora kinase A: insights on their synergistic relationship in pancreatic cancer. BMC Biol. 2017 Feb 13;15(1):10.
Nikhil K, Raza A, Haymour HS, Flueckiger BV, Chu J, Shah K. Aurora Kinase A-YBX1 Synergy Fuels Aggressive Oncogenic Phenotypes and Chemoresistance in Castration-Resistant Prostate Cancer. Cancers (Basel). 2020 Mar;12(3):12.
Shi Y, Solomon LR, Pereda-Lopez A, Giranda VL, Luo Y, Johnson EF, et al. Ubiquitin-specific cysteine protease 2a (USP2a) regulates the stability of Aurora-A. J Biol Chem. 2011 Nov 11;286(45):38960–8.
Mori D, Yamada M, Mimori-Kiyosue Y, Shirai Y, Suzuki A, Ohno S, et al. An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol. 2009 Sep;11(9):1057–68.
Wang F, Wang L, Fisher LA, Li C, Wang W, Peng A. Phosphatase 1 Nuclear Targeting Subunit (PNUTS) Regulates Aurora Kinases and Mitotic Progression. Mol Cancer Res. 2019 Jan;17(1):10–9.
Huang Y, Li T, Ems-McClung SC, Walczak CE, Prigent C, Zhu X, et al. Aurora A activation in mitosis promoted by BuGZ. J Cell Biol. 2018 Jan 2;217(1):107–16.
Liu L, Guo C, Dammann R, Tommasi S, Pfeifer GP. RASSF1A interacts with and activates the mitotic kinase Aurora-A. Oncogene. 2008 Oct 16;27(47):6175–86.
Satinover DL, Leach CA, Stukenberg PT, Brautigan DL. Activation of Aurora-A kinase by protein phosphatase inhibitor-2, a bifunctional signaling protein. Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8625–30.
Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005 Oct 28;20(2):237–49.
Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003 Sep 5;114(5):585–98.
Zhong Y, Yang J, Xu WW, Wang Y, Zheng CC, Li B, et al. KCTD12 promotes tumorigenesis by facilitating CDC25B/CDK1/Aurora A-dependent G2/M transition. Oncogene. 2017 Nov 2;36(44):6177–89.
Lee S, Cimica V, Ramachandra N, Zagzag D, Kalpana GV. Aurora A is a repressed effector target of the chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res. 2011 May 1;71(9):3225–35.
Wu C, Lyu J, Yang EJ, Liu Y, Zhang B, Shim JS. Targeting AURKA-CDC25C axis to induce synthetic lethality in ARID1A-deficient colorectal cancer cells. Nat Commun. 2018 Aug 10;9(1):3212.
Yu Z, Sun Y, She X, Wang Z, Chen S, Deng Z, et al. SIX3, a tumor suppressor, inhibits astrocytoma tumorigenesis by transcriptional repression of AURKA/B. J Hematol Oncol. 2017 Jun 8;10(1):115.
Nowak I, Boratyn E, Student S, Bernhart SF, Fallmann J, Durbas M, et al. MCPIP1 ribonuclease can bind and cleave AURKA mRNA in MYCN-amplified neuroblastoma cells. RNA Biol. 2020 Aug 20:1–13.
Taguchi S, Honda K, Sugiura K, Yamaguchi A, Furukawa K, Urano T. Degradation of human Aurora-A protein kinase is mediated by hCdh1. FEBS Lett. 2002 May 22;519(1–3):59–65.
Park MT, Oh ET, Song MJ, Lee H, Choi EK, Park HJ. NQO1 prevents radiation-induced aneuploidy by interacting with Aurora-A. Carcinogenesis. 2013 Nov;34(11):2470–85.
Jia L, Lee HS, Wu CF, Kundu J, Park SG, Kim RN, et al. SMAD4 suppresses AURKA-induced metastatic phenotypes via degradation of AURKA in a TGFbeta-independent manner. Mol Cancer Res. 2014 Dec;12(12):1779–95.
Zhang C, Qu L, Lian S, Meng L, Min L, Liu J, et al. PRL-3 Promotes Ubiquitination and Degradation of AURKA and Colorectal Cancer Progression via Dephosphorylation of FZR1. Cancer Res. 2019 Mar 1;79(5):928–40.
Irelan JT, Murphy TJ, DeJesus PD, Teo H, Xu D, Gomez-Ferreria MA, et al. A role for IkappaB kinase 2 in bipolar spindle assembly. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):16940–5.
Kiat LS, Hui KM, Gopalan G. Aurora-A kinase interacting protein (AIP), a novel negative regulator of human Aurora-A kinase. J Biol Chem. 2002 Nov 22;277(47):45558–65.
Lim SK, Gopalan G. Antizyme1 mediates AURKAIP1-dependent degradation of Aurora-A. Oncogene. 2007 Oct 11;26(46):6593–603.
Hasanov E, Chen G, Chowdhury P, Weldon J, Ding Z, Jonasch E, et al. Ubiquitination and regulation of AURKA identifies a hypoxia-independent E3 ligase activity of VHL. Oncogene. 2017 Jun 15;36(24):3450–63.
Meehan M, Parthasarathi L, Moran N, Jefferies CA, Foley N, Lazzari E, et al. Protein tyrosine phosphatase receptor delta acts as a neuroblastoma tumor suppressor by destabilizing the aurora kinase A oncogene. Mol Cancer. 2012 Feb 5;11:6.
Johnson EO, Chang KH, de Pablo Y, Ghosh S, Mehta R, Badve S, et al. PHLDA1 is a crucial negative regulator and effector of Aurora A kinase in breast cancer. J Cell Sci. 2011 Aug 15;124(Pt 16):2711–2722.
Tong Y, Ben-Shlomo A, Zhou C, Wawrowsky K, Melmed S. Pituitary tumor transforming gene 1 regulates Aurora kinase A activity. Oncogene. 2008 Oct 23;27(49):6385–95.
Shao S, Wang Y, Jin S, Song Y, Wang X, Fan W, et al. Gadd45a interacts with aurora-A and inhibits its kinase activity. J Biol Chem. 2006 Sep 29;281(39):28943–50.
Katayama H, Zhou H, Li Q, Tatsuka M, Sen S. Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle. J Biol Chem. 2001 Dec 7;276(49):46219–24.
Sarkissian M, Mendez R, Richter JD. Progesterone and insulin stimulation of CPEB-dependent polyadenylation is regulated by Aurora A and glycogen synthase kinase-3. Genes Dev. 2004 Jan 1;18(1):48–61.
Cheng A, Zhang P, Wang B, Yang D, Duan X, Jiang Y, et al. Aurora-A mediated phosphorylation of LDHB promotes glycolysis and tumor progression by relieving the substrate-inhibition effect. Nat Commun. 2019 Dec 5;10(1):5566.
Mandati V, Del Maestro L, Dingli F, Lombard B, Loew D, Molinie N, et al. Phosphorylation of Merlin by Aurora A kinase appears necessary for mitotic progression. J Biol Chem. 2019 Aug 30;294(35):12992–3005.
Wang-Bishop L, Chen Z, Gomaa A, Lockhart AC, Salaria S, Wang J, et al. Inhibition of AURKA Reduces Proliferation and Survival of Gastrointestinal Cancer Cells With Activated KRAS by Preventing Activation of RPS6KB1. Gastroenterology. 2019;156(3):662–675 e7.
Kunitoku N, Sasayama T, Marumoto T, Zhang D, Honda S, Kobayashi O, et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev Cell. 2003 Dec;5(6):853–64.
