Abstract
Background
Chromoanagenesis events encompassing chromoanasynthesis, chromoplexy, and chromothripsis are described in cancers and can result in highly complex chromosomal rearrangements derived from ‘all-at-once’ catastrophic cellular events. The complexity of these rearrangements and the original descriptions in cancer cells initially led to the assumption that it was an acquired anomaly. While rare, these phenomena involving chromosome 1 have been reported a few individuals in a constitutional setting.
Case presentation
Here, we describe a newborn baby who was initially referred for cytogenetic testing for multiple congenital anomalies including cystic encephalomalacia, patent ductus arteriosus, inguinal hernia, and bilateral undescended testicles. Chromosome analysis was performed and revealed a derivative chromosome 1 with an 1q24-q31 segment inserted into 1q42.13 resulting in gain of 1q24-q31. Whole genome SNP microarray analysis showed a complex pattern of copy number variants with four gains and one loss involving 1q24-q31. Mate pair next-generation sequencing analysis revealed 18 chromosome breakpoints, six gains along an 1q24-q31 segment, one deletion of 1q31.3 segment and one deletion of 1q42.13 segment, which is strongly evocative of a chromoanasynthesis event for developing this complex rearrangement. Parental chromosome analyses were performed and showed the same derivative chromosome 1 in the mother.
Conclusions
To our knowledge, our case is the first case with familial constitutional chromoanagenesis involving chromosome 1q24-q42. This report emphasizes the value of performing microarray and mate pair next-generation sequencing analysis for individuals with germline abnormal or complex chromosome rearrangements.
Similar content being viewed by others
Background
Chromoanagenesis events are new types of complex and massive chromosomal and genomic alterations characterized by the simultaneous occurrence of multiple structural rearrangements confined to one or a few chromosomal segments through a single catastrophic cellular event [1,2,3]. The term ‘chromoanagenesis’ was used to describe a new ‘all-at-once’ process, identified by genome sequencing techniques and bioinformatics tools as a new driver of tumorigenesis by which, challenging the well-known mechanism of gradual accumulation of mutations to prefer cell duplication/survival, a single catastrophic event of massive shattering and disordered reassembly of one or few chromosomes induced oncogenic lesions [3]. Therefore, the concept of chromoanagenesis, a form of chromosome rebirth, provides new insight into the nature of complex chromosomal rearrangements. Chromoanagenesis has been encompassed at least three phenomena independent of the underlying mechanism: the chromoplexy, the chromothripsis, and the chromoanasynthesis [1,2,3].
The chromoplexy is characterized by the interdependent occurrence of multiple inter-and intra-chromosomal translocations and deletions [4]. The chromothripsis is defined as a mutational event driven by multiple double-strand breaks occurring in a single catastrophic event between several chromosomes/segments and followed by NHEJ-mediated repair mechanisms (the reassembly of the DNA fragments in random order and orientation to form complex derivative chromosomes) with or without copy number changes [3]. Thus, the chromothripsis-related structural rearrangements usually include deletions, insertions and inversions. Being a chromosome shattering phenomenon, complex genomic rearrangements can occur at a part of or an entire chromosome, or few chromosomes. In contrast to the chromothripsis/chromoplexy, the chromoanasynthesis is a replication based complex rearrangement process that involves serial fork stalling and template switching or microhomology-mediated break-induced replication mechanisms [5,6,7], which can lead to a highly remodeled chromosomes with copy number changes including gains and losses along a single chromosome. Therefore, chromothripsis and chromoanasynthesis could frequently explain the formation of multiple copy number changes on the same chromosome.
The complexity of these rearrangements and the original descriptions in cancer cells initially led to the assumption that chromoanagenesis was an acquired anomaly [3]. While rare, chromoanagenesis-related complex chromosomal rearrangements involving chromosome 1 have been reported a few individuals in a constitutional setting [5, 8,9,10,11,12,13]. Majority of these complex chromosomal rearrangements involve translocations between chromosome 1 and other chromosomes instead of a single rearranged chromosome 1. Furthermore, not all breakpoints were well characterized at a high resolution. Here, we describe the first case of a newborn baby with multiple congenital anomalies and very complex chromosomal rearrangements involving a long arm of chromosome 1 (at 1q24-q42) inherited from his mother. The 18 breakpoints along the long arm of chromosome 1 of our patient were well characterized by whole genome SNP microarray and mate pair next-generation sequencing analyses, which provides new insight into the nature of a chromoanagenesis event.
