Abstract
Congenital portosystemic shunts in foals are rare and only a small number of cases have been described. Detailed description of the course of the shunt is lacking in earlier reports. This is the first detailed description of a computed tomography angiography (CTA) displaying an extra-hepatic splenocaval shunt. A 1-month old colt showing increasing signs of dullness, ataxia, circling, lip-smacking and coordination problems was presented. Hyperammonemia was detected and abdominal CTA revealed an extra-hepatic portocaval shunt. During surgery, ligation of the abnormal vessel could not be achieved, and the foal was euthanized because of complications during surgery. CTA provided a detailed overview of portal vasculature. If a portosystemic shunt is suspected in a foal, CTA can be used to confirm the diagnosis and for surgical planning.
Similar content being viewed by others
Background
A portosystemic shunt is an abnormal vessel between the portal venous system and the systemic circulation, bypassing the liver [1, 2]. Congenital portosystemic shunts can be intra- or extrahepatic. Congenital portosystemic shunts have frequently been reported in small animals [1, 2] but are considered rare in foals. In the last 25 years only four cases of portosystemic shunts in foals have been described [3,4,5]. In two of those cases the shunt was extrahepatic. These foals presented with dullness, intermittent neurological signs, hypersalivation, blindness and disorientation. Hyperammonemia was found in all cases, which makes hepatic encephalopathy a likely cause of the clinical signs and imaging of the portal vein was attempted. The imaging techniques used to explore the course of the shunt included ultrasonography, positive contrast portography and computed tomography angiography (CTA) (Table 1). Unfortunately, the description of the course of the shunts is limited in these reports. CTA was only used in one case of an intrahepatic shunt [4]. This is the first detailed description of the course of an extra-hepatic splenocaval shunt explored using CTA.
Case presentation
A 1-month old Dutch Warmblood colt was presented to the Equine Clinic at Utrecht University (NL) during emergency hours with episodic neurological signs. The neurological signs were first noted after the first day at pasture, at 1 week of age, and included mild ataxia, circling, lip-smacking and biting at the right carpus. During this first visit to the clinic intermittent very mild, but similar, abnormalities were observed at general and neurological clinical examination. Blood chemistry and hematology on admission revealed mild leucocytosis (12.8 × 109/L, reference range 4.7–10.0), an increased lactate concentration (4.2 mmol/L, reference range 0.7–1.2) and low gamma-immunoglobulins (2.7 g/L, reference range 6–19). Trauma could not be excluded and the foal was treated with equine hyperimmune plasma (Hypermune),Footnote 1 flunixin meglumine (Finadyne)Footnote 2 and vitamin E (Equi-vitamin E).Footnote 3 The neurological signs resolved during the initial few hours of hospitalisation and the foal was discharged from the clinic after 48 h, appearing bright and alert, but less lively than might be expected given his age.
Episodic neurological signs were noted by the owner during the following weeks and became more severe, when present. Upon the second admittance to the clinic, 4 weeks later, the foal was dull and demonstrated ataxia, bruxism, circling, kept his head low and appeared to be blind. He had trouble localizing the udder to drink and there were severe bite marks on the teats of the dam. Clinical findings that were noted on repeated neurological examination included intermittent dullness, hypermetria alternated with dysmetria of all four limbs, intermittent bilateral horizontal nystagmus, ptosis, variable presence of the menace response and delayed postural reactions. Clinical signs were suspicious of a metabolic disorder based on the variability of severity, occurrence and suspected origin of deficits. Further diagnostics revealed hyperammonemia (117 µmol/L, reference range 11–55), increased bile acids (53 μmol/L, reference range 1–8.6), unconjugated bilirubinemia (168 μmol/L, reference < 35), and leucocytosis (14.3 × 109/L, reference range 4.7–10). A congenital portosystemic shunt was suspected.
