Abstract
Background
The use of post-transplant cyclophosphamide (PTCy) is highly effective in preventing graft-versus-host disease (GVHD) in the haploidentical (Haplo) transplant setting and is being increasingly used in matched sibling (MSD) and matched unrelated (MUD) transplants. There is no information on the impact of donor types using homogeneous prophylaxis with PTCy.
Methods
We retrospectively compared outcomes of adult patients with acute myeloid leukemia (AML) in first complete remission (CR1) who received a first allogeneic stem cell transplantation (SCT) with PTCy as GVHD prophylaxis from MSD (n = 215), MUD (n = 235), and Haplo (n = 789) donors registered in the EBMT database between 2010 and 2017.
Results
The median follow-up was 2 years. Haplo-SCT carried a significantly increased risk of acute grade II–IV GVHD (HR 1.6; 95% CI 1.1–2.4) and NRM (HR 2.6; 95% CI 1.5–4.5) but a lower risk of relapse (HR 0.7; 95% CI 0.5–0.9) that translated to no differences in LFS (HR 1.1; 95% CI 0.8–1.4) or GVHD/relapse-free survival (HR 1; 95% CI 0.8–1.3). Interestingly, the use of peripheral blood was associated with an increased risk of acute (HR 1.9; 95% CI 1.4–2.6) and chronic GVHD (HR 1.7; 95% CI 1.2–2.4) but a lower risk of relapse (HR 0.7; 95% CI 0.5–0.9).
Conclusions
The use of PTCy in patients with AML in CR1 receiving SCT from MSD, MUD, and Haplo is safe and effective. Haplo-SCT had increased risk of acute GVHD and NRM and lower relapse incidence but no significant difference in survival.
Similar content being viewed by others
Introduction
The use of post-transplant cyclophosphamide (PTCy) has proven to be highly effective in preventing graft-versus-host (GVHD) and reducing non-relapse mortality (NRM) rates in haploidentical (Haplo) hematopoietic stem cell transplant (SCT) [1,2,3].
As a consequence, PTCy is being increasingly used in other allogeneic transplant settings, such as SCT from HLA-matched sibling donors (MSD) [4,5,6], matched unrelated donors (MUD), and mismatched unrelated donors (MMUD) [1,2,3, 7].
Since the first reports of Haplo-SCT using PTCy, there has been significant interest in comparing this platform with those using other donor types such as cord blood from unrelated donors [4,5,6, 8] or bone marrow (BM) or peripheral blood (PB) from MSD, MUD, and MMUD transplants [9,10,11,12,13,14,15]. However, an important limitation of these studies is that each type of transplant received different GVHD prophylaxis. Therefore, comparisons were made of different transplant platforms instead of different donor types. Two prospective studies compared T cell replete Haplo and MSD transplants in acute myeloid leukemia (AML) [16] and acute lymphoblastic leukemia [17] using non-PTCy GVHD prophylaxis with similar results but increased risk of GVHD after Haplo. In addition, safety and feasibility of PTCy-based GVHD prophylaxis in transplants using different donor types was further evaluated in small single-center prospective non-randomized studies showing comparable outcomes in Haplo-SCT compared to 9/10 MUD [18], as well as in MSD, MUD, and MMUD transplants [19].
The aim of this study was to investigate the impact of donor type in the outcome of patients with AML undergoing unmanipulated allogeneic SCT (allo-SCT) using PTCy as GvHD prophylaxis. We analyzed patients who received allo-SCT from MSD, MUD, and Haplo for acute myeloid leukemia (AML) in first complete remission (CR1) and reported to the European Society for Blood and Marrow Transplantation (EBMT) registry from 2010 to 2017.
Patients and methods
Study design and data source
This is a retrospective registry-based analysis on behalf of the Acute Leukaemia Working Party (ALWP) of the EBMT. The EBMT is a voluntary working group of more than 600 transplantation centers that are required to report all consecutive stem cell transplantations and follow-up once a year. Audits are routinely performed to determine the accuracy of the data. All transplantation centers are required to obtain written informed consent before data registration with the EBMT in accordance with the 1975 Helsinki Declaration.
