Abstract
Background
Several retrospective studies have identified risk factors associated with ocular myasthenia gravis (OMG) generalization in non-surgical patients. However, the outcomes of OMG after thymectomy have not been investigated fully. This study aimed to explore the clinical predictors of post-thymectomy OMG prognosis.
Methods
We performed a retrospective review of OMG patients who underwent thymectomy at our institution from January 2012 to December 2021. Kaplan–Meier and Cox proportional hazard regression analyses were used to evaluate associations between clinical features and prognosis. The main outcome measures were OMG conversion, complete stable remission (CSR), and clinical improvement.
Results
Fifty-eight patients were identified for conversion analysis. Thirteen (22.4%) developed generalized myasthenia gravis (GMG) at a median time of 12.7 (3–37.3) months from symptom onset. Repetitive nerve stimulation (RNS)-positivity was associated with increased risk of conversion to GMG (P = 0.002). Patients with histotype B2/B3 thymoma showed a higher risk of conversion (P = 0.002) than did patients with hyperplasia and AB/B1 thymoma. Fifty-two patients fulfilled the criteria for CSR and improvement. Sixteen (30.8%) achieved CSR at a median time of 28.7 (15–54) months after thymectomy. Fifteen (28.8%) showed clinical improvement at last follow up. Patients who achieved CSR showed a younger age of onset (P = 0.022), lower percentage of acetylcholine receptor antibody-seropositivity (P = 0.029). Histologically, patients with thymic hyperplasia and stage I thymoma showed a higher chance of CSR (P = 0.010) than did patients with stage II/III thymoma. Multivariate analysis revealed that RNS-positivity (hazard ratio [HR] 6.007, P = 0.021) and histotype B2/B3 thymoma (HR 4.611, P = 0.048) were associated with OMG conversion. Thymic hyperplasia and stage I thymoma (HR 0.300, P = 0.026) were associated with OMG CSR after thymectomy.
Conclusion
For OMG patients after thymectomy, RNS-positivity and histotype B2/B3 thymoma are independent predictors of conversion to GMG. On the other hand, thymic hyperplasia and stage I thymoma independently predict CSR.
Similar content being viewed by others
Background
Myasthenia gravis (MG) is an autoimmune disease caused by pathogenic autoantibodies to components of the postsynaptic muscle endplate. The typical manifestation is fluctuations in severity of muscle weakness [1]. According to symptoms at disease onset, MG can be further divided into ocular MG (OMG, MGFA class I) and generalized MG (GMG, MGFA Class II–V) [2]. However, 50–65% of OMG patients will develop systemic neuromuscular weakness, indicating conversion to secondary GMG (SGMG), typically within the first 2 years [3].
Patients with pure OMG and SGMG can differ significantly in the clinical course and impairment of daily life. Several retrospective studies have identified certain risk factors associated with OMG conversion, which include sex, older age of onset [4], antibody-seropositivity [5], and the presence of other autoimmune diseases [6]. However, most of these studies recruited patients treated conservatively (with pyridostigmine or immunosuppressants), and data on OMG prognosis after thymectomy are rare. Moreover, the thymus is pathologically linked to MG. A significant correlation of thymoma histologic subtype, Masaoka stage, and MG pathogenesis has been described by Weis et al. [7]. They found that the immunopathology of types B1–B3 thymomas favour lack of self-tolerance and triggering of MG [7]. Whether these differences would translate to differences in the post-thymectomy outcomes of OMG is intriguing. Furthermore, thymomas can vary in size and location in the anterior mediastinum, but it is unknown whether these anatomical features could impact thymoma-associated OMG prognosis [8].
In view of the rarity of studies on surgery in OMG, this study aimed to address two issues pertinent to OMG patients post-thymectomy: we sought to report on long-term post-thymectomy OMG prognosis and to explore factors affecting post-thymectomy OMG outcomes.
Materials and methods
Patient enrolment and definition
We conducted a retrospective study of 82 consecutive OMG patients who underwent thymectomy at Tianjin Medical University General Hospital from January 2012 to December 2021. The diagnosis of OMG was based on typical clinical manifestations, consisting of fluctuating diplopia, ptosis, or both, and at least one positive result in the following tests: (1) Anti-AChR Ab, (2) RNS, or (3) clinical response to edrophonium chloride (Tensilon test) or pyridostigmine.