CTA of the foal’s abdomen was performed to confirm the presence and determine the location of the portosystemic shunt. The foal was premedicated with 0.2 mg fentanyl (Fentadon)Footnote 4 and 10 mg midazolam (Midazolam Actavis)Footnote 5 IV, and anesthesia was induced with 350 mg propofol (Propofol)Footnote 6 IV. The foal was intubated and general anesthesia maintained by isoflurane inhalation (ET isoflurane 1.2%). The animal was positioned in dorsal recumbency on the patient table of a 64-slice CT Scanner (Siemens Somatom Definition AS sliding gantry). CTA included an unenhanced scan and enhanced scans. Enhanced scans were available for the early venous phase and late venous phase. The contrast medium used in this study was iobitridol (Xenetix).Footnote 7 One minute after manual intravenous bolus injection of 100 mL of contrast medium (1 mL/kg) via the jugular vein was completed, the early venous phase was manually started and the foal was scanned from the diaphragm to the mid abdomen. A late venous phase scan of the entire abdomen was initiated 1 min after the previous scan. Scanning direction was from cranial to caudal in both scans. The following technical parameters were used: 120 kV, 340 mAs, 3 mm slices, 0.5 s tube rotation, pitch 0.9, helical acquisition. Images were reconstructed using a soft tissue algorithm (B30F) with a matrix size of 512 × 512 and reconstruction Field Of View of 442 × 442 mm.
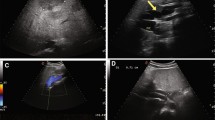
CTA images confirmed the presence of an extra-hepatic portocaval shunt. A short abnormal vessel was identified, linking the portal vein with the caudal vena cava. At the junction of the splenic vein and portal vein, an abnormal vessel arose from the portal vein. The abnormal vessel, with a diameter of 2.5 cm, looped to the left and caudally, entering the left side of the caudal vena cava, just cranial to the left renal vein (Figs. 1 and 2). The total length of the shunt was approximately 5 cm. Cranial to the shunt, a well-developed portal vein with a diameter of 1.1 cm continued, which was joined by the gastroduodenal vein and then branched into the liver. The liver appeared normal in size and shape, with a homogenous attenuation before and after contrast medium administration.
Transverse computed tomographic images of the foal with a single extrahepatic portocaval shunt. The letters (a, b, c and d) denote the right side of the patient and a to d is cranial to caudal. a Cranial to the shunt the remaining portal vein (arrow) enters the liver and the caudal vena cava (asterisk) appears normal in size, shape and position. b A broad and short abnormal vessel arises from the combined caudal and cranial mesenteric veins (arrow), at the level of the junction of the splenic vein (arrowhead) and the mesenteric veins. c The shunt (arrow) merges with the caudal vena cava (asterisk) on the left side. d Caudal to the shunt the caudal vena cava is visible (asterisk)
Illustration of the anatomy of the extrahepatic portocaval shunt. At the level of the junction of the splenic and portal vein, a shunting vessel loops to the left and caudally before merging with the caudal vena cava, just cranial to the left renal vein. 1. Caudal vena cava, 2. portal vein, 3. splenic vein, 4. caudal mesenteric veins, 5. cranial mesenteric veins, 6. splenocaval shunt, 7. gastroduodenal vein, 8. remaining portal branch to liver
Two days after confirmation of the diagnosis, surgery was initiated for partial occlusion of the shunt using a cellophane band. The foal was premedicated with 0.2 mg fentanyl (Fentadon) (see footnote 4) and 100 mg propofol (Propofol) (see footnote 6) IV, and anesthesia was induced with 300 mg propofol (Propofol) (see footnote 6) IV. The foal was intubated and maintained under general anesthesia by isoflurane inhalation (ET isoflurane 1.2%) and a constant rate infusion (CRI) of 0.25 mcg/kg/min fentanyl (Fentadon) (see footnote 4). Adequate blood pressure was maintained using dobutamine CRI (0.1–0.5 mcg/kg/min to effect, Dobutamine-hameln)Footnote 8 and norepinephrine CRI (1.5–3 mcg/kg/min to effect, Noradrenaline CF).Footnote 9 During surgery via midline celiotomy the caecum and colon were exteriorized as far as possible. The assumed portal vein and shunt were identified with great difficulty, due to their very dorsal localisation. Manual pressure on the colon, to keep it out of the surgical site, caused a small rupture of the colonic wall, with subsequent contamination of the abdomen. Given the likelihood that peritonitis would develop, the owner agreed to euthanasia.