Patient eligibility
All adults (age ≥ 18 years) with AML in CR1 at transplantation, reported to Promise-EBMT, who underwent first allogeneic SCT, from an unmanipulated graft, using PT-Cy from Haplo, MUD, or MSD donors between 2010 and 2017, were analyzed. Haplo was defined as recipient-donor number of human leukocyte antigen (HLA) mismatches ≥ 2.
Endpoints and definitions
The primary endpoint was to compare leukemia-free survival (LFS) after MSD, MUD, and Haplo donor transplants. Secondary endpoints were neutrophil engraftment, acute GVHD (aGVHD) and chronic GVHD (cGVHD), relapse incidence, nonrelapse mortality (NRM), GVHD-free and relapse-free survival (GRFS), and overall survival (OS) within the same subgroups and to perform analysis of risk factors for each outcome.
Neutrophil recovery was defined as the first day of an absolute neutrophil count of 0.5 × 109/L lasting for 3 or more consecutive days. aGVHD and cGVHD were defined and graded according to standard criteria [20, 21]. Relapse was defined as disease recurrence and appearance of blasts in the peripheral blood or BM (> 5%) after CR. LFS was calculated until the date of first relapse, death from any cause, or the last follow-up for patients in CR. NRM was defined as death from any cause other than relapse. The composite endpoint GRFS was defined as survival without the following events: stage III–IV aGVHD, severe cGVHD, disease relapse, or death from any cause after SCT [22]. Myeloablative conditioning (MAC) was defined as a regimen containing either total body irradiation with a dose greater than 6 Gray, a total dose of oral busulfan greater than 8 mg/kg, or a total dose of intravenous busulfan > 6.4 mg/kg or melphalan at doses > 140 mg/m2. In addition, regimens containing 2 alkylating agents were considered as MAC. All other regimens were defined as reduced intensity (RIC).
Statistical analysis
Patient characteristics according to donor type were compared using chi-squared tests for categorical and Kruskal-Wallis tests for continuous variables. GRFS, LFS, and OS were estimated using the Kaplan-Meier method. Cumulative incidence functions were used to estimate neutrophil engraftment, aGVHD, cGVHD, relapse incidence, and NRM. Competing risks were death for relapse incidence and neutrophil engraftment, relapse for NRM, and relapse or death for aGVHD and cGVHD. Univariate analyses were done using the log-rank test for LFS, GRFS, and OS and Gray’s test for cumulative incidence. Multivariate analyses were performed using the Cox proportional hazard model.
Donor type, gender, age at transplantation, performance status, cytogenetic risk group according to the Medical Research Council [23], type of AML (primary vs secondary) stem cell source, transplantation year, cytomegalovirus serostatus, and conditioning regimen were included in the final model. To take into account the center effect, we introduced a random effect (also named frailty effect) for each center into the model. The significance level was fixed at .05, and p values were 2-sided. Statistical analyses were performed using R software version 3.2.3 (R Development Core Team, Vienna, Austria) software packages.
Results
Patient and transplantation characteristics
Patient, disease, and transplant characteristics of the overall population and according to donor type are summarized in Table 1. Briefly, a total of 1239 patients were included in the study, of which 789 were transplanted from Haplo, 235 from MUD, and 215 from MSD donors. Median age of patients was 52 years (range, 18–76). Forty-seven (6%), 543 (66%), and 239 (29%) had standard-, intermediate-, and high-risk cytogenetics, respectively. Preferred conditioning regimens were thiotepa, busulfan, and fludarabine for Haplo (n = 371; 47%) and busulfan and fludarabine for MSD (n = 83; 39%) and MUD (n = 102; 43%).