Zheng X, Chi J, Zhi J, Zhang H, Yue D, Zhao J, et al. Aurora-A-mediated phosphorylation of LKB1 compromises LKB1/AMPK signaling axis to facilitate NSCLC growth and migration. Oncogene. 2018 Jan 25;37(4):502–11.
Sarkar S, Brautigan DL, Larner JM. Aurora Kinase A Promotes AR Degradation via the E3 Ligase CHIP. Mol Cancer Res. 2017 Aug;15(8):1063–72.
Chang SS, Yamaguchi H, Xia W, Lim SO, Khotskaya Y, Wu Y, et al. Aurora A kinase activates YAP signaling in triple-negative breast cancer. Oncogene. 2017 Mar 2;36(9):1265–75.
LeRoy PJ, Hunter JJ, Hoar KM, Burke KE, Shinde V, Ruan J, et al. Localization of human TACC3 to mitotic spindles is mediated by phosphorylation on Ser558 by Aurora A: a novel pharmacodynamic method for measuring Aurora A activity. Cancer Res. 2007 Jun 1;67(11):5362–70.
Sehdev V, Katsha A, Arras J, Peng D, Soutto M, Ecsedy J, et al. HDM2 regulation by AURKA promotes cell survival in gastric cancer. Clin Cancer Res. 2014 Jan 1;20(1):76–86.
Jin S, Wang X, Tong T, Zhang D, Shi J, Chen J, et al. Aurora-A enhances malignant development of esophageal squamous cell carcinoma (ESCC) by phosphorylating beta-catenin. Mol Oncol. 2015 Jan;9(1):249–59.
Zheng XQ, Guo JP, Yang H, Kanai M, He LL, Li YY, et al. Aurora-A is a determinant of tamoxifen sensitivity through phosphorylation of ERalpha in breast cancer. Oncogene. 2014 Oct 16;33(42):4985–96.
Moustafa-Kamal M, Gamache I, Lu Y, Li S, Teodoro JG. BimEL is phosphorylated at mitosis by Aurora A and targeted for degradation by betaTrCP1. Cell Death Differ. 2013 Oct;20(10):1393–403.
Dar AA, Belkhiri A, El-Rifai W. The aurora kinase A regulates GSK-3beta in gastric cancer cells. Oncogene. 2009 Feb 12;28(6):866–75.
Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008 Sep 4;455(7209):119–23.
Briassouli P, Chan F, Savage K, Reis-Filho JS, Linardopoulos S. Aurora-A regulation of nuclear factor-kappaB signaling by phosphorylation of IkappaBalpha. Cancer Res. 2007 Feb 15;67(4):1689–95.
Martin B, Chesnel F, Delcros JG, Jouan F, Couturier A, Dugay F, et al. Identification of pVHL as a novel substrate for Aurora-A in clear cell renal cell carcinoma (ccRCC). PLoS One. 2013;8(6):e67071.
Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002 Feb;22(3):874–85.
Alexander KE, Rizkallah R. aurora A Phosphorylation of YY1 during Mitosis Inactivates its DNA Binding Activity. Sci Rep. 2017 Aug 30;7(1):10084.
Du R, Huang C, Chen H, Liu K, Xiang P, Yao N, et al. SDCBP/MDA-9/syntenin phosphorylation by AURKA promotes esophageal squamous cell carcinoma progression through the EGFR-PI3K-Akt signaling pathway. Oncogene. 2020 Jun 22;39(31):5405–19.
Qi D, Wang Q, Yu M, Lan R, Li S, Lu F. Mitotic phosphorylation of SOX2 mediated by Aurora kinase A is critical for the stem-cell like cell maintenance in PA-1 cells. Cell Cycle. 2016 Aug 2;15(15):2009–18.
Chou EJ, Hung LY, Tang CJ, Hsu WB, Wu HY, Liao PC, et al. Phosphorylation of CPAP by Aurora-A Maintains Spindle Pole Integrity during Mitosis. Cell Rep. 2016 Mar 29;14(12):2975–87.
Wu JC, Chen TY, Yu CT, Tsai SJ, Hsu JM, Tang MJ, et al. Identification of V23RalA-Ser194 as a critical mediator for Aurora-A-induced cellular motility and transformation by small pool expression screening. J Biol Chem. 2005 Mar 11;280(10):9013–22.
Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C, et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci. 2004 May 15;117(Pt 12):2523–2531.
Mori D, Yano Y, Toyo-oka K, Yoshida N, Yamada M, Muramatsu M, et al. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol Cell Biol. 2007 Jan;27(1):352–67.
Venoux M, Basbous J, Berthenet C, Prigent C, Fernandez A, Lamb NJ, et al. ASAP is a novel substrate of the oncogenic mitotic kinase Aurora-A: phosphorylation on Ser625 is essential to spindle formation and mitosis. Hum Mol Genet. 2008 Jan 15;17(2):215–24.
Sakai H, Urano T, Ookata K, Kim MH, Hirai Y, Saito M, et al. MBD3 and HDAC1, two components of the NuRD complex, are localized at Aurora-A-positive centrosomes in M phase. J Biol Chem. 2002 Dec 13;277(50):48714–23.
Rong R, Jiang LY, Sheikh MS, Huang Y. Mitotic kinase Aurora-A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene. 2007 Dec 6;26(55):7700–8.
Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004 Jan;36(1):55–62.
Hsueh KW, Fu SL, Chang CB, Chang YL, Lin CH. A novel Aurora-A-mediated phosphorylation of p53 inhibits its interaction with MDM2. Biochim Biophys Acta. 2013 Feb;1834(2):508–15.
Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, et al. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem. 2004 Dec 10;279(50):52175–82.
Yu CT, Hsu JM, Lee YC, Tsou AP, Chou CK, Huang CY. Phosphorylation and stabilization of HURP by Aurora-A: implication of HURP as a transforming target of Aurora-A. Mol Cell Biol. 2005 Jul;25(14):5789–800.
Katayama H, Wang J, Treekitkarnmongkol W, Kawai H, Sasai K, Zhang H, et al. Aurora kinase-A inactivates DNA damage-induced apoptosis and spindle assembly checkpoint response functions of p73. Cancer Cell. 2012 Feb 14;21(2):196–211.
Fu J, Bian M, Xin G, Deng Z, Luo J, Guo X, et al. TPX2 phosphorylation maintains metaphase spindle length by regulating microtubule flux. J Cell Biol. 2015 Aug 3;210(3):373–83.
Toji S, Yabuta N, Hosomi T, Nishihara S, Kobayashi T, Suzuki S, et al. The centrosomal protein Lats2 is a phosphorylation target of Aurora-A kinase. Genes Cells. 2004 May;9(5):383–97.
Lin Y, Richards FM, Krippendorff BF, Bramhall JL, Harrington JA, Bapiro TE, et al. Paclitaxel and CYC3, an aurora kinase A inhibitor, synergise in pancreatic cancer cells but not bone marrow precursor cells. Br J Cancer. 2012 Nov 6;107(10):1692–701.
Zheng FM, Long ZJ, Hou ZJ, Luo Y, Xu LZ, Xia JL, et al. A novel small molecule aurora kinase inhibitor attenuates breast tumor-initiating cells and overcomes drug resistance. Mol Cancer Ther. 2014 Aug;13(8):1991–2003.
Coumar MS, Chu CY, Lin CW, Shiao HY, Ho YL, Reddy R, et al. Fast-forwarding hit to lead: aurora and epidermal growth factor receptor kinase inhibitor lead identification. J Med Chem. 2010 Jul 8;53(13):4980–8.