Discussion and conclusion
Congenital splenocaval extrahepatic shunting in foals has not been described frequently and not in such detail as in this case report. In dogs and cats portocaval shunts are more common and a hereditary cause is suspected in several breeds [6]. The morphology of different portocaval shunts in dogs and cats has been described in detail, and has led to a subdivision of portocaval shunts involving the splenic, left gastric, right gastric or colic vein [7,8,9,10]. According to this classification, the course of the abnormal vessel can be considered a case of splenocaval shunting.
Woodford et al. [3] described the course of an extra-hepatic shunt in a foal, determined using ultrasonography and intra-operative fluoroscopy. Similar to the current case, this shunt coursed caudally and was located in the region of the renal veins. However, the shunt described by Woodford et al. curved to the right instead of to the left as in the present case. The other published description of an extra-hepatic shunt in a foal was considered too limited to enable comparison with the current case [5].
A few of the previously described portosystemic shunts in foals were imaged by ultrasound or positive contrast portography (Table 1). In the current case no attempt was made to identify the shunt by ultrasound as CTA is considered superior for the detection of vascular anomalies and surgical planning [7,8,9,10]. Cross-sectional imaging with CTA provided a clear visualisation of the portosystemic shunt. Also, no attempt was made to visualize the shunt by ultrasound during surgery, since surrounding intestines, filled with gas and feces would have prevented the acquisition of clear images using this modality.
CTA of the portal system is routinely used to confirm a diagnosis of portosystemic shunts in dogs and cats. Different multi-phase computed tomography (CT) protocols have been described to investigate the portal system in companion animals [7,8,9,10,11,12,13,14]. Including the entire abdomen in the scan is recommended to allow identification of acquired extrahepatic shunts and to follow tortuous vessels [11]. In the current case, manual bolus injection of the contrast medium was chosen for practical reasons. Arterial and portal phase were not performed as described in multi-phase CTA in companion animals [7,8,9,10,11,12,13,14]. The timing of all phases was considered to be impossible at that moment because of size related scan duration and necessity for breath hold. Instead, manually started unenhanced, early venous phase and late venous phase scans were performed and provided detailed angiography of the portal veins and its tributaries.
A limitation of this study was the extent of the early venous phase of the CT scan. In this enhanced scan, the area from the diaphragm to the level of the middle of the right kidney was included. As the shunt joined the caudal vena cava caudal to the right renal vein, visualization of the shunt during the early venous phase scan was incomplete. The late venous phase scan did include the entire abdomen, but enhancement of vasculature was reduced compared to the early venous phase scan. Therefore, CTA of portosystemic shunts in foals should be performed of the entire abdomen or at least with inclusion of the kidneys, as portocaval shunts appear to occur in this area and therefore more caudally located than most types of small animal extra-hepatic portocaval shunts [7,8,9,10,11,12,13,14].
This is the first detailed description of CTA performed in a horse with an extra-hepatic splenocaval shunt. CTA provided a detailed overview of the abdominal and portal vasculature. When a portosystemic shunt is suspected in a foal, CTA represents a feasible technique for confirming the diagnosis and to allow surgical planning.
Availability of data and materials
All relevant data generated or analyzed during this study are included in this article and its supplementary information files.
Notes
Equine hyperimmune plasma—Hypermune—Veterinary Immunogenics Ltd, Penrith, Cumbria, England.
Flunixin Meglumine 50 mg/g oral paste—Finadyne—Intervet, Boxmeer, The Netherlands.
Vitamin E 300 g—Equi-vitamin E—Equipharma—Ecuphar, Oostkamp, Belgium.
Fentanyl 0.05 mg/mL—Fentadon—Eurovet Animal Health BV, Bladel, The Netherlands.
Midazolam 5 mg/mL—Midazolam Actavis—Actavis Group PTC, Hafnarfjordur, Iceland.