Outcome measures consisted of development of GMG, complete stable remission (CSR) and clinical improvement, time to OMG conversion (calculated from time of symptom onset) and to CSR (calculated from thymectomy).
Inclusion criteria were an onset age ≥ 18 years, a minimum of 3 months of isolated ocular disease, R0 resection for thymoma, and follow-up duration of at least 2 years: 2 years or more from symptom onset or until GMG developed in conversion analysis, and 2 years or more after thymectomy for CSR and clinical improvement analysis. Patients who were lost to follow-up or who had incomplete clinical data were excluded from this study. We also excluded patients with thymic carcinomas or cysts because these conditions have not been validated to be associated with MG pathogenesis [9].
GMG was defined by any symptoms beyond the extraocular muscles or eyelid, including dysphagia, dysarthria, dyspnoea, dysphonia, neck or extremity weakness, with the above positive serological or physiological testing. CSR was defined as the absence of any symptoms or signs of MG for at least 1 year without any medication for MG. Clinical improvement was defined as a substantial decrease in pre-treatment clinical manifestations or a sustained substantial reduction in MG medications.
Extended thymectomy, defined as resection of the entire thymus and mediastinal fat tissue between both phrenic nerves, was performed for all patients. Indications for thymectomy included thymic abnormality suggestive of thymoma on diagnostic imaging, or in suspicion of hyperplasia but with inadequate response to acetylcholinesterases, resistance to taking immunosuppressive (IS) therapy or with contraindications to or being refractory to IS agents. With respect to OMG patients in whom it was difficult to differentiate thymoma from thymic hyperplasia in thymic imaging, we still recommended thymectomy, to eliminate any possible thymoma. For patients with a high suspicion of thymic hyperplasia, it was recommended that they make their own decision regarding surgery, after a comprehensive explanation of the pros and cons of both medical treatment and surgery.
This study was approved by the ethics committee of Tianjin Medical University General Hospital (Ethical No. IRB2022-WZ-024) and was conducted according to the principles of the Declaration of Helsinki. The need for obtaining patient consent was waived due to the nature of the study.
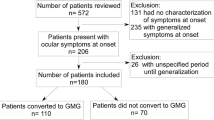
After inclusion and exclusion criteria screening, twenty-four patients were excluded from the study. Therefore, a total of 58 patients were finally identified for OMG conversion study, 52 patients were eligible for CSR analysis. The flowchart of recruitment and exclusion detail was shown in Fig. 1.
Clinical predictors
The following variables that may predict conversion and CSR were evaluated: age, sex, clinical symptoms at onset (diplopia, ptosis, or both), anti-AChR Ab statis, RNS status, thymus histology, Masaoka–Koga Stage of thymoma, tumour location, tumour size, surgical approach, duration of symptoms before surgery, and immunosuppressive treatment (corticosteroids, azathioprine or tacrolimus) after surgery.
Thymus pathology
Thymoma histology was classified according to the WHO criteria [10] by local surgical pathologists. To facilitate analysis, we assigned each thymoma to one of the WHO subtypes (A, AB, B1, B2, B3). However, six cases had combinations of type B1 and B2, and five cases had combinations of type B2 and B3 thymomas. We classified these “B1 plus B2” as B2 thymomas, and “B2 plus B3” as B3 thymomas, when there was any area in which the diagnostic histology of B2 or B3 could be recognized. The classification system of Masaoka–Koga [11] was adopted as the staging system. The stage was determined by reviewing the surgical records and pathologic reports.
Thymoma anatomical features
The tumour location and size were determined from preoperative thoracic imaging examinations. If the tumour boundary exceeded the left/right sternal border, it was defined as left or right thymoma, respectively. Both maximum and mean tumour diameter (measured as the average diameter of the anteroposterior, vertical, and transverse span length) were evaluated.
Serological testing
Both AchR and MuSK Abs were tested in all patients. The presence of AChR Abs was considered positive with the titre > 0.5 nmol/L, and the presence of MuSK Ab was considered when the titre > 0.01 nmol/L, as assessed in radioimmune assays.