Case presentation
During the pregnancy of the proband by a 23-year-old G1P0 mother, prenatal ultrasound of the fetus revealed congenital anomalies including dilated right cerebral ventricle (suspected germinal matrix hemorrhage), pyelectasis, echogenic bowel, and hypoplastic nasal bone. 36-week gestational fetal MRI revealed cystic encephalomalacia in region of left caudate nucleus and caudothalamic groove appears more conspicuous, likely reflecting sequela of germinal matrix hemorrhage, similar prominent left lateral ventricles without evidence of obstructive hydrocephalus, and mild left fetal pyelocaliectasis.
The proband was born at 37-week-6-day gestational age by cesarean due to breech presentation, and abnormal prenatal ultrasound/MRI findings. The mother was group B streptococcus positive. At birth, his weight was 2.915 kg, his length is 47 cm, and his occipital–frontal circumference (OFC) was 34.5 cm. His Apgars were 7 and 8 at 1 and 5 min, respectively. He had decreased respiratory effort after birth and requiring blow by oxygen briefly. His echo showed small patent ductus arteriosus with left to right shunting, ductal velocity indicating elevated pulmonary artery pressures, and insufficient tricuspid valve regurgitation for estimation of right ventricular systolic pressure. His ultrasound revealed resolving left germinal matrix hemorrhage. He had no acute hemorrhage, no pyelectasis, no echogenic bowel, and a normal size of the ventricles. His MRI revealed periventricular white matter cystic encephalomalacic change at the left frontal horn and caudothalamic groove, likely representing sequela of prior germinal matrix hemorrhage. He had bilateral undescended testes with right testis located within the inguinal canal, and the left testis seen coursing between the left lower pelvis and upper inguinal canal.
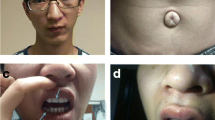
At the age of 4 months, his height was 55.9 cm (<1st centile; 50th centile for a 1-month old), his weight was 5.1 kg (<1st centile; 50th centile for an 1.5-month old), and his OFC was 41.4 cm (38th centile). He had failure to thrive, developmental delay, severe tracheomalacia, stridor/difficulty breathing along with decreased oral intake, bilateral inguinal, gastroesophageal reflux disease, and bilaterally undescended testes. He also had possible seizure disorder, increased tone, and abnormal rigid movements with significant jitteriness and frequent myoclonic jerks. His anterior fontanelle was open and flat. He had facial dysmorphisms including small nose and depressed nasal bridge with possible hypertelorism.
His mother had intellectual disability and lived with her mother. Paternal grandmother and paternal great grandmother had seizure. Father’s family members had attention deficit hyperactivity disorder, anxiety, and depression disorders. No cancer-related disorders were found on either side of the family.
Results
His blood karyotype is 46,XY,der(1)ins(1;1)(q42.13;q24q31), with interstitial gain of the 1q24-q31 segment (Fig. 1). Genome-wide SNP-microarray (CytoscanHD chip, Thermo Fisher Scientific using Chromosome Analysis Suite (ChAS) version 3.3 for the SNP analysis) demonstrates 1q24.1q25.1(166,124,606-175,038,371)× 3,1q25.3q31.1(182,388,825-186,693,330)× 3,1q31.2(192,446,379-192,799,227)× 3,1q31.3(195,686,410-195,745,969)× 3, 1q31.3(195,828,516-195,876,205)× 1, which are 8.9 Mb gain of 1q24.1-q25.1, 4.3 Mb gain of 1q25.3-q31.1, 352 Kb gain of 1q31.2, 60 Kb gain of 1q31.3 and 48 Kb loss of 1q31.3 (Fig. 2a). Therefore, the propositus had an unbalanced derivative chromosome 1 with four gains and one loss along the 1q24-q31 region (Fig. 2a). His father had a normal karyotype, while his mother carried the same derivative chromosome 1 as observed in her son (Fig. 1) and her CytoScanHD SNP microarray (Thermo Fisher Scientific, Waltham, MA) revealed the same gains and loss along the 1q24-q31 region as her son, further supporting that her son’s derivative chromosome 1 was inherited from the mother.
