Abstract
Background
Although bronchiectasis has been recognised as a feature of some patients with Alpha1-Antitrypsin deficiency the prevalence and characteristics are not widely known. We wished to determine the prevalence of bronchiectasis and patient characteristics. The first cohort of patients recruited to the EARCO (European Alpha1 Research Collaboration) International Registry data base by the end of 2021 was analysed for radiological evidence of both emphysema and bronchiectasis as well as baseline demographic features.
Results
Of the first 505 patients with the PiZZ genotype entered into the data base 418 (82.8%) had a reported CT scan. There were 77 (18.4%) with a normal scan and 38 (9.1%) with bronchiectasis alone. These 2 groups were predominantly female never smokers and had lung function in the normal range. The remaining 303 (72.5%) ZZ patients all had emphysema on the scan and 113 (27%) had additional evidence of bronchiectasis.
Conclusions
The data indicates the bronchiectasis alone is a feature of 9.1% of patients with the PiZZ genotype of Alpha1-antitrypsin deficiency but although emphysema is the dominant lung pathology bronchiectasis is also present in 27% of emphysema cases and may require a different treatment strategy.
Similar content being viewed by others
Background
Alpha-1 antitrypsin (AAT) is a polyvalent protein with many putative functions [1] but thought to be primarily an irreversible inhibitor of neutrophil serine proteinases [2]. These enzymes particularly neutrophil elastase (NE) and proteinase 3 (PR3), have been shown to induce emphysematous and airway changes similar to features of chronic obstructive pulmonary disease (COPD) when instilled into the lungs of experimental animals [2].
Initial studies reported by Eriksson [3] using trypsin inhibitory capacity as the marker of AAT deficiency demonstrated that individuals and family members had clinical features varying from none to severe early onset emphysema and bronchiectasis (Bx). This resulted in widespread testing and reporting of individuals especially those with severe early onset basal panlobular emphysema, sporadic case reports [4] and analysed cohorts [5,6,7,8] of those with Bx.
Many patients with COPD have significant bacterial colonisation of the airways and a corresponding neutrophilic load characterised by purulent (elastase positive) secretions [9, 10]. Radiologically these patients often have Bx [10] which lead to the concept that the increased neutrophilic load and hence excess local NE release could damage airways, impair host defences and facilitate bacterial colonisation [11] leading to a self- perpetuating cycle of events [12]. It therefore seems logical that AAT deficiency itself would add another amplifying factor to the pathophysiology of Bx. Indeed in COPD, AAT deficiency is associated with greater airways inflammation (particularly during exacerbations) and detectable active NE even in the stable state [13]; AAT augmentation abrogates this increased inflammation [14].
However, a recent publication has suggested that routine testing of Bx patients for AAT deficiency is not recommended as it is rarely fruitful (< 10%) and does not influence management [15]. This has been questioned [16] as the prevalence of deficiency in such cohorts is clearly higher than expected in the UK indigenous general population [17] and indeed may influence current and future management as systemic AAT augmentation abrogates the airways inflammatory process in AATD [14] and hence excessive putative local airway damage.
The recent establishment of EARCO (European Alpha1 Research Collaboration) International Registry, a deep phenotyping data base of AAT deficiency patients sponsored by the European Respiratory Society (ERS) enables us to estimate the prevalence of Bx in AAT deficiency as a baseline to understanding its nature, impact and management. The current article presents the initial findings of patients recruited to EARCO up to December 2021 [18].
Methods
Recruitment
Patients with AAT deficiency were recruited to the ongoing international multicentre observational study to document the natural history of AATD and the impact of augmentation therapy (EARCO study, IRAS ID: 265,728, www.clinicaltrials.gov (ID: NCT04180319)). This clinical research collaboration of the ERS has previously been described in detail by Greulich et al. [19].