Haplo patients were older (p < 0.001) and had higher proportion of secondary AML (p < 0.001), while differences in gender, performance status, and cytogenetic risk category were not statistically significant. Regarding transplant characteristics, Haplo-SCT recipients received more frequently MAC (p = 0.006) and BM as stem cell source (p < 0.001), while the proportion of in vivo T cell depletion was higher in MUD transplants (p < 0.001). Although the vast majority (93%) of Haplo patients received GvHD prophylaxis with PTCy combined with 2 other immunosuppressive (IS) drugs, only 47% and 26% of MUD and MSD patients received such combination, respectively. In contrast, a higher proportion of MSD and MUD patients received 1 or no additional IS drugs than Haplo patients (p < 0.001).
Engraftment
Cumulative incidence of neutrophil recovery at 60 days was 94% (95% CI; 92–95) for Haplo, 98% (95% CI; 95–99) for MUDs, and 98% (95% CI; 94–99) for MSDs (p = 0.12). The median time of neutrophil recovery was 19 days (range, 2–63), 20 days (range, 2–48), and 19 days (range, 5–64) for Haplo, MUD, and MSD, respectively.
GvHD
The cumulative incidence of aGvHD grades II–IV at 100 days was 26% (95% CI; 23–29) for Haplo, 28% (95% CI; 22–34) for MUD, and 17% (95% CI; 12–23) for MSD (p = 0.03). The cumulative incidence of aGvHD grades III–IV was 9% (95% CI; 7–12) for Haplo, 8% (95% CI; 5–11) for MUD, and 6% (95% CI; 4–10) for MSD (p = 0.2) (Table 2). In multivariable analysis (Table 3), Haplo was associated with an increased risk of aGvHD grades II–IV, when compared with MSD (HR 1.6; 95% CI, 1.08–2.37; p = 0.02). The use of PB as the stem cell source was also associated with a higher risk of aGvHD grades II–IV (HR 1.93; 95% CI, 1.39–2.67; p < 0.001) and grades III–IV (HR 1.86; 95% CI, 1.1–3.15; p = 0.02) (Table 4).
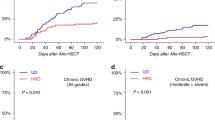
The cumulative incidence of cGvHD at 2 years was 30% (95% CI; 26–33), 32% (95% CI; 25–39), and 34% (95% CI; 26–41) (p = 0.3) for Haplo, MUD, and MSD, respectively (Table 2). The cumulative incidence of extensive type cGvHD was 10% (95% CI; 8–13) for Haplo, 18% (95% CI; 13–25) for MUD, and 14% (95% CI; 9–20) for MSD (p = 0.003) (Table 2). In multivariable analysis (Table 4), use of PB was independently associated with a higher risk of cGvHD (HR 1.71; 95% CI, 1.23–2.39; p = 0.001) and extensive cGvHD (HR 1.86; 95% CI, 1.15–3.01; p = 0.01). Female donor to male recipient showed also an increased risk of extensive cGvHD (HR 1.71; 95% CI, 1.14–2.57; p = 0.009). Donor type was not associated with the risk of cGvHD or extensive cGvHD in multivariate analysis.
Relapse
The median time to relapse was 192 days (range, 3–1750). The cumulative incidence of relapse at 2 years was 23% (95% CI; 20–26) for Haplo, 25% (95% CI; 19–31) for MUD, and 33% (95% CI; 26–41) for MSD (p = 0.02) (Table 2) (Fig. 1). In multivariable analysis (Table 3), Haplo was associated with a lower risk of relapse when compared with MSD (HR 0.67; 95% CI, 0.48–0.93; p = 0.02). Other variables associated with a decreased risk of relapse were use of MAC (HR 0.56; 95% CI, 0.43–0.77; p < 0.001), PB as the stem cell source (HR 0.74; 95% CI, 0.45–0.99; p = 0.04), and standard or intermediate cytogenetic risk category (HR 0.58; 95% CI, 0.43–0.78; p < 0.001) (Table 4).