Copy number changes on the long arm of chromosome 1q24-q31 region. a Genome-wide SNP microarray revealed four gains (blue blocks) and one loss (red block), the X axis is the long arm of chromosome 1q23.3-1q31.3 region, the Y axis is for the copy number, blue and red blocks are for gain and loss of 1q, respectively; b Consistent with Genome-wide SNP microarray, mate pair next-generation sequencing also revealed four gains and one loss. Blue and red lines are for gain and loss of 1q, respectively
To further characterize the derivative chromosome 1, mate pair next-generation sequencing [11, 12] of the proband was performed and revealed complex rearrangements involving chromosome 1q. The derivative chromosome 1 had complicated rearrangements with four gains and one deletion along the 1q24-q31 region (Fig. 2b) and 18 breakpoints along 1q24–31 and 1q42 regions, seq [GRCh38] inv.(1)(pter- > q31.3(195,857, 632)::q31.3(195,906,366)- > q42.12(225,475,803)::q31.3(195,717,189)- > q31.3(195, 784,837)::q25.3(182,416,826)- > q31.1 (186,739,165)::q25.1(175,081,899)- > q24.3(172,866,607)::q24.1(166,154,922)- > q24.3(172,866,584)::q42.12(225,476,068)- > q42.13(229,372,123)::q31.2(192,827,141)- > q31.2(192,831,551)::q31.2(192,827,064)- > q31.2(192,473,950)::q42.13(229,387,971)- > qter), resulting in six gains and two losses along 1q (Fig. 3).
Characterization of the derivative chromosome 1 by mate pair next-generation sequencing. From left to right: ideogram of chromosome 1, replicating chromosome 1 showing 1q24-q42 region, sister chromatid/homolog chromatid of chromosome 1, and chromosome 1q24–42 had 18 breakpoints, nine chromosome junctions, six gains and two losses, suggesting replication-mediated chromoanasynthesis by fork stalling and template switching or microhomology-mediated break-induced replication as responsible to form a highly complex chromosome 1
Discussion and conclusions
Chromoanagenesis events can lead to complex and massive chromosomal rearrangements and contains chromoplexy, chromothripsis, and chromoanasynthesis events [2]. The chromoplexy can frequently create chromosomal translocations and deletions [4]. The chromothripsis usually produces deletions, insertions and inversions. Deletions are generally more common than gains of genetic material through a typical chromothripsis event. In contrast to the chromothripsis / chromoplexy, the chromoanasynthesis can typically lead to a highly remodeled chromosomes with copy number changes along a single chromosome [5,6,7]. Gains are more common than deletions in a usual chromoanasynthesis event. Our patient had multiple gains and losses clustered on a single chromosome arm (the long arm of chromosome 1), which support the complex 1q rearrangement in our patient may arise through either a chromothripsis or a chromoanasynthesis event. Since the complex 1q rearrangements in our patient had more gains (six gains) than deletions (two losses), which may support the presence of a chromoanasynthesis event as responsible to form a highly complex rearrangement of chromosome 1 with copy number changes by fork stalling and template switching and microhomology-mediated break-induced replication (Fig. 3).