Briefly patients over the age of 18 gave written informed consent for their clinical data to be collected during routine assessments at secondary care sites in Belgium, Czech Republic, Croatia, Estonia, Italy, The Netherlands, Poland, Portugal, Romania, Spain, Sweden, Switzerland, Turkey and United Kingdom. All subjects with confirmed AAT deficiency, as defined by serum AAT levels of less than 11microM (50 mg/dl), and/or proteinase inhibitor genotypes ZZ, SZ and heterozygotes or homozygotes for other rare deficient variants were eligible for inclusion in EARCO. Data included extensive baseline demographics as reported recently [19]. For the current study the data base up to December 2021 was searched for all patients with a PiZZ genotype who had undergone a reported CT densitometry scan. Patients were divided into 4 groups namely those with a reported normal scan, evidence of emphysema alone, those with Bx alone and those with reported emphysema and Bx.
Assessment of CT scans
Bronchiectatic change (namely tubular, cystic or varicose) and the presence of emphysema were reported together with the distribution as either upper or lower zone dominant or widespread. Baseline CT scan data was used to compare prevalence and type of bronchiectasis seen in PiZZ both with and without CT confirmed emphysema.
Statistical analysis
Patient data for the 4 radiological groups outlined above were analysed using Mann-Whitney U test for FEV1, gas transfer and other quantitative variables. Categorical data were analysed using Chi-squared test and p values < 0.05 were accepted as statistically different.
Results
Data was collected from of the first 860 individual patients recruited to the EARCO registry up to December 2021, of whom 505 had a confirmed PiZZ genotype and 418 (82.8%) of these had a reported CT scan. The baseline characteristics of the whole cohort of PiZZ subjects and the 418 with a CT scan report is shown in Table 1. The majority of patients were never or ex-smokers at the time of recruitment with only 1.2% currently smoking at baseline. Normal scans were described for 77 patients (18.4%). Bx alone was reported on CT scan for 38 PiZZ patients (9.1%) and emphysema alone was reported for 190 patients (45.5%) but was also present with Bx for 113 patients (27%). Overall 303 patients had emphysema reported on their CT scan (72.5%) and of these 39.6% were described as lower zone in distribution, 21.8% upper zone and 38.6% widespread. The bronchiectasis was not characterised in 42% of those with Bx alone or when present with emphysema. It was described as tubular when present alone (36.8%) or with emphysema (36.6%) and cystic (13.2% and 13.6%) alone or with emphysema respectively. When not generalised it was reported as distributed mainly in the lower zones, 43.4% when present alone and 54.5% of those with emphysema, consistent with the archetypal distribution of emphysema in AATD.
Of the 87 patients in the PiZZ group who had no CT scan report the demographics were similar to the group as a whole with 59.8% male a mean age of 47.8 years (SD = 16.0). Most (52.9%) were never smokers and 47.1% ex-smokers with a mean pack year history of 16.0 (SD = 16.5). Of these 87 patients the average FEV1 was 88.3% (SD 31.2). Kco was available for 54 of the 87 patients with a mean value of 76.7% predicted (SD 20.9). The baseline characteristics of those with CT scan reports were similar to the total PiZZ cohort (Table 1).
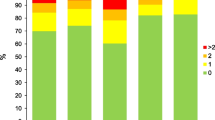
The four groups of PiZZ subjects defined by the CT findings are summarised in Table 2. There were some demographic differences between the 4 groups. Those with a normal scan were predominantly female, mainly never smokers and younger with generally near normal lung function compared to the groups with emphysema (p < 0.001). The demographics for this “normal” group was similar to the group of patients who had Bx alone (Table 2). The patients with emphysema were older on average (p < 0.001) with a slight male preponderance (p = 0.09), consisted of fewer never smokers and had a greater smoking history compared to those with a normal scan or Bx alone (p < 0.001). In addition, these 2 emphysema groups (with and without Bx) had moderate airflow obstruction and significantly reduced gas transfer as expected (p < 0.001 compared to those with normal scans or Bx alone). The patients with emphysema and Bx were more likely to be male (p = 0.021), smokers (p < 0.001) and with reduced lung function compared to those with Bx alone (p < 0.001 all measures). The average lung function data corrected for age sex and height for the 4 groups is summarised in Figs. 1 and 2.