NRM and causes of death
The cumulative incidence of NRM at 2 years was 23% (95% CI; 20–26) for Haplo, 14% (95% CI; 9–19) for MUD, and 10% (95% CI; 6–15) for MSD (p < 0.001) (Table 2) (Fig. 2). In multivariable analysis (Table 3), Haplo was associated with an increased risk of NRM (HR 2.6; 95% CI, 1.5–4.49; p < 0.001). The other factor associated with an increased NRM was higher recipient’s age per 10 years (HR 1.35; 95% CI, 1.19–1.54; p < 0.001) (Table 4).
At the last follow-up, 432 patients had died, of which 290 (65%) were due to a variety of non-relapse causes, 222 (73%) in Haplo, 36 (52%) in MUD, and 32 (47%) in MSD. The main causes of transplant-related deaths were infections and GvHD, being 107 (39%) and 40 (14%) in Haplo, 13 (20%) and 8 (12%) in MUD, and, 10 (15%) and 11 (16%) in MSD cohorts, respectively (Table 5).
Survival
For the entire cohort, LFS, OS, and GRFS at 2 years were 56% (95%CI; 53–59), 63% (95%CI; 60–66), and 45% (95%CI; 42–48), respectively.
LFS was 54% (95% CI; 51–58) for Haplo, 62% (95% CI; 55–69) for MUD, and 57% (95% CI; 49–65) for MSD (p = 0.2) (Fig. 3). In multivariable analysis (Table 4), variables associated with better LFS were MAC (HR 0.71; 95% CI, 0.58–0.88; p = 0.001), good- or intermediate-risk cytogenetics (HR 0.73; 95% CI, 0.58–0.92; p = 0.007), and good performance status (HR 0.8; 95% CI, 0.64–0.99; p = 0.04), while higher recipient’s age per 10 years (HR 1.1; 95% CI, 1.02–1.18; p = 0.02) and positive CMV serostatus of the recipient (HR 1.27; 95% CI, 1–1.6; p = 0.04) showed worse outcome.
OS was 61% (95% CI; 58–65) for Haplo, 68% (95% CI; 62–75) for MUD, and 64% (95% CI; 56–72) for MSD (p = 0.1) (Figure 4). Variable that independently correlated with better OS in multivariable analysis were MAC (HR 0.76; 95% CI, 0.6–0.96; p = 0.02) and good- or intermediate-risk cytogenetics (HR 0.7; 95% CI, 0.54–0.9; p = 0.005), while higher recipient’s age per 10 years was associated with poorer survival (HR 1.22; 95% CI, 1.12–1.33; p < 0.001) (Table 4).
GRFS was 46% (95% CI; 42–50) for Haplo, 42% (95% CI; 35–50) for MUD, and 45% (95% CI; 37–53) for MSD (p = 0.9) (Table 2). For GRFS, MAC (HR 0.79; 95% CI, 0.66–0.95; p = 0.01), good- or intermediate-risk cytogenetics (HR 0.78; 95% CI, 0.63–0.96; p = 0.02), and good performance status (HR 0.8; 95% CI, 0.67–0.97; p = 0.02) were associated with improved outcome (Table 4).
Discussion
The use of PTCy for GvHD prophylaxis in patients with AML in CR1 receiving SCT from MSD, MUD, and Haplo is safe and effective, resulting in low rates of GVHD, especially chronic, in all transplant settings. Using this approach, our results demonstrate that patients undergoing Haplo-SCT had higher rates of aGVHD and NRM, but lower relapse incidence. As seen in other transplant scenarios, PB was also associated with more GVHD and less relapse.
Due to the retrospective nature of a registry-based study, some potential bias cannot be completely ruled out. In order to minimize one of the most important, such as the disease status at transplantation, all patients included in the analysis had AML in CR1. Although all patients received PTCy prophylaxis as the main inclusion criteria for the study, a variety of conditioning regimens were used and there were obvious differences in additional GvHD prevention strategies, such as in vivo TCD or the addition of other IS drugs, depending of the type of donor. In fact, Haplo patients received more frequently MAC, BM, and PTCy with 2 IS drugs, while a higher proportion of MUD patients received in vivo TCD. Although some of these variables could be adjusted in multivariable analysis, TCD and combination of IS drugs for GvHD prophylaxis were strongly associated with type of donor and their effect could not be evaluated. Despite all these pitfalls, we aimed to compare MSD, MUD, and Haplo using a homogeneous GvHD prophylaxis with PTCy in a large series of patients, which allowed us to segregate the effect of donor from the effect of GvHD prophylaxis.