Patients with chromoanasynthesis-mediated rearrangements have been reported to display developmental delay, intellectual disability, dysmorphic features, or relatively mild phenotypic effects [1, 14], and the severity of clinical presentation will depend on dosage-sensitive genes located in these gain and loss regions. Our patient had six gains and two losses at the highly rearranged 1q (Table 1). Although no genes locate at one loss and two gains, the four gains in our patient contain ~ 258 RefSeq genes, 89 OMIM genes, and 20 disease genes (Table 1). Among eight autosomal dominant disease genes, he PRRX1 gene (OMIM: 167420) encodes a homeobox gene, expresses in specific temporal and spatial patterns, functions as transcriptional regulators of developmental processes [15], and has important roles during the patterning of the first pharyngeal arch and mandibulofacial development [16]. Heterozygous loss-of-function mutation in the PRRX1 gene can lead to agnathia-otocephaly complex (OMIM: 202650), which is a rare and lethal condition characterized by mandibular hypoplasia or agnathia, ventromedial auricular malposition (melotia) and/or auricular fusion (synotia), microstomia with oroglossal hypoplasia or aglossia, holoprosencephaly, and skeletal, genitourinary, and cardiovascular anomalies [17]. Gain of the PRRX1 gene has been reported in patients with intellectual disability, global developmental delay, seizures, autistic behavior, plagiocephaly, cardiomyopathy, and facial dysmorphism (DECIPHER patients 2365, 264469, 277957, 285697, 285898, 293723, 317779, 332725). The gains in our patient also contain two autosomal dominant disease genes associated with eye disorders, heterozygous mutation in the MYOC (OMIM: 601652) and the HMCN1 (OMIM: 608548) genes have been associated with one form of primary open angle glaucoma 1A (OMIM: 137750) and susceptibility to age-related macular degeneration-1 (OMIM: 603075), respectively. Smaller gains as our patients have also been reported in patients with intellectual disability, global developmental delay, autistic behavior, and facial dysmorphism (DECIPHER patients 273428, 293641, 343360, 362331, and ClinVar patient nsv530079, etc.).
Beside six gains and two losses, our patient had 18 breakpoints and nine chromosome junctions (Table 2). Two breakpoints locate at introns of two genes, FAM78B and TNN (OMIM: 617472). The FAM78B gene is novel with unknown function. It is 109 Kb in size, has 261 amino acids, codes two exons, and makes a 29.8 KDa protein [18]. The TNN gene encodes an extracellular matrix glycoprotein with a characteristic structure consisting of an N-terminal cysteine-rich segment, EGF-like repeats, fibronectin type III repeats, and C-terminal fibrinogen-like domain [19]. The TNN gene expresses in all brain regions, with a graded staining pattern in the hippocampal CA3 region and may associate with cell migration and neurite growth [19]. It has been associated with low bone mineral density and primary myopathies [20, 21]. While our patient had no genes located at one loss, two gains and 16 breakpoints of the abnormal chromosome 1 (Table 2), it is possible that positional effects may influence the expression of nearby dosage-sensitive genes, contributing to abnormal phenotype.
In order to understand genome architecture at rearrangements’ breakpoints and role of unusual DNA sequences such as low-copy repeats or tandem repeats in chromoanagenesis, we checked for all repeat elements at the distal and proximal sites of the 18 breakpoints using RepeatMasker [http://www.repeatmasker.org] and Repbase update programs [22]. We detected a variety of repeats at 17 out of 18 breakpoints, which include long interspersed nuclear elements (LINE, a total of 6), short interspersed nuclear elements (SINE, a total of 5), long terminal repeat elements (LTR, a total of 4), and other simple repeat elements (a total of 2) (Table 2). These repetitive sequences create points of genomic instability and may serve as substrates for chromosomal rearrangements [23, 24]. Our patient had LINE sequences and Alu repeats at breakpoints (Table 2). LINE-1 s (L1 s) are endogenous mutagens and have both DNA endonuclease [25] and reverse-transcriptase activities [26]. L1 is capable of mobilizing itself [27, 28] and other retrotransposons such as Alu [29, 30]. There is also a correlation between retrotransposon sequences and genomic structural variants [31,32,33,34] and segmental duplications [35]. In particular, L1-mediated retrotransposition and homologous recombination between Alu repeats may serve as potential mutagens in the genome [36]. The abundance of these elements at breakpoints in our patient may suggest an association of active and inactive retrotransposons at a chromoanagenesis event. Further studies of other breakpoint junctions involved in constitutional chromoanagenesis cases will be necessary to elucidate the role of these endogenous mutagens in chromoanagenesis formation.