Shionome Y, Lin WH, Shiao HY, Hsieh HP, Hsu JT, Ouchi T. A novel aurora-A inhibitor, BPR1K0609S1, sensitizes colorectal tumor cells to 5-fluorofracil (5-FU) treatment. Int J Biol Sci. 2013;9(4):403–11.
Ndolo KM, Park KR, Lee HJ, Yoon KB, Kim YC, Han SY. Characterization of the Indirubin Derivative LDD970 as a Small Molecule Aurora Kinase A Inhibitor in Human Colorectal Cancer Cells. Immune Netw. 2017 Apr;17(2):110–5.
Nair JS, Ho AL, Schwartz GK. The induction of polyploidy or apoptosis by the Aurora A kinase inhibitor MK8745 is p53-dependent. Cell Cycle. 2012 Feb 15;11(4):807–17.
Shionome Y, Yan L, Liu S, Saeki T, Ouchi T. Integrity of p53 associated pathways determines induction of apoptosis of tumor cells resistant to Aurora-A kinase inhibitors. PLoS One. 2013;8(1):e55457.
Du J, Yan L, Torres R, Gong X, Bian H, Marugan C, et al. Aurora A-Selective Inhibitor LY3295668 Leads to Dominant Mitotic Arrest, Apoptosis in Cancer Cells, and Shows Potent Preclinical Antitumor Efficacy. Mol Cancer Ther. 2019 Dec;18(12):2207–19.
Cheung CH, Lin WH, Hsu JT, Hour TC, Yeh TK, Ko S, et al. BPR1K653, a novel Aurora kinase inhibitor, exhibits potent anti-proliferative activity in MDR1 (P-gp170)-mediated multidrug-resistant cancer cells. PLoS One. 2011;6(8):e23485.
Liu W, Lu Y, Chai X, Liu X, Zhu T, Wu X, et al. Antitumor activity of TY-011 against gastric cancer by inhibiting Aurora A, Aurora B and VEGFR2 kinases. J Exp Clin Cancer Res. 2016 Nov 25;35(1):183.
Hsu YC, Coumar MS, Wang WC, Shiao HY, Ke YY, Lin WH, et al. Discovery of BPR1K871, a quinazoline based, multi-kinase inhibitor for the treatment of AML and solid tumors: Rational design, synthesis, in vitro and in vivo evaluation. Oncotarget. 2016 Dec 27;7(52):86239–56.
Yu T, Tagat JR, Kerekes AD, Doll RJ, Zhang Y, Xiao Y, et al. Discovery of a Potent, Injectable Inhibitor of Aurora Kinases Based on the Imidazo-[1,2-a]-Pyrazine Core. ACS Med Chem Lett. 2010 Aug 12;1(5):214–8.
Hoang NT, Phuong TT, Nguyen TT, Tran YT, Nguyen AT, Nguyen TL, et al. In Vitro Characterization of Derrone as an Aurora Kinase Inhibitor. Biol Pharm Bull. 2016 Jun 1;39(6):935–45.
Emanuel S, Rugg CA, Gruninger RH, Lin R, Fuentes-Pesquera A, Connolly PJ, et al. The in vitro and in vivo effects of JNJ-7706621: a dual inhibitor of cyclin-dependent kinases and aurora kinases. Cancer Res. 2005 Oct 1;65(19):9038–46.
Carry JC, Clerc F, Minoux H, Schio L, Mauger J, Nair A, et al. SAR156497, an exquisitely selective inhibitor of aurora kinases. J Med Chem. 2015 Jan 8;58(1):362–75.
Zhang C, Wu X, Zhang M, Zhu L, Zhao R, Xu D, et al. Small molecule R1498 as a well-tolerated and orally active kinase inhibitor for hepatocellular carcinoma and gastric cancer treatment via targeting angiogenesis and mitosis pathways. PLoS One. 2013;8(6):e65264.
Lin ZZ, Hsu HC, Hsu CH, Yeh PY, Huang CY, Huang YF, et al. The Aurora kinase inhibitor VE-465 has anticancer effects in pre-clinical studies of human hepatocellular carcinoma. J Hepatol. 2009 Mar;50(3):518–27.
Chan F, Sun C, Perumal M, Nguyen QD, Bavetsias V, McDonald E, et al. Mechanism of action of the Aurora kinase inhibitor CCT129202 and in vivo quantification of biological activity. Mol Cancer Ther. 2007 Dec;6(12 Pt 1):3147–57.
Faisal A, Vaughan L, Bavetsias V, Sun C, Atrash B, Avery S, et al. The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo. Mol Cancer Ther. 2011 Nov;10(11):2115–23.
Bavetsias V, Large JM, Sun C, Bouloc N, Kosmopoulou M, Matteucci M, et al. Imidazo[4,5-b] pyridine derivatives as inhibitors of Aurora kinases: lead optimization studies toward the identification of an orally bioavailable preclinical development candidate. J Med Chem. 2010 Jul 22;53(14):5213–28.
Soncini C, Carpinelli P, Gianellini L, Fancelli D, Vianello P, Rusconi L, et al. PHA-680632, a novel Aurora kinase inhibitor with potent antitumoral activity. Clin Cancer Res. 2006 Jul 1;12(13):4080–9.
Rawson TE, Ruth M, Blackwood E, Burdick D, Corson L, Dotson J, et al. A pentacyclic aurora kinase inhibitor (AKI-001) with high in vivo potency and oral bioavailability. J Med Chem. 2008 Aug 14;51(15):4465–75.
D'Alise AM, Amabile G, Iovino M, Di Giorgio FP, Bartiromo M, Sessa F, et al. Reversine, a novel Aurora kinases inhibitor, inhibits colony formation of human acute myeloid leukemia cells. Mol Cancer Ther. 2008 May;7(5):1140–9.
Kantarjian HM, Schuster MW, Jain N, Advani A, Jabbour E, Gamelin E, et al. A phase 1 study of AMG 900, an orally administered pan-aurora kinase inhibitor, in adult patients with acute myeloid leukemia. Am J Hematol. 2017 Jul;92(7):660–7.
Carducci M, Shaheen M, Markman B, Hurvitz S, Mahadevan D, Kotasek D, et al. A phase 1, first-in-human study of AMG 900, an orally administered pan-Aurora kinase inhibitor, in adult patients with advanced solid tumors. Invest New Drugs. 2018 Dec;36(6):1060–71.
Mita M, Gordon M, Rejeb N, Gianella-Borradori A, Jego V, Mita A, et al. A phase l study of three different dosing schedules of the oral aurora kinase inhibitor MSC1992371A in patients with solid tumors. Target Oncol. 2014 Sep;9(3):215–24.
Graux C, Sonet A, Maertens J, Duyster J, Greiner J, Chalandon Y, et al. A phase I dose-escalation study of MSC1992371A, an oral inhibitor of aurora and other kinases, in advanced hematologic malignancies. Leuk Res. 2013 Sep;37(9):1100–6.
Raymond E, Alexandre J, Faivre S, Goldwasser F, Besse-Hammer T, Gianella-Borradori A, et al. A phase I schedule dependency study of the aurora kinase inhibitor MSC1992371A in combination with gemcitabine in patients with solid tumors. Invest New Drugs. 2014 Feb;32(1):94–103.