PTCy was highly effective in preventing acute and chronic GvHD in MSD, MUD, and Haplo-SCT and seems to compare favorably with standard GvHD prophylaxis with calcineurin inhibitor and methotrexate in MSD and MUD transplants [24]. In fact, the incidence of GvHD after PTCy seems similar to that reported with anti-T cell globulin in both scenarios [25], with the potential advantage of avoiding complications associated with prolonged TCD. An interesting observation was the higher incidence of acute grades II–IV and a trend towards a higher severe acute GvHD of Haplo-SCT compared to MSD transplants. It should be noted that most previous studies had not been able to demonstrate this effect [9, 11, 14, 26], probably due to the use of different GvHD prophylaxis for each procedure. In fact, Haplo was associated with increased risk of GVHD compared to MSD in two prospective studies using similar non-PTCy transplant platforms [16, 17]. Particularly relevant was the low overall chronic and chronic extensive GvHD observed in our study in all cohorts, as it has been previously reported with PTCy [1, 4, 7, 24, 27]. We should highlight that we did not observe increased risk of chronic GvHD in Haplo in multivariable analysis. We could speculate that PTCy abrogates the detrimental effect of HLA disparity for this particular outcome, but we should also consider that most patients in the Haplo cohort received PTCy with a double combination of IS drugs, while patients in the MSD and MUD cohorts received less intensive GvHD prophylaxis. In fact, a recent study of the ALWP-EBMT has recently reported that the addition of IS drugs to PTCy enhances its effect and reduces the risk of severe chronic GvHD, reducing mortality and improving survival [6].
The most important observation from our study is that, using PTCy as GVHD prophylaxis, NRM was higher in the Haplo setting compared with the MSD and MUD cohorts. Previous studies comparing Haplo (with PTCy) with MSD and MUD transplants (with standard GvHD prophylaxis) have reported discrepant results in terms of NRM. While some studies reported a higher rate of NRM in Haplo [11, 15], some others reported similar [12, 14] or even improved outcomes [10]. The reasons of these discrepancies remain unexplained and are probably multifactorial, but differences in transplant platforms could explain, at least in part, some of these results. Under similar GvHD prophylaxis, a greater HLA disparity in the Haplo compared with the MSD and MUD settings could explain a higher NRM. This finding suggests that HLA-matched donors, when available, should remain as the first choice. Although the negative impact of Haplo in NRM was partially counterbalanced with a decreased incidence of relapse that translated in similar LFS, other strategies aiming at reducing relapse such as maintenance or MRD-guided therapy with a growing targeted therapy strategies could be investigated.
The fact that Haplo was associated with lower risk of relapse deserves special attention. It is possible that this was a spurious finding since more patients in the Haplo cohort died from NRM and were therefore no longer at risk of relapse. However, Haplo procedure could have offered enhanced anti-leukemic efficacy, intriguingly in a way that was independent of chronic GVHD. Superior graft-versus-leukemia effect of Haplo compared to MSD transplants for high-risk AML has already been observed in previous comparative studies. Two prospective trials with biological randomization from China showed decreased posttransplant minimal residual disease (MRD) positivity [28] or relapse [29] in patients undergoing Haplo, particularly relevant for those with detectable pretransplant MRD. In addition, a recent retrospective study of EBMT also showed decreased relapse incidence in patients with high-risk cytogenetics undergoing Haplo [15].
The immunological pressure of Haplo grafts has been illustrated with the observation that loss of the mismatched HLA haplotype is a frequent mechanism of escape associated with relapse [30]. The biological explanation is unknown but NK-mediated alloreactivity has been previously proposed to induce enhanced efficacy and GVHD protection in the context of T cell-depleted Haplo-SCT [31]. The hypothesis of an increased anti-leukemic efficacy independent of GvHD of Haplo-SCT compared to matched donors in the context of PTCy should be further explored from a clinical and biological point of view.