To our knowledge, our case is the first case with familial constitutional chromoanagenesis involving chromosome 1q24-q42. Constitutional chromoanagenesis have likely been underestimated in a constitutional setting. Microarray and mate pair next-generation sequencing technologies can be used to accurately detect such complexity. Further characterization of these breakpoint junctions in our patient will help understand the molecular mechanisms responsible for this process of massive genomic rearrangement of chromosome 1q.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All authors read and approved the final manuscript.
Abbreviations
- 1q:
-
The long arm of chromosome 1
- cm:
-
Centimeter
- Kg:
-
Kilogram
- MRI:
-
Magnetic resonance imaging
- NHEJ:
-
Non-homologous end joining
- OFC:
-
Occipital–frontal circumference
- OMIM:
-
Online Mendelian Inheritance in Man
- SNP:
-
Single nucleotide polymorphism
References
Fukami M, Kurahashi H. Clinical consequences of chromothripsis and other catastrophic cellular events. Methods Mol Biol. 2018;1769:21–33.
Holland AJ, Cleveland DW. Chromoanagenesis and cancer: mechanisms and consequences of localized, complex chromosomal rearrangements. Nat Med. 2012;18(11):1630–8.
Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144(1):27–40.
Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153(3):666–77.
Liu P, Erez A, Nagamani SC, Dhar SU, Kolodziejska KE, Dharmadhikari AV, et al. Chromosome catastrophes involve replication mechanisms generating complex genomic rearrangements. Cell. 2011;146(6):889–903.
Lee JA, Carvalho CM, Lupski JR. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell. 2007;131(7):1235–47.
Hastings PJ, Ira G, Lupski JR. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet. 2009;5(1):e1000327.
Chiang C, Jacobsen JC, Ernst C, Hanscom C, Heilbut A, Blumenthal I, et al. Complex reorganization and predominant non-homologous repair following chromosomal breakage in karyotypically balanced germline rearrangements and transgenic integration. Nat Genet. 2012;44(4):390–7, S1.
Gamba BF, Richieri-Costa A, Costa S, Rosenberg C, Ribeiro-Bicudo LA. Chromothripsis with at least 12 breaks at 1p36.33-p35.3 in a boy with multiple congenital anomalies. Mol Gen Genomics. 2015;290(6):2213–6.
Fontana P, Genesio R, Casertano A, Cappuccio G, Mormile A, Nitsch L, et al. Loeys-Dietz syndrome type 4, caused by chromothripsis, involving the TGFB2 gene. Gene. 2014;538(1):69–73.
Kloosterman WP, Guryev V, van Roosmalen M, Duran KJ, de Bruijn E, Bakker SC, et al. Chromothripsis as a mechanism driving complex de novo structural rearrangements in the germline. Hum Mol Genet. 2011;20(10):1916–24.
Kloosterman WP, Tavakoli-Yaraki M, van Roosmalen MJ, van Binsbergen E, Renkens I, Duran K, et al. Constitutional chromothripsis rearrangements involve clustered double-stranded DNA breaks and nonhomologous repair mechanisms. Cell Rep. 2012;1(6):648–55.
Weckselblatt B, Hermetz KE, Rudd MK. Unbalanced translocations arise from diverse mutational mechanisms including chromothripsis. Genome Res. 2015;25(7):937–47.
Suzuki E, Shima H, Toki M, Hanew K, Matsubara K, Kurahashi H, et al. Complex X-chromosomal rearrangements in two women with ovarian dysfunction: implications of chromothripsis/chromoanasynthesis-dependent and -independent origins of complex genomic alterations. Cytogenet Genome Res. 2016;150(2):86–92.
Kern MJ, Argao EA, Birkenmeier EH, Rowe LB, Potter SS. Genomic organization and chromosome localization of the murine homeobox gene Pmx. Genomics. 1994;19(2):334–40.
Celik T, Simsek PO, Sozen T, Ozyuncu O, Utine GE, Talim B, et al. PRRX1 is mutated in an otocephalic newborn infant conceived by consanguineous parents. Clin Genet. 2012;81(3):294–7.