Dent SF, Gelmon KA, Chi KN, Jonker DJ, Wainman N, Capier CA, et al. NCIC CTG IND.181: phase I study of AT9283 given as a weekly 24 hour infusion in advanced malignancies. Invest New Drugs. 2013 Dec;31(6):1522–9.
Foran J, Ravandi F, Wierda W, Garcia-Manero G, Verstovsek S, Kadia T, et al. A phase I and pharmacodynamic study of AT9283, a small-molecule inhibitor of aurora kinases in patients with relapsed/refractory leukemia or myelofibrosis. Clin Lymphoma Myeloma Leuk. 2014 Jun;14(3):223–30.
Moreno L, Marshall LV, Pearson AD, Morland B, Elliott M, Campbell-Hewson Q, et al. A phase I trial of AT9283 (a selective inhibitor of aurora kinases) in children and adolescents with solid tumors: a Cancer Research UK study. Clin Cancer Res. 2015 Jan 15;21(2):267–73.
Vormoor B, Veal GJ, Griffin MJ, Boddy AV, Irving J, Minto L, et al. A phase I/II trial of AT9283, a selective inhibitor of aurora kinase in children with relapsed or refractory acute leukemia: challenges to run early phase clinical trials for children with leukemia. Pediatr Blood Cancer. 2017 Jun;64(6).
Hay AE, Murugesan A, DiPasquale AM, Kouroukis T, Sandhu I, Kukreti V, et al. A phase II study of AT9283, an aurora kinase inhibitor, in patients with relapsed or refractory multiple myeloma: NCIC clinical trials group IND.191. Leuk Lymphoma. 2016;57(6):1463–6.
Schoffski P, Aftimos P, Dumez H, Deleporte A, De Block K, Costermans J, et al. A phase I study of two dosing schedules of oral BI 847325 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016 Jan;77(1):99–108.
Diamond JR, Bastos BR, Hansen RJ, Gustafson DL, Eckhardt SG, Kwak EL, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of ENMD-2076, a novel angiogenic and Aurora kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2011 Feb 15;17(4):849–60.
Yee KW, Chen HW, Hedley DW, Chow S, Brandwein J, Schuh AC, et al. A phase I trial of the aurora kinase inhibitor, ENMD-2076, in patients with relapsed or refractory acute myeloid leukemia or chronic myelomonocytic leukemia. Invest New Drugs. 2016 Oct;34(5):614–24.
Matulonis UA, Lee J, Lasonde B, Tew WP, Yehwalashet A, Matei D, et al. ENMD-2076, an oral inhibitor of angiogenic and proliferation kinases, has activity in recurrent, platinum resistant ovarian cancer. Eur J Cancer. 2013 Jan;49(1):121–31.
Diamond JR, Eckhardt SG, Pitts TM, van Bokhoven A, Aisner D, Gustafson DL, et al. A phase II clinical trial of the Aurora and angiogenic kinase inhibitor ENMD-2076 for previously treated, advanced, or metastatic triple-negative breast cancer. Breast Cancer Res. 2018 Aug 2;20(1):82.
Lheureux S, Tinker A, Clarke B, Ghatage P, Welch S, Weberpals JI, et al. A Clinical and Molecular Phase II Trial of Oral ENMD-2076 in Ovarian Clear Cell Carcinoma (OCCC): A Study of the Princess Margaret Phase II Consortium. Clin Cancer Res. 2018 Dec 15;24(24):6168–74.
Veitch Z, Zer A, Loong H, Salah S, Masood M, Gupta A, et al. A phase II study of ENMD-2076 in advanced soft tissue sarcoma (STS). Sci Rep. 2019 May 14;9(1):7390.
Abou-Alfa GK, Mayer R, Venook AP, O'Neill AF, Beg MS, LaQuaglia M, et al. Phase II Multicenter, Open-Label Study of Oral ENMD-2076 for the Treatment of Patients with Advanced Fibrolamellar Carcinoma. Oncologist. 2020, Mar 10.
Traynor AM, Hewitt M, Liu G, Flaherty KT, Clark J, Freedman SJ, et al. Phase I dose escalation study of MK-0457, a novel Aurora kinase inhibitor, in adult patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011 Feb;67(2):305–14.
Giles FJ, Swords RT, Nagler A, Hochhaus A, Ottmann OG, Rizzieri DA, et al. MK-0457, an Aurora kinase and BCR-ABL inhibitor, is active in patients with BCR-ABL T315I leukemia. Leukemia. 2013 Jan;27(1):113–7.
Seymour JF, Kim DW, Rubin E, Haregewoin A, Clark J, Watson P, et al. A phase 2 study of MK-0457 in patients with BCR-ABL T315I mutant chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Cancer J. 2014 Aug 15;4:e238.
Amin M, Minton SE, LoRusso PM, Krishnamurthi SS, Pickett CA, Lunceford J, et al. A phase I study of MK-5108, an oral aurora a kinase inhibitor, administered both as monotherapy and in combination with docetaxel, in patients with advanced or refractory solid tumors. Invest New Drugs. 2016 Feb;34(1):84–95.
Dees EC, Infante JR, Cohen RB, O'Neil BH, Jones S, von Mehren M, et al. Phase 1 study of MLN8054, a selective inhibitor of Aurora A kinase in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011 Apr;67(4):945–54.
Macarulla T, Cervantes A, Elez E, Rodriguez-Braun E, Baselga J, Rosello S, et al. Phase I study of the selective Aurora A kinase inhibitor MLN8054 in patients with advanced solid tumors: safety, pharmacokinetics, and pharmacodynamics. Mol Cancer Ther. 2010 Oct;9(10):2844–52.
Schoffski P, Jones SF, Dumez H, Infante JR, Van Mieghem E, Fowst C, et al. Phase I, open-label, multicentre, dose-escalation, pharmacokinetic and pharmacodynamic trial of the oral aurora kinase inhibitor PF-03814735 in advanced solid tumours. Eur J Cancer. 2011 Oct;47(15):2256–64.
Steeghs N, Eskens FA, Gelderblom H, Verweij J, Nortier JW, Ouwerkerk J, et al. Phase I pharmacokinetic and pharmacodynamic study of the aurora kinase inhibitor danusertib in patients with advanced or metastatic solid tumors. J Clin Oncol. 2009 Oct 20;27(30):5094–101.
Cohen RB, Jones SF, Aggarwal C, von Mehren M, Cheng J, Spigel DR, et al. A phase I dose-escalation study of danusertib (PHA-739358) administered as a 24-hour infusion with and without granulocyte colony-stimulating factor in a 14-day cycle in patients with advanced solid tumors. Clin Cancer Res. 2009 Nov 1;15(21):6694–701.
Borthakur G, Dombret H, Schafhausen P, Brummendorf TH, Boissel N, Jabbour E, et al. A phase I study of danusertib (PHA-739358) in adult patients with accelerated or blastic phase chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant or intolerant to imatinib and/or other second generation c-ABL therapy. Haematologica. 2015 Jul;100(7):898–904.
Meulenbeld HJ, Bleuse JP, Vinci EM, Raymond E, Vitali G, Santoro A, et al. Randomized phase II study of danusertib in patients with metastatic castration-resistant prostate cancer after docetaxel failure. BJU Int. 2013 Jan;111(1):44–52.
Schoffski P, Besse B, Gauler T, de Jonge MJ, Scambia G, Santoro A, et al. Efficacy and safety of biweekly i.v. administrations of the Aurora kinase inhibitor danusertib hydrochloride in independent cohorts of patients with advanced or metastatic breast, ovarian, colorectal, pancreatic, small-cell and non-small-cell lung cancer: a multi-tumour, multi-institutional phase II study. Ann Oncol. 2015 Mar;26(3):598–607.
Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010 Jun 24;115(25):5202–13.
Liu Y, Hawkins OE, Su Y, Vilgelm AE, Sobolik T, Thu YM, et al. Targeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-kappaB impairs this drug-induced senescence. EMBO Mol Med. 2013 Jan;5(1):149–66.
Ding YH, Zhou ZW, Ha CF, Zhang XY, Pan ST, He ZX, et al. Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des Devel Ther. 2015;9:425–64.
Zhu Q, Yu X, Zhou ZW, Zhou C, Chen XW, Zhou SF. Inhibition of Aurora A Kinase by Alisertib Induces Autophagy and Cell Cycle Arrest and Increases Chemosensitivity in Human Hepatocellular Carcinoma HepG2 Cells. Curr Cancer Drug Targets. 2017;17(4):386–401.
Sehdev V, Peng D, Soutto M, Washington MK, Revetta F, Ecsedy J, et al. The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Mol Cancer Ther. 2012 Mar;11(3):763–74.
Brockmann M, Poon E, Berry T, Carstensen A, Deubzer HE, Rycak L, et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell. 2013 Jul 8;24(1):75–89.
Li Y, Li X, Pu J, Yang Q, Guan H, Ji M, et al. c-Myc Is a Major Determinant for Antitumor Activity of Aurora A Kinase Inhibitor MLN8237 in Thyroid Cancer. Thyroid. 2018 Dec;28(12):1642–54.
Kurokawa C, Geekiyanage H, Allen C, Iankov I, Schroeder M, Carlson B, et al. Alisertib demonstrates significant antitumor activity in bevacizumab resistant, patient derived orthotopic models of glioblastoma. J Neurooncol. 2017 Jan;131(1):41–8.
Zhou N, Singh K, Mir MC, Parker Y, Lindner D, Dreicer R, et al. The investigational Aurora kinase A inhibitor MLN8237 induces defects in cell viability and cell-cycle progression in malignant bladder cancer cells in vitro and in vivo. Clin Cancer Res. 2013 Apr 1;19(7):1717–28.
Carol H, Boehm I, Reynolds CP, Kang MH, Maris JM, Morton CL, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011 Nov;68(5):1291–304.
Pitts TM, Bradshaw-Pierce EL, Bagby SM, Hyatt SL, Selby HM, Spreafico A, et al. Antitumor activity of the aurora a selective kinase inhibitor, alisertib, against preclinical models of colorectal cancer. Oncotarget. 2016 Aug 2;7(31):50290–301.
Cervantes A, Elez E, Roda D, Ecsedy J, Macarulla T, Venkatakrishnan K, et al. Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora a kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012 Sep 1;18(17):4764–74.
Dees EC, Cohen RB, von Mehren M, Stinchcombe TE, Liu H, Venkatakrishnan K, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012 Sep 1;18(17):4775–84.
Kelly KR, Shea TC, Goy A, Berdeja JG, Reeder CB, McDonagh KT, et al. Phase I study of MLN8237--investigational Aurora A kinase inhibitor--in relapsed/refractory multiple myeloma, non-Hodgkin lymphoma and chronic lymphocytic leukemia. Invest New Drugs. 2014 Jun;32(3):489–99.
Friedberg JW, Mahadevan D, Cebula E, Persky D, Lossos I, Agarwal AB, et al. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol. 2014 Jan 1;32(1):44–50.
Mosse YP, Lipsitz E, Fox E, Teachey DT, Maris JM, Weigel B, et al. Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a Children's Oncology Group Phase I Consortium study. Clin Cancer Res. 2012 Nov 1;18(21):6058–64.
Matulonis UA, Sharma S, Ghamande S, Gordon MS, Del Prete SA, Ray-Coquard I, et al. Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol. 2012 Oct;127(1):63–9.
Melichar B, Adenis A, Lockhart AC, Bennouna J, Dees EC, Kayaleh O, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol. 2015 Apr;16(4):395–405.
Dickson MA, Mahoney MR, Tap WD, D'Angelo SP, Keohan ML, Van Tine BA, et al. Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. Ann Oncol. 2016 Oct;27(10):1855–60.
Beltran H, Oromendia C, Danila DC, Montgomery B, Hoimes C, Szmulewitz RZ, et al. A Phase II Trial of the Aurora Kinase A Inhibitor Alisertib for Patients with Castration-resistant and Neuroendocrine Prostate Cancer: Efficacy and Biomarkers. Clin Cancer Res. 2019 Jan 1;25(1):43–51.
Barr PM, Li H, Spier C, Mahadevan D, LeBlanc M, Ul Haq M, et al. Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108. J Clin Oncol. 2015 Jul 20;33(21):2399–404.
O'Connor OA, Ozcan M, Jacobsen ED, Roncero JM, Trotman J, Demeter J, et al. Randomized Phase III Study of Alisertib or Investigator's Choice (Selected Single Agent) in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma. J Clin Oncol. 2019 Mar 10;37(8):613–23.
Tentler JJ, Bradshaw-Pierce EL, Serkova NJ, Hasebroock KM, Pitts TM, Diamond JR, et al. Assessment of the in vivo antitumor effects of ENMD-2076, a novel multitargeted kinase inhibitor, against primary and cell line-derived human colorectal cancer xenograft models. Clin Cancer Res. 2010 Jun 1;16(11):2989–98.
Fletcher GC, Brokx RD, Denny TA, Hembrough TA, Plum SM, Fogler WE, et al. ENMD-2076 is an orally active kinase inhibitor with antiangiogenic and antiproliferative mechanisms of action. Mol Cancer Ther. 2011 Jan;10(1):126–37.
Wang X, Sinn AL, Pollok K, Sandusky G, Zhang S, Chen L, et al. Preclinical activity of a novel multiple tyrosine kinase and aurora kinase inhibitor, ENMD-2076, against multiple myeloma. Br J Haematol. 2010 Aug;150(3):313–25.
Diamond JR, Eckhardt SG, Tan AC, Newton TP, Selby HM, Brunkow KL, et al. Predictive biomarkers of sensitivity to the aurora and angiogenic kinase inhibitor ENMD-2076 in preclinical breast cancer models. Clin Cancer Res. 2013 Jan 1;19(1):291–303.
Ionkina AA, Tentler JJ, Kim J, Capasso A, Pitts TM, Ryall KA, et al. Efficacy and Molecular Mechanisms of Differentiated Response to the Aurora and Angiogenic Kinase Inhibitor ENMD-2076 in Preclinical Models of p53-Mutated Triple-Negative Breast Cancer. Front Oncol. 2017;7:94.
Shimomura T, Hasako S, Nakatsuru Y, Mita T, Ichikawa K, Kodera T, et al. MK-5108, a highly selective Aurora-A kinase inhibitor, shows antitumor activity alone and in combination with docetaxel. Mol Cancer Ther. 2010 Jan;9(1):157–66.
Chefetz I, Holmberg JC, Alvero AB, Visintin I, Mor G. Inhibition of Aurora-A kinase induces cell cycle arrest in epithelial ovarian cancer stem cells by affecting NFkB pathway. Cell Cycle. 2011 Jul 1;10(13):2206–14.
Shan W, Akinfenwa PY, Savannah KB, Kolomeyevskaya N, Laucirica R, Thomas DG, et al. A small-molecule inhibitor targeting the mitotic spindle checkpoint impairs the growth of uterine leiomyosarcoma. Clin Cancer Res. 2012 Jun 15;18(12):3352–65.