Despite the risks and benefits of BM over PB have been widely investigated, the effect of the stem cell source on transplant outcomes deserves special consideration. In MUD transplants, BM reduced the risk of chronic GVHD in a randomized study [32] and improved long-term GRFS and overall survival in a large retrospective registry study [33]. In Haplo-SCT, the use of PB resulted in an increased risk of acute GvHD, uncertain impact of chronic GvHD, and decreased risk of relapse in patients with acute leukemia, but not with lymphoma [34, 35]. In the present study, we confirm that PB was associated with increased risk of acute and chronic GvHD, less relapses, but no final influence on NRM and survival.
Although most retrospective studies comparing MAC with RIC in patients with AML have suggested similar survival, since the latter has been associated with increased relapse but reduced NRM [36, 37], we observed a significant reduction of relapse with MAC that translated into improved survival when compared with RIC. Unfortunately, the only randomized study, designed to address this issue in patients with AML, was closed to patient accrual early due to excess of relapse and reduced survival in the RIC cohort [38]. Since the efficacy of RIC in SCT mainly relies on graft-versus-leukemia effect, it may be particularly relevant to increase conditioning intensity in transplant platforms with effective GvHD control such as with the use of PTCy.
Conclusion
In patients with AML undergoing allo-SCT, PTCy for GvHD prophylaxis showed promising outcomes. Future studies comparing PTCy standard regimens are warranted to establish the standard of care. In this specific scenario, Haplo-SCT had increased risk of acute GVHD and NRM that was counterbalanced by a lower relapse incidence that translated into no significant difference in LFS and OS. Haplo-SCT offers a good alternative to matched donor transplants.
Availability of data and materials
The dataset supporting the conclusions of this article are available in the ALWP of EBMT in Paris, Saint Antoine Hospital.
Abbreviations
- aGVHD:
-
Acute GVHD
- allo-SCT:
-
Allogeneic stem cell transplantation
- ALWP:
-
Acute Leukaemia Working Party
- AML:
-
Acute myeloid leukemia
- BM:
-
Bone marrow
- cGVHD:
-
Chronic GVHD
- CR1:
-
First complete remission
- EBMT:
-
European Society for Blood and Marrow Transplantation
- GRFS:
-
GVHD-free and relapse-free survival
- GVHD:
-
Graft-versus-host
- Haplo:
-
Haploidentical donors
- HLA:
-
Human leukocyte antigen
- LFS:
-
Leukemia-free survival
- MAC:
-
Myeloablative conditioning
- MSD:
-
Matched sibling donors
- MUD:
-
Matched unrelated
- NRM:
-
Nonrelapse mortality
- PB:
-
Peripheral blood
- PTCy:
-
Post-transplant cyclophosphamide
- RIC:
-
Reduced intensity conditioning
- SCT:
-
Stem cell transplantation
- OS:
-
Overall survival
References
Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Tr. 2008;14(6):641–50.
Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Tr. 2010;16(4):482–9.
Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Tr. 2013;19(1):117–22.
Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–30.
Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–505.
Ruggeri A, Labopin M, Bacigalupo A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11(1):40.
Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152–61.
Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(9):1891–900.
Bashey A, Zhang X, Sizemore CA, et al. T-cell–replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–6.
Ciurea SO, Zhang M-J, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–40.
Versluis J, Labopin M, Ruggeri A, et al. Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv. 2017;1(7):477–85.
Piemontese S, Ciceri F, Labopin M, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017;10(1):24.
Baron F, Labopin M, Ruggeri A, et al. Impact of donor type in patients with AML given allogeneic hematopoietic cell transplantation after low-dose TBI based regimen. Clin Cancer Res. 2018;24(12):clincanres.3622.2017.
Rashidi A, Hamadani M, Zhang M-J, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019;3(12):1826–36.