Electrodiagnostic testing
The RNS test was routinely conducted in the bilateral orbicularis oculi muscle for OMG. A decrement of more than 10% with 3-Hz stimulation was considered to represent a positive result.
Post-thymectomy treatment
Pyridostigmine alone or in combination with prednisone were prescribed by neurologists, according to OMG symptoms before surgery Generally, prednisone was recommended for patients with diplopia. After symptomatic control was achieved, the dose was tapered over months to the minimum effective dose or withdrawal. When corticosteroids were ineffective, or when side effects limited their use, or when contraindications precluded their use entirely, additional immunosuppressive agents, such as tacrolimus, azathioprine, mycophenolate mofetil, or methotrexate were considered.
Statistical analysis
Categorical variables were analysed using the chi-square and Fisher’s exact test. Continuous variables were analysed with two-tailed t-tests. Cumulative incidence of OMG conversion and CSR were analysed using the Kaplan–Meier method and the log-rank test. Univariate and multivariate Cox proportional hazards regression analysis was applied to determine the factors affecting OMG conversion and CSR during the follow-up period. A P value < 0.05 was considered statistically significant. All data analyses were performed using SPSS Statistics for Mac version 25.0 (IBM Corp, Armonk, NY).
Results
Baseline demographics and clinical features
Clinical features of converted OMG and pure OMG
The median follow-up duration after thymectomy in our 58 OMG patients was 59.3 (range 9–114.5) months. At last review, 13 (22.4%) patients had developed GMG at a median conversion time of 9.2 (range 1.4–32.9) months after thymectomy, and 12.7 (range 3–37.3) months after symptom onset. Eleven of these 13 patients (84.6%) developed GMG within 2 years of symptom onset. Of the 58 included patients, 24 (42.3%) were men and 34 (57.7%) were women, with a median age at symptom onset of 54.7 years. The clinical characteristics of the 13 OMG patients who converted to GMG and the 45 pure OMG patients are displayed in Table 1. No significant difference was observed in terms of sex, age, OMG symptoms at onset (ptosis/diplopia), disease duration before surgery, surgical approach, tumour location, tumour size, immunosuppressive treatment after surgery, and post-operative follow-up duration. Acetylcholine receptor antibody (AchR Ab)-seropositivity showed a trend for increased risk of conversion to GMG (P = 0.085), although no statistically significant difference was reached. RNS-positivity was associated with an increased risk of conversion to GMG, with 84.6% of those with RNS-positivity converting to GMG, as compared with 35.6% of patients who were RNS-negative (P = 0.002). We did not find a difference in conversion rate between OMG with thymoma and hyperplasia (12/44, 27.3% vs. 1/14, 7.1%; P = 0.228, Table 1). However, patients with histological subtype B2/B3 thymoma showed a statistically significant increase in the risk of conversion (P = 0.002) compared to the group of patients with either hyperplasia or subtype AB/B1 thymoma.
Clinical features of CSR-OMG and non-CSR OMG
Of the 52 patients who could be included in the CSR analysis, 16 (30.8%) achieved CSR at a median time of 28.7 (range 15–54) months after thymectomy. Fifteen (28.8%) patients showed improvement at the last follow-up. No significant difference was observed between the CSR-OMG and non-CSR OMG patients in terms of sex, OMG symptoms at onset, disease duration before surgery, surgical approach, tumour location, tumour size, or immunosuppressive treatment after surgery. However, patients who achieved CSR had a younger age of onset (48.3 ± 13.7 vs. 57.5 ± 12.6 years, P = 0.022).
We then used receiver operating characteristic (ROC) curves to explore the best threshold for age (60.5 years, area under the ROC curve [AUC] 0.703, 95% confidence interval [CI] 0.561–0.846; sensitivity: 47.2%; specificity: 87.5%) (Fig. 2). The AchR Ab-seropositivity was associated with a decreased probability of CSR (P = 0.029). RNS-positivity was associated with a trend for decreased probability of CSR (P = 0.063). Patients with thymic hyperplasia or stage I thymoma showed a significantly higher chance of achieving CSR (P = 0.010) compared with patients with stage II/III thymoma. Post-operative follow-up duration was markedly longer in the CSR group (78.3 ± 28.6 vs. 58.3 ± 25.4 months, P = 0.015), indicating that, with the extension of follow-up time, more patients would achieve CSR and that 2 years may not be long enough to determine whether CSR can be reached. We believe that this follow-up duration difference also explained the surgical approach outcomes between these two groups, as unilateral video-assisted thoracoscopic surgery was routinely performed from 2012 to 2017 in our centre, while the sub-xiphoid method became mainstream in the recent 4 years (Table 2).