Howard S, Berdini V, Boulstridge JA, Carr MG, Cross DM, Curry J, et al. Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity. J Med Chem. 2009 Jan 22;52(2):379–88.
Tanaka R, Squires MS, Kimura S, Yokota A, Nagao R, Yamauchi T, et al. Activity of the multitargeted kinase inhibitor, AT9283, in imatinib-resistant BCR-ABL-positive leukemic cells. Blood. 2010 Sep 23;116(12):2089–95.
Qi W, Liu X, Cooke LS, Persky DO, Miller TP, Squires M, et al. AT9283, a novel aurora kinase inhibitor, suppresses tumor growth in aggressive B-cell lymphomas. Int J Cancer. 2012 Jun 15;130(12):2997–3005.
Petersen W, Liu J, Yuan L, Zhang H, Schneiderjan M, Cho YJ, et al. Dasatinib suppression of medulloblastoma survival and migration is markedly enhanced by combining treatment with the aurora kinase inhibitor AT9283. Cancer Lett. 2014 Nov 1;354(1):68–76.
Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004 Mar;10(3):262–7.
Cheetham GM, Charlton PA, Golec JM, Pollard JR. Structural basis for potent inhibition of the Aurora kinases and a T315I multi-drug resistant mutant form of Abl kinase by VX-680. Cancer Lett. 2007 Jun 28;251(2):323–9.
Huang XF, Luo SK, Xu J, Li J, Xu DR, Wang LH, et al. Aurora kinase inhibitory VX-680 increases Bax/Bcl-2 ratio and induces apoptosis in Aurora-A-high acute myeloid leukemia. Blood. 2008 Mar 1;111(5):2854–65.
Arlot-Bonnemains Y, Baldini E, Martin B, Delcros JG, Toller M, Curcio F, et al. Effects of the Aurora kinase inhibitor VX-680 on anaplastic thyroid cancer-derived cell lines. Endocr Relat Cancer. 2008 Jun;15(2):559–68.
Lin YG, Immaneni A, Merritt WM, Mangala LS, Kim SW, Shahzad MM, et al. Targeting aurora kinase with MK-0457 inhibits ovarian cancer growth. Clin Cancer Res. 2008 Sep 1;14(17):5437–46.
Hose D, Reme T, Meissner T, Moreaux J, Seckinger A, Lewis J, et al. Inhibition of aurora kinases for tailored risk-adapted treatment of multiple myeloma. Blood. 2009 Apr 30;113(18):4331–40.
Donato NJ, Fang D, Sun H, Giannola D, Peterson LF, Talpaz M. Targets and effectors of the cellular response to aurora kinase inhibitor MK-0457 (VX-680) in imatinib sensitive and resistant chronic myelogenous leukemia. Biochem Pharmacol. 2010 Mar 1;79(5):688–97.
Carpinelli P, Ceruti R, Giorgini ML, Cappella P, Gianellini L, Croci V, et al. PHA-739358, a potent inhibitor of Aurora kinases with a selective target inhibition profile relevant to cancer. Mol Cancer Ther. 2007 Dec;6(12 Pt 1):3158–68.
Gontarewicz A, Balabanov S, Keller G, Colombo R, Graziano A, Pesenti E, et al. Simultaneous targeting of Aurora kinases and Bcr-Abl kinase by the small molecule inhibitor PHA-739358 is effective against imatinib-resistant BCR-ABL mutations including T315I. Blood. 2008 Apr 15;111(8):4355–64.
Modugno M, Casale E, Soncini C, Rosettani P, Colombo R, Lupi R, et al. Crystal structure of the T315I Abl mutant in complex with the aurora kinases inhibitor PHA-739358. Cancer Res. 2007 Sep 1;67(17):7987–90.
Li JP, Yang YX, Liu QL, Zhou ZW, Pan ST, He ZX, et al. The pan-inhibitor of Aurora kinases danusertib induces apoptosis and autophagy and suppresses epithelial-to-mesenchymal transition in human breast cancer cells. Drug Des Devel Ther. 2015;9:1027–62.
He SJ, Shu LP, Zhou ZW, Yang T, Duan W, Zhang X, et al. Inhibition of Aurora kinases induces apoptosis and autophagy via AURKB/p70S6K/RPL15 axis in human leukemia cells. Cancer Lett. 2016 Nov 28;382(2):215–30.
Fraedrich K, Schrader J, Ittrich H, Keller G, Gontarewicz A, Matzat V, et al. Targeting aurora kinases with danusertib (PHA-739358) inhibits growth of liver metastases from gastroenteropancreatic neuroendocrine tumors in an orthotopic xenograft model. Clin Cancer Res. 2012 Sep 1;18(17):4621–32.
Wang H, Tian L, Goldstein A, Liu J, Lo HC, Sheng K, et al. Bone-in-culture array as a platform to model early-stage bone metastases and discover anti-metastasis therapies. Nat Commun. 2017 Apr 21;8:15045.
Qi W, Cooke LS, Liu X, Rimsza L, Roe DJ, Manziolli A, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011 Apr 1;81(7):881–90.
Sehdev V, Katsha A, Ecsedy J, Zaika A, Belkhiri A, El-Rifai W. The combination of alisertib, an investigational Aurora kinase A inhibitor, and docetaxel promotes cell death and reduces tumor growth in preclinical cell models of upper gastrointestinal adenocarcinomas. Cancer. 2013 Feb 15;119(4):904–14.
Chinn DC, Holland WS, Mack PC. Anticancer activity of the Aurora A kinase inhibitor MK-5108 in non-small-cell lung cancer (NSCLC) in vitro as monotherapy and in combination with chemotherapies. J Cancer Res Clin Oncol. 2014 Jul;140(7):1137–49.
Graff JN, Higano CS, Hahn NM, Taylor MH, Zhang B, Zhou X, et al. Open-label, multicenter, phase 1 study of alisertib (MLN8237), an aurora A kinase inhibitor, with docetaxel in patients with solid tumors. Cancer. 2016 Aug 15;122(16):2524–33.
Do TV, Xiao F, Bickel LE, Klein-Szanto AJ, Pathak HB, Hua X, et al. Aurora kinase A mediates epithelial ovarian cancer cell migration and adhesion. Oncogene. 2014 Jan 30;33(5):539–49.
Payton M, Bush TL, Chung G, Ziegler B, Eden P, McElroy P, et al. Preclinical evaluation of AMG 900, a novel potent and highly selective pan-aurora kinase inhibitor with activity in taxane-resistant tumor cell lines. Cancer Res. 2010 Dec 1;70(23):9846–54.
Falchook G, Coleman RL, Roszak A, Behbakht K, Matulonis U, Ray-Coquard I, et al. Alisertib in Combination With Weekly Paclitaxel in Patients With Advanced Breast Cancer or Recurrent Ovarian Cancer: A Randomized Clinical Trial. JAMA Oncol. 2019 Jan 1;5(1):e183773.
Kelly KR, Nawrocki ST, Espitia CM, Zhang M, Yang JJ, Padmanabhan S, et al. Targeting Aurora A kinase activity with the investigational agent alisertib increases the efficacy of cytarabine through a FOXO-dependent mechanism. Int J Cancer. 2012 Dec 1;131(11):2693–703.
Brunner AM, Blonquist TM, DeAngelo DJ, McMasters M, Fell G, Hermance NM, et al. Alisertib plus induction chemotherapy in previously untreated patients with high-risk, acute myeloid leukaemia: a single-arm, phase 2 trial. Lancet Haematol. 2020 Feb;7(2):e122–e33.