Faye-Petersen O, David E, Rangwala N, Seaman JP, Hua Z, Heller DS. Otocephaly: report of five new cases and a literature review. Fetal Pediatr Pathol. 2006;25(5):277–96.
Gregory SG, Barlow KF, McLay KE, Kaul R, Swarbreck D, Dunham A, et al. The DNA sequence and biological annotation of human chromosome 1. Nature. 2006;441(7091):315–21.
Neidhardt J, Fehr S, Kutsche M, Lohler J, Schachner M. Tenascin-N: characterization of a novel member of the tenascin family that mediates neurite repulsion from hippocampal explants. Mol Cell Neurosci. 2003;23(2):193–209.
Han YY, Zhao LJ, Lin Y, He H, Tian Q, Zhu W, et al. Multiple analyses indicate the specific association of NR1I3, C6 and TNN with low hip BMD risk. J Genet Genomics. 2017;44(6):327–30.
Evila A, Arumilli M, Udd B, Hackman P. Targeted next-generation sequencing assay for detection of mutations in primary myopathies. Neuromuscul Disord. 2016;26(1):7–15.
Jurka J. Repbase update: a database and an electronic journal of repetitive elements. Trends Genet. 2000;16(9):418–20.
George CM, Alani E. Multiple cellular mechanisms prevent chromosomal rearrangements involving repetitive DNA. Crit Rev Biochem Mol Biol. 2012;47(3):297–313.
Weckselblatt B, Rudd MK. Human structural variation: mechanisms of chromosome rearrangements. Trends Genet. 2015;31(10):587–99.
Feng Q, Moran JV, Kazazian HH Jr, Boeke JD. Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell. 1996;87(5):905–16.
Mathias SL, Scott AF, Kazazian HH Jr, Boeke JD, Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991;254(5039):1808–10.
Moran JV, Holmes SE, Naas TP, DeBerardinis RJ, Boeke JD, Kazazian HH Jr. High frequency retrotransposition in cultured mammalian cells. Cell. 1996;87(5):917–27.
Kazazian HH Jr. Mobile elements: drivers of genome evolution. Science. 2004;303(5664):1626–32.
Kajikawa M, Okada N. LINEs mobilize SINEs in the eel through a shared 3′ sequence. Cell. 2002;111(3):433–44.
Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet. 2003;35(1):41–8.
Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet. 2002;3(5):370–9.
Sen SK, Han K, Wang J, Lee J, Wang H, Callinan PA, et al. Human genomic deletions mediated by recombination between Alu elements. Am J Hum Genet. 2006;79(1):41–53.
Lee J, Han K, Meyer TJ, Kim HS, Batzer MA. Chromosomal inversions between human and chimpanzee lineages caused by retrotransposons. PLoS One. 2008;3(12):e4047.
Deininger PL, Batzer MA. Alu repeats and human disease. Mol Genet Metab. 1999;67(3):183–93.
Bailey JA, Liu G, Eichler EE. An Alu transposition model for the origin and expansion of human segmental duplications. Am J Hum Genet. 2003;73(4):823–34.
Nazaryan-Petersen L, Bertelsen B, Bak M, Jonson L, Tommerup N, Hancks DC, et al. Germline Chromothripsis driven by L1-mediated Retrotransposition and Alu/Alu homologous recombination. Hum Mutat. 2016;37(4):385–95.
Acknowledgements
The authors greatly thank the cytogenetics technologists for their participation in this project.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MG and EW performed cytogenetic and Genome-wide SNP-microarray studies. YZ wrote the manuscript. NH, BP, MW performed mate pair next-generation sequencing experiments. CG and NG managed the patient and critically revised the paper. All authors revised, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the ethical standard of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Informed verbal consent for publication was obtained from the patient’s parents.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gudipati, M.A., Waters, E., Greene, C. et al. Stable transmission of complex chromosomal rearrangements involving chromosome 1q derived from constitutional chromoanagenesis. Mol Cytogenet 12, 43 (2019). https://doi.org/10.1186/s13039-019-0455-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13039-019-0455-z