Cumulative probability of OMG conversion and CSR
The cumulative probability of OMG conversion in the sample of 58 patients, according to RNS, AchR Ab status, and thymus histology (B2/B3 versus hyperplasia + AB/B1), was calculated by using the Kaplan–Meier method. Among these variables, RNS-positivity (P = 0.002) and histological subtype of B2/B3 thymoma (P = 0.008) were associated with increased risk of OMG conversion as compared with patients with RNS-negativity and histotype hyperplasia + AB/B1 thymoma (Fig. 3).
Kaplan–Meier curve of the cumulative probability of conversion to GMG after onset of symptoms in in different patient groups. A Patients with negative and positive RNS results (P = 0.002). B patients with different thymic histology (P = 0.008). GMG, generalized myasthenia gravis. RNS, repetitive nerve stimulation
The cumulative probability of achieving CSR was also studied. The analysis showed that AchR Ab-seronegativity (P = 0.048) was associated with a higher chance of achieving CSR. Patients with hyperplasia and stage I thymoma showed an increased chance of achieving CSR as compared to those with stage II–III thymoma (P = 0.014) (Fig. 4).
Multivariate analysis
Both univariate and multivariate analysis, using a Cox proportional hazard model, were performed to verify prognostic factors associated with OMG conversion and CSR. Among the variables considered, RNS-positivity (hazard ratio [HR] 6.007, 95% CI 1.316–27.412, P = 0.021) and histotype B2/B3 thymomas (HR 4.611, 95% CI 1.010–21.043, P = 0.048) were significantly associated with OMG conversion. On the other hand, thymic hyperplasia and Masaoka–Koga Stage I thymoma (HR 0.300, 95% CI 0.104–0.864, P = 0.026) were independently associated with CSR in OMG patients after thymectomy (Table 3). We further performed subgroup analysis for patients with thymomatous OMG, and found by univariable Cox regression analysis that RNS positivity was a significant positive predictor for OMG conversion (HR 5.274, 95% CI 1.54–24.106, P = 0.032), and an age at onset > 60 years was a significant negative predictor for achieving CSR (HR 0.124, 95% CI 0.016–0.981, P = 0.048). In multivariate Cox analysis, only RNS positivity was a significant positive predictor for OMG conversion (HR 5.017, 95% CI 1.094–22.997, P = 0.038). Thymoma histotype B2/B3 showed a positive association with OMG conversion compared to AB/B1, and stage II/III thymoma showed a negative association with CSR compared to the stage I counterpart. However, neither reached statistical significance (Table 4).
Discussion
To the best of our knowledge, no previous study had simultaneously targeted predictors of GMG conversion and CSR after thymectomy in OMG patients. Our retrospective study showed that RNS-positivity and World Health Organization (WHO) subtype B2/B3 thymomas were significantly associated with post-thymectomy OMG conversion. On the other hand, thymic hyperplasia and Masaoka–Koga Stage I thymoma were independently associated with post-thymectomy OMG CSR.
Thymectomy is required for patients with thymoma. Its role in non-thymomatous OMG (NTOMG), however, remains highly controversial [12]. In our department, patients with OMG underwent thymectomy on condition of thymus abnormalities found on diagnostic imaging. Fourteen patients with pathologically confirmed thymic hyperplasia were included in our study. Thymectomy has already been shown to result in better clinical outcomes and reduced corticosteroid requirements in non-thymomatous AchR-seropositive GMG [13]. The same potential benefits may also apply in OMG patients, particularly in those with thymic hyperplasia. Correlations between the degree of follicular hyperplasia and the level of anti-AChR Abs suggest a causal relationship between thymic hyperplasia and MG [14]. Wong et al. [6] identified that thymic hyperplasia was a significant predictive factor for conversion to GMG in OMG patients who have not undergone thymectomy. Wang et al. [15] reported that OMG patients with thymus hyperplasia progressed more rapidly than did those with other thymus pathology. Patients with thymic hyperplasia in our study cohort showed a satisfying post-thymectomy outcome (only 1 of 14 patients converted to GMG, whereas 6 of 13 achieved CSR), which was in accord with some other previous studies [16, 17]. Histologically, MG was reportedly associated mainly with type B thymomas and tended to be more frequent in type B2 or B3 thymomas [7]. Our study supported the correlation between thymus pathology and OMG prognosis.