Prevalence in patients with the SZ genotype
The milder SZ genotype is not considered an at risk variant for COPD in the absence of heavy smoking. However similar data was documented within the EARCO database for a number of such patients (n = 235) of whom 62.1% also had a CT scan. Of these 41.8% were reported as normal, 11.6% had bronchiectatic changes alone, 32.2% emphysema alone and 14.4% both Bx and Emphysema. These patients had a higher smoking history than the ZZ cohort (overall 30.55 pack years; SD 27.6) and predominantly normal spirometry (see Table 3).
Discussion
The baseline data from this initial analysis of the first 860 patients uploaded to the EARCO data base identified 505 PiZZ patients of whom 418 had undergone CT scanning as part of their disease characterisation and of these 38 had evidence of Bx alone which is similar to the prevalence found by Eriksson [3] in his initial 23 patient cohort analysis. Interestingly the small sub cohort reported here was predominantly female and with little smoking history (which may partly explain the lack of emphysema) and predominantly normal lung function. This sex and smoking difference is reminiscent of historical studies where smoking men with the same symptoms but airflow obstruction were often assumed to have COPD and investigated no further, whereas non-smoking females underwent more extensive investigations including radiographic bronchograms. It was often mooted that this reflected a selection bias dependant on investigation whereas here that bias seems unlikely.
Clearly Bx alone is a feature of a proportion of patients with PiZZ AATD and is similar to the prevalence described by Carreto et al. in specialist Bx clinics [15] which suggests that continued testing in Bx for this associated genetic defect should still be continued. Whether this leads to different management to non-deficient Bx patients requires much more individual characterisation including bacterial colonisation, airways neutrophilia (and serine proteinase activity), as well as exacerbation history. Whereas this is important in all patients with exacerbations the presence of AATD will likely have an increased inflammatory load [12] amenable (at least in part) to AAT augmentation intravenously [13], by the inhaled route [20] or with more recent oral antiproteinase strategies in development [21].
However with the advent of CT scanning as a non-invasive GOLD standard more cases of Bx are being identified with up to 30% of COPD patients having both emphysema and Bx [22] suggesting it is a significant comorbidity especially in severity subgroups [23]. In the current study 113 (27%) of PiZZ patients had Bx associated with emphysema which is also similar to the prevalence of easily visible changes described by Parr et al. in a smaller but highly characterised cohort [15], although the prevalence of minor changes that fulfilled the Naidich criteria for Bx [24] was almost universal. In non-deficient COPD this association influences both mortality [25] and recurrent exacerbations [26]. Indeed early studies of COPD patients with productive purulent sputum suggesting significant bacterial colonisation [9] is associated with a high prevalence of Bx [10]. On this basis it has been suggested that such COPD patients with Bx should be considered for additional treatment strategies as for patients with Bx alone [22] Whether these features are also true of a significant proportion of AATD patients remains to be determined but it is tempting to speculate that (as in COPD) such patients have increased inflammation and may benefit from antiproteinase therapy and specifically Cathepsin C inhibitors [27] that may reduce both the inflammation and proteinase load in Bx but also influence the proteinase dependant emphysema in AATD patients.
The increasing recognition of the associations of Bx with COPD has raised the concept that this represents a treatable trait and the arguments above provide a strategy in both non deficient and AAT deficient patients. More recently a workshop has described the ROSE criteria [28] for the key components to determine the implications of the combination and its verification. As this recent work was not published when EARCO was established the essential components of the ROSE score were not mandatory in the data base and much of the features of ROSE have not been systematically collected to date. Also, COPD related to AATD (although highly susceptible to smoking which is a key component of the ROSE score) can also develop in never smokers and hence a “ROSE score” may require further adjustment for this component. Clearly this association and score needs further exploration in AATD as well as non-deficient COPD as mentioned by the authors [28].