Fathi AT, Wander SA, Blonquist TM, Brunner AM, Amrein PC, Supko J, et al. Phase I study of the aurora A kinase inhibitor alisertib with induction chemotherapy in patients with acute myeloid leukemia. Haematologica. 2017 Apr;102(4):719–27.
Mahadevan D, Stejskal A, Cooke LS, Manziello A, Morales C, Persky DO, et al. Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012 Apr 15;18(8):2210–9.
Kelly KR, Friedberg JW, Park SI, McDonagh K, Hayslip J, Persky D, et al. Phase I Study of the Investigational Aurora A Kinase Inhibitor Alisertib plus Rituximab or Rituximab/Vincristine in Relapsed/Refractory Aggressive B-cell Lymphoma. Clin Cancer Res. 2018 Dec 15;24(24):6150–9.
Park SI, Lin CP, Ren N, Angus SP, Dittmer DP, Foote M, et al. Inhibition of Aurora A Kinase in Combination with Chemotherapy Induces Synthetic Lethality and Overcomes Chemoresistance in Myc-Overexpressing Lymphoma. Target Oncol. 2019 Oct;14(5):563–75.
Sak M, Zumbar CT, King PD, Li X, Mifsud CS, Usubalieva A, et al. Cytotoxic synergy between alisertib and carboplatin versus alisertib and irinotecan are inversely dependent on MGMT levels in glioblastoma cells. J Neurooncol. 2019 Jun;143(2):231–40.
Kozyreva VK, Kiseleva AA, Ice RJ, Jones BC, Loskutov YV, Matalkah F, et al. Combination of Eribulin and Aurora A Inhibitor MLN8237 Prevents Metastatic Colonization and Induces Cytotoxic Autophagy in Breast Cancer. Mol Cancer Ther. 2016 Aug;15(8):1809–22.
Santo L, Hideshima T, Cirstea D, Bandi M, Nelson EA, Gorgun G, et al. Antimyeloma activity of a multitargeted kinase inhibitor, AT9283, via potent Aurora kinase and STAT3 inhibition either alone or in combination with lenalidomide. Clin Cancer Res. 2011 May 15;17(10):3259–71.
DuBois SG, Marachelian A, Fox E, Kudgus RA, Reid JM, Groshen S, et al. Phase I Study of the Aurora A Kinase Inhibitor Alisertib in Combination With Irinotecan and Temozolomide for Patients With Relapsed or Refractory Neuroblastoma: A NANT (New Approaches to Neuroblastoma Therapy) Trial. J Clin Oncol. 2016 Apr 20;34(12):1368–75.
DuBois SG, Mosse YP, Fox E, Kudgus RA, Reid JM, McGovern R, et al. Phase II Trial of Alisertib in Combination with Irinotecan and Temozolomide for Patients with Relapsed or Refractory Neuroblastoma. Clin Cancer Res. 2018 Dec 15;24(24):6142–9.
Tao Y, Zhang P, Frascogna V, Lecluse Y, Auperin A, Bourhis J, et al. Enhancement of radiation response by inhibition of Aurora-A kinase using siRNA or a selective Aurora kinase inhibitor PHA680632 in p53-deficient cancer cells. Br J Cancer. 2007 Dec 17;97(12):1664–72.
Moretti L, Niermann K, Schleicher S, Giacalone NJ, Varki V, Kim KW, et al. MLN8054, a small molecule inhibitor of aurora kinase a, sensitizes androgen-resistant prostate cancer to radiation. Int J Radiat Oncol Biol Phys. 2011 Jul 15;80(4):1189–97.
Venkataraman S, Alimova I, Tello T, Harris PS, Knipstein JA, Donson AM, et al. Targeting Aurora Kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. J Neurooncol. 2012 May;107(3):517–26.
Shiomitsu K, Sajo E, Rubin C, Sehgal I. The radiosensitizing effect of the aurora kinase inhibitors, ENMD-2076, on canine mast cell tumours in vitro. Vet Comp Oncol. 2016 Mar;14(1):13–27.
Song A, Andrews DW, Werner-Wasik M, Kim L, Glass J, Bar-Ad V, et al. Phase I trial of alisertib with concurrent fractionated stereotactic re-irradiation for recurrent high grade gliomas. Radiother Oncol. 2019 Mar;132:135–41.
Cha TL, Chuang MJ, Wu ST, Sun GH, Chang SY, Yu DS, et al. Dual degradation of aurora A and B kinases by the histone deacetylase inhibitor LBH589 induces G2-M arrest and apoptosis of renal cancer cells. Clin Cancer Res. 2009 Feb 1;15(3):840–50.
Park JH, Jong HS, Kim SG, Jung Y, Lee KW, Lee JH, et al. Inhibitors of histone deacetylases induce tumor-selective cytotoxicity through modulating Aurora-A kinase. J Mol Med (Berl). 2008 Jan;86(1):117–28.
Fiskus W, Wang Y, Joshi R, Rao R, Yang Y, Chen J, et al. Cotreatment with vorinostat enhances activity of MK-0457 (VX-680) against acute and chronic myelogenous leukemia cells. Clin Cancer Res. 2008 Oct 1;14(19):6106–15.
Dai Y, Chen S, Venditti CA, Pei XY, Nguyen TK, Dent P, et al. Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate. Blood. 2008 Aug 1;112(3):793–804.
Fiskus W, Hembruff SL, Rao R, Sharma P, Balusu R, Venkannagari S, et al. Co-treatment with vorinostat synergistically enhances activity of Aurora kinase inhibitor against human breast cancer cells. Breast Cancer Res Treat. 2012 Sep;135(2):433–44.
Kretzner L, Scuto A, Dino PM, Kowolik CM, Wu J, Ventura P, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Res. 2011 Jun 1;71(11):3912–20.
Zullo KM, Guo Y, Cooke L, Jirau-Serrano X, Mangone M, Scotto L, et al. Aurora A Kinase Inhibition Selectively Synergizes with Histone Deacetylase Inhibitor through Cytokinesis Failure in T-cell Lymphoma. Clin Cancer Res. 2015 Sep 15;21(18):4097–109.
Paller CJ, Wissing MD, Mendonca J, Sharma A, Kim E, Kim HS, et al. Combining the pan-aurora kinase inhibitor AMG 900 with histone deacetylase inhibitors enhances antitumor activity in prostate cancer. Cancer Med. 2014 Oct;3(5):1322–35.
Siddiqi T, Frankel P, Beumer JH, Kiesel BF, Christner S, Ruel C, et al. Phase 1 study of the Aurora kinase A inhibitor alisertib (MLN8237) combined with the histone deacetylase inhibitor vorinostat in lymphoid malignancies. Leuk Lymphoma. 2020 Feb;61(2):309–17.
Shah KN, Bhatt R, Rotow J, Rohrberg J, Olivas V, Wang VE, et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat Med. 2019 Jan;25(1):111–8.
Cancer M, Drews LF, Bengtsson J, Bolin S, Rosen G, Westermark B, et al. BET and Aurora Kinase A inhibitors synergize against MYCN-positive human glioblastoma cells. Cell Death Dis. 2019 Nov 21;10(12):881.
Felgenhauer J, Tomino L, Selich-Anderson J, Bopp E, Shah N. Dual BRD4 and AURKA Inhibition Is Synergistic against MYCN-Amplified and Nonamplified Neuroblastoma. Neoplasia. 2018 Oct;20(10):965–74.