Although no correlation was detected between OMG generalization and AChR Ab status, our study showed that patients who were seronegative for AChR Abs were more likely to achieve CSR after thymectomy. Using standard assays, AChR Abs are detectable in approximately 50% of patients with OMG and in nearly 90% of patients with GMG [2]. Anti-muscle-specific receptor tyrosine kinase (MuSK) Abs are rarely found in isolated OMG [18]. Only one patient who was AChR Ab-seronegative was anti-MuSK Ab-seropositive in our study. We did not incorporate this variable into our analysis because of this small proportion. However, one must be aware that many “seronegative” cases may harbour an autoantibody that is not detected by conventional means. The diagnostic yield of AChR Ab testing is significantly increased by cell-based assays [19], rather than by the typical radioimmunoprecipitation technique. Moreover, other autoantibodies may also exist. Anti-LRP4 Abs have been detected in about 20% of “double-negative” MG [20]. Antibodies directed against cortactin (a postsynaptic protein required for clustering of AChRs) have been identified in 24% of double-seronegative OMG patients [21]. Whether the presence of these antibodies could have an effect on OMG prognosis requires further validation.
The presence of comorbidities at disease onset has been described as a risk factor for conversion to GMG [6]. We did not incorporate this variable into analysis because of the small sample size (only 6 of 58 patients had another comorbid autoimmune disease). This link will need to be further validated externally for more robust evidence.
At our institution, we consider a minimum of 3 months of isolated ocular disease as an inclusion criterion. This 3-month duration is in keeping with the views of other studies [6]. Monsul et al. [22] suggested this time interval as the limit for purely ocular symptoms before classifying a patient as having OMG. However, studies by Kupersmith [23] and Mee et al. [24] included patients who developed GMG within 3 months of symptom onset. This is an important question that should be clarified in future studies.
Our analyses did not identify age as a risk factor for OMG conversion. The effect of age on OMG conversion to GMG is paradoxical among studies. Feng et al. [4] revealed that the onset age correlated with the conversion rate, and the threshold age was 43 years. On the other hand, Wong et al. did not show age as a risk factor in either univariable or multivariable analysis [6]. However, we did find that younger patients showed a greater chance of achieving CSR than did the older patients. ROC curve analysis indicated that the best threshold for age was 60.5 years (Fig. 2), which is older than the usual age limit for distinguishing early from late onset MG [2]. We attributed this cut-off age discrepancy to demographic characteristic differences. Because most patients in our study had thymoma-related OMG, 50 years may not be suitable as a cut-off age in our study cohort, or in an OMG with thymoma cohort. Moreover, Sarkkinen et al. [25] recently reported that ectopic germinal centres, which can predict better post-thymectomy outcomes in NTMG, are more prevalent in early onset NTMG thymus. In consideration of the potential differences in thymic histopathology in NTMG patients of different ages, we further analysed the proportion of early-onset and late-onset cases in our NTOMG sample set. Early onset cases accounted for almost the same proportion as late onset counterparts, both in terms of conversion and of CSR analysis (Tables 1 and 2). For OMG patients with hyperplasia, it remains unclear whether age can affect post-thymectomy outcomes; this needs further investigation.
Our study showed that RNS-positivity was strongly associated with OMG conversion in univariable analysis (P = 0.002), log-rank test (P = 0.002), and multi-variable COX regression analysis (P = 0.022). Moreover, RNS-negativity tended to be associated with a greater likelihood of CSR, although the difference did not reach statistical significance. The association between RNS and OMG conversion was reported by other studies [26]. Kim. et al. [27] revealed that an abnormal RNS test, particularly in the limb muscles, was an independent predictor of the conversion from OMG to GMG. In addition, an abnormal RNS test has been reported to be related to a shortened time to conversion to GMG [28]. Since limb muscles were not routinely tested for OMG in our study, whether performing RNS testing in orbicularis oculi would be sufficient for evaluating neurotransmission in extraocular muscles needs further evaluation.