The current study has the strengths of being an in depth characterisation of AAT deficient patients across many specialist groups and countries and is therefore reflective of the current patient population. It emphasises that many patients do not fulfil the archetypical AATD patient with basal panlobular emphysema, supporting widespread AAT testing in all patients with COPD and those who present with Bx alone. However it does have some weaknesses. In particular, not all patients had CT scans at baseline (although those who did not, had similar baseline characteristics of those who did). In addition data on airway colonisation, and nature and frequency of exacerbations are not mandatory fields on the EARCO data base, leading to missing data. The nature and distribution of emphysema as well as characteristics of the Bx are requested within EARCO, but again not mandatory, and should also be described to strict criteria as part of subsequent retrospective and prospective analyses to complete understanding of this combination phenotype and its management. Finally, tests to confirm that other causes of Bx had been excluded were rarely formally reported in the EARCO database, although sites confirmed that it was routine practice to screen for immunodeficiency, take a detailed history of past infections and consider autoimmune or other familial causes.
The current retrospective analysis has concentrated on those with the recognised “at risk” PiZZ genotype. Although EARCO includes other rarer severe deficiency genotypes the numbers are currently too few for meaningful comparisons with the core ZZ cohort.
The milder SZ genotype is not considered “at risk” for COPD in the absence of heavy smoking [29] and currently not recommended for augmentation therapy. In the EARCO data base of the 235 SZ patients documented the cohort had a higher smoking history but a greater proportion of normal scans. and predominantly normal spirometry. Bronchiectasis alone and Emphysema with and without bronchiectasis was also observed.
The reasons for testing these patients for AAT genotype (although permitted for the EARCO data base) is currently unknown but it is clearly important to determine any influence of acquisition bias in a prospective study to explore the nature and impact of these findings in the SZ genotype.
Conclusions
In conclusion AAT deficiency is associated with Bx alone in up to 10% of PiZZ individuals and 27% of those with emphysema. This should become a routine part of patients’ assessment and become a feature of in depth future clinical study and management. In particular this should include microbiology the airways inflammation compared to non-deficient COPD as well as the impact on progression and health status and the need for, and effect of antiproteinase therapy.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to individual patient privacy and lack of consent but are available from the corresponding author on reasonable request.
Abbreviations
- AAT:
-
Alpha-1 antitrypsin
- NE:
-
Neutrophil elastase
- PR3:
-
Proteinase 3
- COPD:
-
Chronic obstructive pulmonary disease
- Bx:
-
Bronchiectasis
- AATD:
-
Alpha-1 antitrypsin deficiency
- EARCO:
-
European Alpha1 Research Collaboration) International Registry
- ERS:
-
European Respiratory Society
References
Stockley RA. α1-antitrypsin: a polyfunctional protein? Lancet Resp Med. 2015;3(5):341–3.
Crisford H, Sapey E, Stockley RA. Proteinase 3; a potential target in chronic obstructive pulmonary disease and other chronic inflammatory disease. Respir Res. 2018;19:180.
Eriksson S. Pulmonary emphysema and alpha-1 antitrypsin deficiency. Acta Med Scand. 1964;175:197–205.
Guest PJ, Hansell DM. High resolution computed tomography (HRCT) in emphysema associated with a1-antitrypsin deficiency. Clin Radiol. 1992;45:260–6.
Parr DG, Guest PG, Reynolds JH, Dowson LJ, Stockley RA. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2007;15(12):1215–21.