Vilgelm AE, Pawlikowski JS, Liu Y, Hawkins OE, Davis TA, Smith J, et al. Mdm2 and aurora kinase a inhibitors synergize to block melanoma growth by driving apoptosis and immune clearance of tumor cells. Cancer Res. 2015 Jan 1;75(1):181–93.
Kojima K, Konopleva M, Tsao T, Nakakuma H, Andreeff M. Concomitant inhibition of Mdm2-p53 interaction and Aurora kinases activates the p53-dependent postmitotic checkpoints and synergistically induces p53-mediated mitochondrial apoptosis along with reduced endoreduplication in acute myelogenous leukemia. Blood. 2008 Oct 1;112(7):2886–95.
Ratushny V, Pathak HB, Beeharry N, Tikhmyanova N, Xiao F, Li T, et al. Dual inhibition of SRC and Aurora kinases induces postmitotic attachment defects and cell death. Oncogene. 2012 Mar 8;31(10):1217–27.
Alcaraz-Sanabria A, Nieto-Jimenez C, Corrales-Sanchez V, Serrano-Oviedo L, Andres-Pretel F, Montero JC, et al. Synthetic Lethality Interaction Between Aurora Kinases and CHEK1 Inhibitors in Ovarian Cancer. Mol Cancer Ther. 2017 Nov;16(11):2552–62.
Brewer Savannah KJ, Demicco EG, Lusby K, Ghadimi MP, Belousov R, Young E, et al. Dual targeting of mTOR and aurora-A kinase for the treatment of uterine Leiomyosarcoma. Clin Cancer Res. 2012 Sep 1;18(17):4633–45.
Liu LL, Long ZJ, Wang LX, Zheng FM, Fang ZG, Yan M, et al. Inhibition of mTOR pathway sensitizes acute myeloid leukemia cells to aurora inhibitors by suppression of glycolytic metabolism. Mol Cancer Res. 2013 Nov;11(11):1326–36.
Lee JW, Parameswaran J, Sandoval-Schaefer T, Eoh KJ, Yang DH, Zhu F, et al. Combined Aurora Kinase A (AURKA) and WEE1 Inhibition Demonstrates Synergistic Antitumor Effect in Squamous Cell Carcinoma of the Head and Neck. Clin Cancer Res. 2019 Jun 1;25(11):3430–42.
Casari I, Domenichini A, Sestito S, Capone E, Sala G, Rapposelli S, et al. Dual PDK1/Aurora Kinase A Inhibitors Reduce Pancreatic Cancer Cell Proliferation and Colony Formation. Cancers (Basel). 2019 Oct 31;11(11).
Daniele S, Sestito S, Pietrobono D, Giacomelli C, Chiellini G, Di Maio D, et al. Dual Inhibition of PDK1 and Aurora Kinase A: An Effective Strategy to Induce Differentiation and Apoptosis of Human Glioblastoma Multiforme Stem Cells. ACS Chem Neurosci. 2017 Jan 18;8(1):100–14.
Caputo E, Miceli R, Motti ML, Tate R, Fratangelo F, Botti G, et al. AurkA inhibitors enhance the effects of B-RAF and MEK inhibitors in melanoma treatment. J Transl Med. 2014 Jul 31;12:216.
Horwacik I, Durbas M, Boratyn E, Wegrzyn P, Rokita H. Targeting GD2 ganglioside and aurora A kinase as a dual strategy leading to cell death in cultures of human neuroblastoma cells. Cancer Lett. 2013 Dec 1;341(2):248–64.
Durbas M, Pabisz P, Wawak K, Wisniewska A, Boratyn E, Nowak I, et al. GD2 ganglioside-binding antibody 14G2a and specific aurora A kinase inhibitor MK-5108 induce autophagy in IMR-32 neuroblastoma cells. Apoptosis. 2018 Oct;23(9–10):492–511.
Liu Y, Hawkins OE, Vilgelm AE, Pawlikowski JS, Ecsedy JA, Sosman JA, et al. Combining an Aurora Kinase Inhibitor and a Death Receptor Ligand/Agonist Antibody Triggers Apoptosis in Melanoma Cells and Prevents Tumor Growth in Preclinical Mouse Models. Clin Cancer Res. 2015 Dec 1;21(23):5338–48.
Yin T, Zhao ZB, Guo J, Wang T, Yang JB, Wang C, et al. Aurora A Inhibition Eliminates Myeloid Cell-Mediated Immunosuppression and Enhances the Efficacy of Anti-PD-L1 Therapy in Breast Cancer. Cancer Res. 2019 Jul 1;79(13):3431–44.
Donnella HJ, Webber JT, Levin RS, Camarda R, Momcilovic O, Bayani N, et al. Kinome rewiring reveals AURKA limits PI3K-pathway inhibitor efficacy in breast cancer. Nat Chem Biol. 2018 Aug;14(8):768–77.
Venkatakrishnan K, Zhou X, Ecsedy J, Mould DR, Liu H, Danaee H, et al. Dose selection for the investigational anticancer agent alisertib (MLN8237): Pharmacokinetics, pharmacodynamics, and exposure-safety relationships. J Clin Pharmacol. 2015 Mar;55(3):336–47.
Dai Y, Xu C, Sun X, Chen X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017 Jun 21;46(12):3830–52.
Inchanalkar S, Deshpande NU, Kasherwal V, Jayakannan M, Balasubramanian N. Polymer Nanovesicle-Mediated Delivery of MLN8237 Preferentially Inhibits Aurora Kinase A To Target RalA and Anchorage-Independent Growth in Breast Cancer Cells. Mol Pharm. 2018 Aug 6;15(8):3046–59.
Lu M, Wang C, Wang J. Tanshinone I induces human colorectal cancer cell apoptosis: The potential roles of Aurora A-p53 and survivin-mediated signaling pathways. Int J Oncol. 2016 Aug;49(2):603–10.
Ma ZL, Zhang BJ, Wang DT, Li X, Wei JL, Zhao BT, et al. Tanshinones suppress AURKA through up-regulation of miR-32 expression in non-small cell lung cancer. Oncotarget. 2015 Aug 21;6(24):20111–20.
Gong Y, Li Y, Abdolmaleky HM, Li L, Zhou JR. Tanshinones inhibit the growth of breast cancer cells through epigenetic modification of Aurora A expression and function. PLoS One. 2012;7(4):e33656.
Grover A, Singh R, Shandilya A, Priyandoko D, Agrawal V, Bisaria VS, et al. Ashwagandha derived withanone targets TPX2-Aurora A complex: computational and experimental evidence to its anticancer activity. PLoS One. 2012;7(1):e30890.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82073075, 81802795, 31301144); the Key Scientific Research Project Plan of Colleges and Universities in Henan Province (No. 18A310034); the Science and Technology Project of Henan Province (No. 182102310324, 202102310206); and the Training plan for Young Backbone Teachers of Zhengzhou University (No. 2018ZDGGJS037).
Author information
Authors and Affiliations
Contributions
All authors made substantial, direct and intellectual contribution to the review. Under the direction of the corresponding authors, Ruijuan Du orgnanized and wrote this review, Chuntian Huang collected data and Kangdong Liu provided editorial assistance. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Du, R., Huang, C., Liu, K. et al. Targeting AURKA in Cancer: molecular mechanisms and opportunities for Cancer therapy. Mol Cancer 20, 15 (2021). https://doi.org/10.1186/s12943-020-01305-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-020-01305-3