Previous studies have reported the effect of prednisone in delaying onset of GMG as well as its sustained benefit in reducing the incidence of GMG and controlling diplopia [22, 23]. Generally, patients with diplopia were recommended to receive prednisone therapy, as in our study cohort. Safety concerns about corticosteroids usage include the development of hypertension, diabetes mellitus, osteoporosis, gastrointestinal disorders, or infectious illness, which are common with the chronic use of moderate to high doses of the drug. The rate of post-operative prednisone usage did not differ among our OMG sample set, both in conversion and CSR analysis. The administration of corticosteroids to eliminate extraocular muscle limitation and diplopia continues to be controversial. Core issues revolve around selection of clear responders and the balance between benefits and adverse events [3].
Limited studies have discussed the importance of tumour size and location in determining clinicopathological features or its relationship to thymoma or MG prognosis. Tian et al. reported that thymomas located in the superior mediastinum were more likely to be associated with disease progression and tumour recurrence than those located in the inferior mediastinum [29]. Okumura et al. [30] showed that tumour size determines both recurrence-free survival and disease-specific survival after surgical treatment for thymoma, with a higher incidence of recurrence in patients with thymoma > 5.0 cm and of death in patients with thymoma > 8.0 cm. None of these studies ever mentioned the correlation of thymoma anatomic features with MG. Our study intended to reveal an association between thymoma location/size with OMG prognosis, although statistical significance was not achieved.
Few studies have specifically studied OMG remission after thymectomy. Liu et al. [16] reviewed 110 OMG patients who underwent extended transsternal thymectomy: 26.4% achieved CSR and 58.2% showed improvement. Surprisingly, no patient converted to GMG during the follow-up period. Unlike in our study cohort, nearly all (95.7%) of their patients were non-thymomatous. The rationale for their high rate of surgery in OMG without thymoma is unclear. Mineo et al. [31] retrospectively reviewed 47 NTOMG patients after thymectomy, of whom 64% achieved stable remission. Robert et al. [32] documented 61 OMG patients treated with thymectomy, who were followed for a mean duration of 9 years, and reported a cure, defined as asymptomatic status without the need for medication, in 51%. Thymomatous OMG accounted for 19.7% of all their cases. The discrepancy of thymus pathology might be the main difference among these studies. This may partly explain the higher remission rate in some of these studies. As stated above, OMG patients with hyperplasia seems to achieve a better outcome than their counterparts with thymoma. Huang et al. [33] reported the highest CSR rate (70.8%) in OMG patients after thymectomy. The histopathology of the thymus, however, was unclear in their study. Overall, the reasons for discrepancy in the OMG remission rate among studies are uncertain and may be complicated. The heterogeneity in demographics, pre-operative symptoms, definition of remission, peri-operative treatment, and thymus pathology may collaborate in contributing to this discrepancy. Among these studies, only Liu et al. [34] explored potential predictors of remission in OMG patients after thymectomy. They found that only age at onset of 40 years or younger could serve as a predictor of OMG remission. However, the onset age of this study cohort was markedly younger (median 40, range 5–79 years). More importantly, only 17.6% of patients had OMG with thymoma, which is a marked difference from our study. Overall, studies on prognosis of thymomatous OMG after thymectomy are scarce, and our study made a significant contribution in this area.
The limitations of this study should be acknowledged. First, the current study had a retrospective design, which may have caused selection bias. Second, the number of patients with post-thymectomy OMG was small. The risk factors identified from this limited sample size may not be generalizable for all patients. Third, given the difference in follow-up duration between groups in our CSR analysis, our inclusion criterion of a follow-up period of 2 years may have caused selection bias, which might have influenced the CSR rate and other associated risk factors. Fourth, only AChR-Ab and MuSK-Ab status was tested in our study. Whether other autoantibodies, such as anti-LRP4 Abs and anti-cortactin Abs would present as confounding factors remains unknown. Finally, considering the different aetiology and pathogenesis of thymoma and thymus hyperplasia, the rationale of combining patients with hyperplasia and specific subtypes of thymoma is debatable.