Eden E, Choate R, Barker A, Addrizzo-Harris D, Aksamit TR, Daley CL, Daniles MLA, DiMaggio A, Fennelly K, Griffith DE, Johnson MM, Knowles MR, Metersky ML, Noone PG, O’Donnell AE, Olivier KN, Salathe MA, Schmid A, Thomashow B, Tino G, Turino GM, Winthrop KL. The clinical features of bronchiectasis associated with alpha-1 antitrypsin deficiency, common variable immunodeficiency and primary ciliary dyskinesia – results from the U.S. Bronchiectasis Research Registry. Chronic Obstr Pulm Dis. 2019;6:145–53.
Cuvelier A, Muir JF, Hellot MF, Benhamou D, Martin JP, Benichou J, Sesboue R. Distribution of alpha(1)-antitrypsin alleles in patients with bronchiectasis. Chest. 2000;117:415–9.
Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, Polverino E, Van de kerkhove C, Rutherford R, Davison J, Rosales E, Pesci A, Restrepo MI, Torres A, Aliberti S. Etiology of non–cystic fibrosis bronchiectasis in adults and its correlation to Disease Severity. Ann Am Thorac Soc. 2015;12(12):1764–70.
Hill AT, Campbell EJ, Hill SL, Bayley D, Stockley RA. Association between airway bacterial load and markers of inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–95.
Cooke JC, Currie DC, Morgan AD, Kerr IH, Delany D, Strickland B, Cole PJ. Role of computed tomography in diagnosis of bronchiectasis. Thorax. Apr; 1987;42(4):272–7.
Whitters D, Stockley RA. Immunity and bacterial colonization in bronchiectasis. Thorax. 2012;67:1006–13.
Stockley RA. Bronchiectasis. Medicine. 1999;27(10):113–6.
Hill AT, Bayley DL, Campbell EJ, Hill SL, Stockley RA. Airways inflammation in chronic bronchitis: the effects of smoking and α1-antitrypsin deficiency. Eur Respir J. 2000;15:886–90.
Stockley RA, Bayley DL, Unsal I, Dowson L. The effect of augmentation therapy on bronchial inflammation in α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2002;165:1494–8.
Carreto L, Morrison M, Donovan J, Finch S, Tan GL, Fardon T, Wilson R, Furrie E, Loebinger M, Chalmers JD. Utility of routine screening for alpha-1 antitrypsin deficiency in patients with bronchiectasis. Thorax. 2020;75(7):592–3.
https://doi.org/10.1136/thoraxjnl-2019-214195. Rapid response.
De Serres FJ, Blanco I. Prevalence of α1-antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis. 2012;6(5):277–95.
Miravitlles M, Turner AM, Torres-Duran M, Tanash H, Rodríguez-García C, López-Campos JL, Chlumsky J, Guimaraes C, Rodríguez-Hermosa JL, Corsico A, Martinez-González C, Hernández-Pérez JM, Bustamante A, Parr DG, Casas-Maldonado F, Hecimovic A, Janssens W, Lara B, Barrecheguren M, González C, Stolk J, Esquinas C, Clarenbach CF. Clinical and functional characteristics of individuals with alpha-1 antitrypsin deficiency: EARCO international registry. Respir Res. 2022;16(1):352.
Greulich T, Altraja A, Barrecheguren M, Bals R, Chlumsky J, Chorostowska-Wynimko J, Clarenbach C, Corda L, Corsico AG, Ferrarotti I, Esquinas C, Gouder C, Hećimović A, Ilic A, Ivanov Y, Janciauskiene S, Janssens W, Kohle MR, Krams A, Lara B, Mahadeva R, McElvaney G, Mornex J-F, O’Hara K, Parr D, Piitulainen E, Schmid-Scherzer K, Seersholm N, Stockley RA, Stolk J, Sucena M, Tanash H, Turner A, Ulmeanu R, Wilkens M, Yorgancioğlu A, Zaharie A, Miravitlles M, on behalf of the EARCO Clinical Research Collaboration. Protocol for the EARCO Registry: a pan-european observational study in patients with α1-antitrypsin deficiency. ERJ Open Res. 2020;6(1):00181–2019.