Salvatore D, Labopin M, Ruggeri A, et al. Outcomes of hematopoietic stem cell transplantation from unmanipulated haploidentical versus matched sibling donor in patients with acute myeloid leukemia in first complete remission with intermediate or high-risk cytogenetics: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2018;103(8):haematol.2018.189258.
Wang Y, Liu Q-F, Xu L-P, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956–62.
Wang Y, Liu Q-F, Xu L-P, et al. Haploidentical versus matched-sibling transplant in adults with Philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III randomized study. Am Assoc Cancer Res. 2016;22(14):3467–76.
Gaballa S, Ge I, Fakih RE, et al. Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer. 2016;122(21):3316–26.
Moiseev IS, Pirogova OV, Alyanski AL, et al. Risk-adapted GVHD prophylaxis with post-transplantation cyclophosphamide in adults after related, unrelated, and haploidentical transplantations. Eur J Haematol. 2018;100(5):395–402.
GLUCKSBERG H, STORB R, FEFER A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donor. S. Transplantation. 1974;18(4):295–304.
Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15(6):825–8.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP–EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2015;51(4):610–1.
Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–65.
Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen–identical siblings versus human leukocyte antigen–allelic–matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24(36):5695–702.
Kröger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. New Engl J Medicine. 2016;374(1):43–53.
Martínez C, Gayoso J, Canals C, et al. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: a registry study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol Official J Am Soc Clin Oncol. 2017;35(30):3425–32.
Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64.
Yu S, Huang F, Wang Y, et al. Haploidentical transplantation might have superior graft-versus-leukemia effect than HLA-matched sibling transplantation for high-risk acute myeloid leukemia in first complete remission: a prospective multicentre cohort study. Leukemia. 2019:1–11.
Chang Y-J, Wang Y, Liu Y-R, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017;10(1):134.
Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. New Engl J Med. 2009;361(5):478–88.
Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic Transplants. Science. 2002;295(5562):2097–100.
Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. New Engl J Medicine. 2012;367(16):1487–96.
Alousi A, Wang T, Hemmer MT, et al. Peripheral blood versus bone marrow from unrelated donors: bone marrow allografts have improved long-term overall and graft-versus-host disease-free, relapse-free survival. Biol Blood Marrow Tr. 2018;25(2):270–8.
Bashey A, Zhang M-J, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell–replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35(26):3002–9.
Ruggeri A, Labopin M, Bacigalupo A, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018;124(7):1428–37.
Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia. 2005;19(12):2304–12.
Ringdén O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–7.
Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35(11):JCO.2016.70.709.
Jacoby E, Chen A, Loeb DM, et al. Single-agent post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis after human leukocyte antigen–matched related bone marrow transplantation for pediatric and young adult patients with hematologic malignancies. Biol Blood Marrow Tr. 2016;22(1):112–8.
Mielcarek M, Furlong T, O’Donnell PV, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127(11):1502–8.
Acknowledgements
The authors thank Emmanuelle Polge from the office of the ALWP of EBMT for data collection.
Funding
No funding
Author information
Authors and Affiliations
Consortia
Contributions
Jaime Sanz, Arnon Nagler, and Mohamad Mohty: designed the study. Jacques-Emmanuel Galimard: performed statistical analysis. Myriam Labopin: helped for the interpretation of the results. Jaime Sanz: wrote the manuscript. Boris Afanasyev, Emanuele Angelucci, Fabio Ciceri, Didier Blaise, Jan J. Cornelissen, Ellen Meijer, J.L. Diez-Martin, Yener Koc, Montserrat Rovira, Luca Castagna and Mohamad Mohty: provided cases for the study. All authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The scientific board of the ALWP of EBMT approved this study. All patients gave written informed consent for the use of their data.
Consent for publication
Not applicable for individual patient data. This is a pooled analysis.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sanz, J., Galimard, JE., Labopin, M. et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: a comparative study of the ALWP EBMT. J Hematol Oncol 13, 46 (2020). https://doi.org/10.1186/s13045-020-00882-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-020-00882-6