Propofol 10 mg/mL—Propofol—Fresenius Kabi, Zeist, The Netherlands.
Xenetix 350 mg/mL—Iobitridiol—Guerbet Nederland BV, Gorinchem, The Netherlands.
Dobutamine 5 mg/mL—Dobutamine-hameln—Hameln pharma plus GmbH, Hameld, Germany.
Norepinephrine 1 mg/mL—Noradrenaline CF—Centrafarm BV, Etten-Leur, The Netherlands.
Abbreviations
- CRI:
-
constant rate infusion
- CT:
-
computed tomography
- CTA:
-
computed tomography angiography
- IV:
-
intravenous
References
Santilli RA, Gerboni G. Diagnostic imaging of congenital porto-systemic shunts in dogs and cats: a review. Vet J. 2003;166:7–18.
Hunt GB. Effect of breed on anatomy of portosystemic shunts resulting from congenital diseases in dogs and cats: a review of 242 cases. Aust Vet J. 2004;82:746–9.
Woodford NS, Hotson Moore A, Renfrew H, Tulloch L, Casey M. Surgical management of an extrahepatic portosystemic shunt in a foal: a multidisciplinary problem. Equine Vet Educ. 2017;29:243–8.
Hug SA, Guerrero TG, Makara M, Kummer M, Grest P, Bettschart R, et al. Diagnosis and surgical cellophane banding of an intrahepatic congenital portosystemic shunt in a foal. J Vet Intern Med. 2012;26:171–7.
Fortier LA, Fubini SL, Flanders JA, Divers TJ. The diagnosis and surgical correction of congenital portosystemic vascular anomalies in two calves and two foals. Vet Surg. 1996;25:154–60.
Levy JK, Bunch SE, Komtebedde J. Portosystemic shunts. In: Bonagura JD, Twedt DC, editors. Kirk’s current veterinary therapy XIV, small animal practice. Philadelphia: W.B. Saunders; 1995. p. 581–6.
White RN, Parry AT. Morphology of congenital portosystemic shunts involving the right gastric vein in dogs and cats. J Small Anim Pract. 2015;56:430–40.
White RN, Parry AT. Morphology of congenital portosystemic shunts emanating from the left gastric vein in dogs and cats. J Small Anim Pract. 2013;54:459–67.
White RN, Parry AT. Morphology of congenital portosystemic shunts involving the left colic vein in dogs and cats. J Small Anim Pract. 2016;57:247–54.
White RN, Parry AT. Morphology of splenocaval congenital portosystemic shunts in dogs and cats. J Small Anim Pract. 2016;57:28–32.
Zwingenberger A. CT diagnosis of portosystemic shunts. The veterinary clinics of North America. Small Anim Pract. 2009;39:783–92.
Frank P, Mahaffey M, Egger C, Cornell KK. Helical computed tomographic portography in ten normal dogs and ten dogs with a portosystemic shunt. Vet Radiol Ultrasound. 2003;44:392–400.
Or M, Ishigaki K, de Rooster H, Kutara K, Asano K. Determination of Porto-Azygos shunt anatomy in dogs by computed tomography angiography. Vet Surg. 2016;45:1005–12.
Zwingenberger AL, Schwarz T, Saunders HM. Helical computed tomographic angiography of canine portosystemic shunts. Vet Radiol Ultrasound. 2005;46:27–32.
Acknowledgements
We are grateful to Bianca Desirée Willems for digitizing and editing Fig. 2.
Prior publication
Data have not been published previously.
Funding
The authors declare that there were no funding and support.
Author information
Authors and Affiliations
Contributions
LCK and IDW performed clinical examination and work-up of the foal. JE and AK performed surgery. DSW and SV performed the diagnostic imaging. DSW, LCK and SV drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since this study concerned the treatment of a patient with clinical signs it did not require official or institutional ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Willems, D.S., Kranenburg, L.C., Ensink, J.M. et al. Computed tomography angiography of a congenital extrahepatic splenocaval shunt in a foal. Acta Vet Scand 61, 39 (2019). https://doi.org/10.1186/s13028-019-0474-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13028-019-0474-0