Stolk J, Tov N, Chapman KR, Fernandez P, MacNee W, Hopkinson NS, Piitulainen E, Seersholm N, Vogelmeier CF, Bals R, McElvaney G, Stockley RA. Efficacy and safety of inhaled α1-antitrypsin in patients with severe α1-antitrypsin deficiency and frequent exacerbations of COPD. Eur Respir J. 2019;21(5):1900673.
Pye A, Turner AM. Experimental and investigational drugs for the treatment of alpha-1 antitrypsin deficiency. Expert Opin Investig Drugs. 2019;28(10):891–902.
Whitters D, Stockley RA. Bronchiectasis in older patients with chronic obstructive pulmonary disease: prevalence, diagnosis and therapeutic management. Drugs Aging. 2013;30(4):215–25.
Martínez-García MA, Miravitlles M. Bronchiectasis in COPD patients. More than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–11.
Naidich DP. High-resolution computed tomography of cystic lung disease. Semin Roentgenol. 1991;26:151–74.
Martínez-García MA, de la Rosa Carrillo D, Soler-Cataluña JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–31.
Patel IS, Vlahos I, Wilkinson TMA, Loyd-Owen SJ, Donaldson GC, Wilks M, Reznek RH, Wedzicha JA. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. Aug 2004;15(4):400–7.
Chalmers JD, Haworth CS, Metersky ML, Loebinger MR, Blasi F, Sibila O, O’Donnell AE, Sullivan EJ, Mange KC, Fernandez C, Zou J, Daley CL, WILLOW Investigators. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med. 2020;383(22):2127–37.
Traversi L, Miravitlles M, Martinez-Garcia MA, et al. ROSE: radiology, obstruction, symptoms and exposure – a Delphi consensus definition of the association of COPD and bronchiectasis by the EMBARC Airways Working Group. ERJ Open Res. 2021;7:00399–2021.
Franciosi AN, Hobbs BD, McElvaney OJ, Molloy K, Hersh C, Clarke L, Gunaratnam C, Silverman EK, Carroll TP, McElvaney NG. Clarifying the risk of lung disease in SZ alpha1 antitrypsin deficiency. Am J Respir Crit Care Med. 2020;202(1):73–82.
Acknowledgements
The authors would like to thank the patients who participated in this study and the EARCO study investigators (listed below) all of whom contributed > 10 patients to the EARCO data base. We wish to acknowledge Elise Heuvelin from the ERS office (Lausanne, Switzerland) for her support in the management of EARCO, and Gemma Vilagut and Christina Founti (Bioclever, Barcelona, Spain) for their support in EARCO data monitoring. List of EARCO study investigators: María Torres-Duran, Servicio de Neumología. Hospital Álvaro Cunqueiro. NeumoVigo I+i Research Group, IIS Galicia Sur, Vigo, Spain; Hanan Tanash: Department of Respiratory Medicine and Allergology, Skåne University Hospital, Lund University, Malmö, Sweden; Carlota Rodríguez-García: Servicio de Neumología, Complejo Hospitalario Clínico-Universitario de Santiago, Santiago de Compostela, Spain; José Luis López-Campos: Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES). Instituto de Salud Carlos III, Madrid, Spain. Unidad Médico-Quirúrgica de Enfermedades Respiratorias. Instituto de Biomedicina de Sevilla (IBiS). Hospital Universitario Virgen del Rocío/Universidad de Sevilla, Spain; Jan Chlumsky: Department of Pneumology, Thomayer Hospital, First Faculty of Medicine, Charles University, Prague, Czech Republic; Catarina Guimaraes: Pulmonology Department. Hospital da Senhora da Oliveira, Guimarães, Portugal; Juan Luis Rodríguez-Hermosa: Servicio de Neumología. Hospital Clínico de San Carlos. Departamento de Medicina, Facultad de Medicina, Universidad Complutense de Madrid, Madrid, Spain. Research Institute of Hospital Clínico San Carlos (IdISSC), Madrid, Spain; Angelo Corsico: Pneumology Unit, IRCCS San Matteo Hospital Foundation, Pavia, Italy.. Department of Internal Medicine and Therapeutics, University of Pavia, Pavia, Italy; Cristina