In conclusion, we revealed the impact of thymus pathology and thymoma anatomical features on prognosis of OMG after thymectomy. We found that RNS-positivity and histotype B2/B3 thymoma were independent predictors of OMG conversion to GMG. On the other hand, thymic hyperplasia and stage I thymoma independently predicted CSR after thymectomy. Patients with a younger age of onset and who are negative for anti-AchR Abs may also have a higher chance of achieving CSR. Further prospective studies with a larger number of patients are warranted to validate our findings.
Availability of data and material
All data were retrieved from the medical record database of Tianjin Medical University General Hospital. Please contact the corresponding author for data requests.
Abbreviations
- AchR Ab:
-
Acetylcholine receptor antibody
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CSR:
-
Complete stable remission
- GMG:
-
Generalized myasthenia gravis
- HR:
-
Hazard ratio
- MG:
-
Myasthenia gravis
- MuSK Ab:
-
Muscle-specific receptor tyrosine kinase antibody
- NTOMG:
-
Non-thymomatous ocular myasthenia gravis
- OMG:
-
Ocular myasthenia gravis
- RNS:
-
Repetitive nerve stimulation
- ROC:
-
Receiver operating characteristic
- SGMG:
-
Secondary generalized myasthenia gravis
References
Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30.
Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–36.
Wong SH, Huda S, Vincent A, Plant GT. Ocular myasthenia gravis: controversies and updates. Curr Neurol Neurosci Rep. 2014;14(1):421.
Feng X, Huan X, Yan C, Song J, Lu J, Zhou L, et al. Adult ocular myasthenia gravis conversion: a single-center retrospective analysis in China. Eur Neurol. 2020;83(2):182–8.
Mazzoli M, Ariatti A, Valzania F, Kaleci S, Tondelli M, Nichelli PF, et al. Factors affecting outcome in ocular myasthenia gravis. Int J Neurosci. 2018;128(1):15–24.
Wong SH, Petrie A, Plant GT. Ocular myasthenia gravis: toward a risk of generalization score and sample size calculation for a randomized controlled trial of disease modification. J Neuroophthalmol. 2016;36(3):252–8.
Weis CA, Yao X, Deng Y, Detterbeck FC, Marino M, Nicholson AG, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol. 2015;10(2):367–72.
Amini A, Rusthoven CG. Thymoma: does tumor size matter? J Thorac Dis. 2019;11(Suppl 15):S2005–7.
Kim SH, Koh IS, Minn YK. Pathologic finding of thymic carcinoma accompanied by myasthenia gravis. J Clin Neurol. 2015;11(4):372–5.
Marx A, Chan JK, Coindre JM, Detterbeck F, Girard N, Harris NL, et al. The 2015 world health organization classification of tumors of the thymus: continuity and changes. J Thorac Oncol. 2015;10(10):1383–95.
Detterbeck FC, Nicholson AG, Kondo K, Van Schil P, Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol. 2011;6(7 Suppl 3):S1710–6.
Narayanaswami P, Sanders DB, Wolfe G, Benatar M, Cea G, Evoli A, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–22.
Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375(6):511–22.
Berrih-Aknin S, Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun. 2014;52:90–100.
Wang L, Zhang Y, He M. Clinical predictors for the prognosis of myasthenia gravis. BMC Neurol. 2017;17(1):77.
Liu Z, Feng H, Yeung SC, Zheng Z, Liu W, Ma J, et al. Extended transsternal thymectomy for the treatment of ocular myasthenia gravis. Ann Thorac Surg. 2011;92(6):1993–9.
Zhu K, Li J, Huang X, Xu W, Liu W, Chen J, et al. Thymectomy is a beneficial therapy for patients with non-thymomatous ocular myasthenia gravis: a systematic review and meta-analysis. Neurol Sci. 2017;38(10):1753–60.
Galassi G, Mazzoli M, Ariatti A, Kaleci S, Valzania F, Nichelli PF. Antibody profile may predict outcome in ocular myasthenia gravis. Acta Neurol Belg. 2018;118(3):435–43.
Rodríguez Cruz PM, Al-Hajjar M, Huda S, Jacobson L, Woodhall M, Jayawant S, et al. Clinical features and diagnostic usefulness of antibodies to clustered acetylcholine receptors in the diagnosis of seronegative myasthenia gravis. JAMA Neurol. 2015;72(6):642–9.
Zisimopoulou P, Evangelakou P, Tzartos J, Lazaridis K, Zouvelou V, Mantegazza R, et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun. 2014;52:139–45.
Cortés-Vicente E, Gallardo E, Martínez M, Díaz-Manera J, Querol L, Rojas-García R, et al. Clinical characteristics of patients with double-seronegative myasthenia gravis and antibodies to cortactin. JAMA Neurol. 2016;73(9):1099–104.
Monsul NT, Patwa HS, Knorr AM, Lesser RL, Goldstein JM. The effect of prednisone on the progression from ocular to generalized myasthenia gravis. J Neurol Sci. 2004;217(2):131–3.
Kupersmith MJ. Ocular myasthenia gravis: treatment successes and failures in patients with long-term follow-up. J Neurol. 2009;256(8):1314–20.
Mee J, Paine M, Byrne E, King J, Reardon K, O’Day J. Immunotherapy of ocular myasthenia gravis reduces conversion to generalized myasthenia gravis. J Neuroophthalmol. 2003;23(4):251–5.
Sarkkinen J, Dunkel J, Tuulasvaara A, Huuskonen A, Atula S, Kekäläinen E, et al. Ectopic germinal centers in the thymus accurately predict prognosis of myasthenia gravis after thymectomy. Mod Pathol. 2022;3:4458.
Ding J, Zhao S, Ren K, Dang D, Li H, Wu F, et al. Prediction of generalization of ocular myasthenia gravis under immunosuppressive therapy in Northwest China. BMC Neurol. 2020;20(1):238.
Kim KH, Kim SW, Shin HY. Initial repetitive nerve stimulation test predicts conversion of ocular myasthenia gravis to generalized myasthenia gravis. J Clin Neurol. 2021;17(2):265–72.
Kısabay A, Özdemir HN, Gökçay F, Çelebisoy N. Risk for generalization in ocular onset myasthenia gravis: experience from a neuro-ophthalmology clinic. Acta Neurol Belg. 2021;6:7780.
Tian D, Shiiya H, Sato M, Sun CB, Anraku M, Nakajima J. Tumor location may affect the clinicopathological features and prognosis of thymomas. Thorac Cancer. 2019;10(11):2096–105.
Okumura M, Yoshino I, Yano M, Watanabe SI, Tsuboi M, Yoshida K, et al. Tumour size determines both recurrence-free survival and disease-specific survival after surgical treatment for thymoma. Eur J Cardiothorac Surg. 2019;56(1):174–81.
Mineo TC, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. J Thorac Cardiovasc Surg. 2013;145(5):1319–24.
Roberts PF, Venuta F, Rendina E, De Giacomo T, Coloni GF, Follette DM, et al. Thymectomy in the treatment of ocular myasthenia gravis. J Thorac Cardiovasc Surg. 2001;122(3):562–8.
Huang CS, Hsu HS, Huang BS, Lee HC, Kao KP, Hsu WH, et al. Factors influencing the outcome of transsternal thymectomy for myasthenia gravis. Acta Neurol Scand. 2005;112(2):108–14.
Liu X, Zhou W, Hu J, Hu M, Gao W, Zhang S, et al. Prognostic predictors of remission in ocular myasthenia after thymectomy. J Thorac Dis. 2020;12(3):422–30.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology: Jinwei Zhang and Peng Zhang. Data Curation and investigation: Zeyang Zhang, Hui Zhang and Yuantao Cui. Formal Analysis: Jinwei Zhang, Zeyang Zhang, Yuan Chen and Peng Lv. Supervision and Visualization: Peng Zhang. Writing: Jinwei Zhang. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Tianjin Medical University General Hospital (Ethical No. IRB2022-WZ-024) and conducted according to the principles of the Declaration of Helsinki. The need for patient consent was waived.
Consent for publication
All authors have read and approved the content, and agreed to submit the article in consideration for publication.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, J., Zhang, Z., Zhang, H. et al. Thymectomy in ocular myasthenia gravis—prognosis and risk factors analysis. Orphanet J Rare Dis 17, 309 (2022). https://doi.org/10.1186/s13023-022-02454-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-022-02454-y