Martinez-González: Pneumology Department, Hospital Universitario Central de Asturias, Instituto de Investigacion Sanitaria del Principado de Asturias. Oviedo, Spain; José María Hernández-Pérez: Pneumology Department, Hospital Universitario Nuestra Señora de La Candelaria, Santa Cruz de Tenerife, Spain; Ana Bustamante: Pneumology Section. Hospital Sierrallana-TresMares. Cantabria. Spain. ; David G. Parr: Department of Respiratory Medicine, University Hospitals of Coventry and Warwickshire, Clifford Bridge Road, Coventry, UK; Francisco Casas-Maldonado: Servicio de Neumología. Hospital Clínico Universitario San Cecilio. Departamento de Medicina, Facultad de Medicina, Universidad de Granada, Granada, Spain; Ana Hecimovic: University Hospital Center Zagreb, Clinic for Respiratory Diseases, Zagreb, Croatia. University of Zagreb, School of Medicine, Zagreb, Croatia; Wim Janssens: Katholieke Universiteit (KU) Leuven, Laboratory of Respiratory Diseases, Department of Chronic Disease, Metabolism and Ageing, Leuven, Belgium. University Hospitals Leuven, Department of Respiratory Diseases, Leuven, Belgium; Beatriz Lara: Department of Respiratory Medicine, University Hospitals of Coventry and Warwickshire, Clifford Bridge Road, Coventry, UK; Miriam Barrecheguren: Pneumology Department, Hospital Universitari Vall d'Hebron; Vall d’Hebron Institut de Recerca (VHIR), Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain; Cruz González: Servicio de Neumología. Hospital Clínico Universitario. Instituto de Investigación INCLIVA, Valencia, Spain; Jan Stolk: Department of Pulmonology, Leiden University Medical Center, Leiden, The Netherlands; Christian F. Clarenbach: Division of Pulmonology, University Hospital Zurich, Zurich, Switzerland.
Funding
The International EARCO registry is funded by unrestricted grants of Grifols, CSL Behring, Kamada, pH Pharma and Takeda to the European Respiratory Society (ERS).
Author information
Authors and Affiliations
Consortia
Contributions
RAS proposed the study and was the major author interpreting the data, writing and preparing the manuscript for publication. AP analysed the patient data and contributed to the preparation of the manuscript. JDS performed statistical analysis on the patient data. AMT is the UK lead for EARCO, contributed to data interpretation and revised the manuscript. MM is the chair of EARCO, contributed to data interpretation and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
EARCO received ethics approval in individual countries and all participants gave written informed consent for their data to be included in the EARCO database. In UK the study was approved by London-Camden and Kings Cross Research Ethics Committee, REC Ref: 19/LO/1106.
Consent for publication
Not applicable.
Competing interests
Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, CSL Behring, Inhibrx, Ferrer, Menarini, Mereo Biopharma, Spin Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi, Zambon and Grifols and research grants from Grifols. Alice M Turner has received either grants or speaker fees from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Chiesi, CSL Behring, Takeda, Vertex and Grifols Biotherapeutics. Robert A Stockley has received research grants from Mereo BioPharma and CSL Behring, consulting fees from Mereo BioPharma, CSL Behring, Vertex, Inhibrx and chairs the DSMB for Takeda.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stockley, R.A., Pye, A., De Soyza, J. et al. The prevalence of bronchiectasis in patients with alpha-1 antitrypsin deficiency: initial report of EARCO. Orphanet J Rare Dis 18, 243 (2023). https://doi.org/10.1186/s13023-023-02830-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-023-02830-2