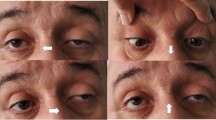
Abstract
The majority of patients with myasthenia gravis (MG) initially present with ocular symptoms. An unresolved question is whether there are clinical features at onset to guide clinicians to predict an individual patient’s conversion risk from ocular MG (OMG) to generalized disease, or “secondary generalized MG” (SGMG), that is, a prognostic model. In light of the emerging theory that early corticosteroids may have a risk-modifying effect, the factors associated with secondary SGMG previously reported should be revisited. Studies showing potential risk-modifying effects of corticosteroids are useful, though flawed, owing to the heterogeneous retrospective studies and methods of reporting. Updates on other potential immunosuppressive agents are also discussed. Thymectomy in OMG has been recently reported in a few studies to be useful. MG associated with antibodies to muscle-specific kinase, usually associated with severe generalized MG, can cause a pure OMG syndrome. Recent serological developments in seronegative patients have also revealed antibodies to clustered anti-acetylcholine receptor and lipoprotein receptor-related protein-4.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Garlepp MJ, Dawkins RL, Christiansen FT, Lawton J, Luciani G, McLeod J, et al. Autoimmunity in ocular and generalised myasthenia gravis. J Neuroimmunol. 1981;1:325–32.
Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357:2122–8.
• Serra A, Ruff R, Kaminski H, Leigh RJ. Factors contributing to failure of neuromuscular transmission in myasthenia gravis and the special case of the extraocular muscles. Ann N Y Acad Sci. 2011;1233:26–33. This review summarizes the factors contributing to the increased susceptibility of the extraocular muscles to neuromuscular transmission failure.
Serra A, Ruff RL, Leigh RJ. Neuromuscular transmission failure in myasthenia gravis: decrement of safety factor and susceptibility of extraocular muscles. Ann N Y Acad Sci. 2012;1275:129–35.
Sanmuganathan PS. Myasthenic syndrome of snake envenomation: a clinical and neurophysiological study. Postgrad Med J. 1998;74:596–9.
Bever CT, Aquino AV, Penn AS, Lovelace RE, Rowland LP. Prognosis of ocular myasthenia. Ann Neurol. 1983;14:516–9.
Ferguson FR, Hutchinson EC, Liversedge LA. Myasthenia gravis; results of medical management. Lancet. 1955;269:636–9.
Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37:141–9.
Oosterhuis HJ. The natural course of myasthenia gravis: a long term follow up study. J Neurol Neurosurg Psychiatr. 1989;52:1121–7.
Evoli A, Tonali P, Bartoccioni E, Monaco LM. Ocular myasthenia: diagnostic and therapeutic problems. Acta Neurol Scand. 1988;77:31–5.
Grob D, Arsura EL, Brunner NG, Namba T. The course of myasthenia gravis and therapies affecting outcome. Ann N Y Acad Sci. 1987;505:472–99.
Kupersmith MJ. Ocular myasthenia gravis: treatment successes and failures in patients with long-term follow-up. J Neurol. 2009;256:1314–20.
Kupersmith MJ, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol. 2003;60:243–8.
Mee J, Paine M, Byrne E, King J, Reardon K, O'Day J. Immunotherapy of ocular myasthenia gravis reduces conversion to generalized myasthenia gravis. J Neuroophthalmol. 2003;23:251–5.
Sommer N, Sigg B, Melms A, Weller M, Schepelmann K, Herzau V, et al. Ocular myasthenia gravis: response to long-term immunosuppressive treatment. J Neurol Neurosurg Psychiatr. 1997;62:156–62.
Kupersmith MJ, Moster M, Bhuiyan S, Warren F, Weinberg H. Beneficial effects of corticosteroids on ocular myasthenia gravis. Arch Neurol. 1996;53:802–4.
Monsul NT, Patwa HS, Knorr AM, Lesser RL, Goldstein JM. The effect of prednisone on the progression from ocular to generalized myasthenia gravis. J Neurol Sci. 2004;217:131–3.
Agius MA. Treatment of ocular myasthenia with corticosteroids: yes. Arch Neurol. 2000;57:750–1.
Chavis PS, Stickler DE, Walker A. Immunosuppressive or surgical treatment for ocular Myasthenia Gravis. Arch Neurol. 2007;64:1792–4.
Gilbert ME, De Sousa EA, Savino PJ. Ocular Myasthenia Gravis treatment: the case against prednisone therapy and thymectomy. Arch Neurol. 2007;64:1790–2.
Kaminski HJ, Daroff RB. Treatment of ocular myasthenia: steroids only when compelled. Arch Neurol. 2000;57:752–3.
Roach ES. Treating ocular Myasthenia Gravis with inadequate evidence. Arch Neurol. 2007;64:1794–5.
Grob D, Brunner NG, Namba T. The natural course of myasthenia gravis and effect of therapeutic measures. Ann N Y Acad Sci. 1981;377:652–69.
Steinberg AD, Krieg AM, Takashi T, Gourley MF. Timing of immunosuppression in the natural history of autoimmune diseases. J Autoimmun. 1992;5(Suppl A):197–203.
Grob D. Course and management of myasthenia gravis. J Am Med Assoc. 1953;153:529–32.
Kaplan I, Blakely BT, Pavlath GK, Travis M, Blau HM. Steroids induce acetylcholine receptors on cultured human muscle: implications for myasthenia gravis. Proc Natl Acad Sci U S A. 1990;87:8100–4.
Askanas V, McFerrin J, Park-Matsumoto YC, Lee CS, Engel WK. Glucocorticoid increases acetylcholinesterase and organization of the postsynaptic membrane in innervated cultured human muscle. Exp Neurol. 1992;115:368–75.
Oosterhuis H, Bethlem J. Neurogenic muscle involvement in myasthenia gravis a clinical and histopathological study. J Neurol Neurosurg Psychiatr. 1973;36:244–54.
Barker L, Minakaran N, Patel L, Plant GT. Fixed ophthalmoplegia and extraocular muscle atrophy in acetylcholine receptor antibody-positive myasthenia gravis. Abstracts of the International Neuro-ophthalmology Society Meeting 2012. Neuro-ophthalmology. 2012;36(S1):47–8.
Okamoto K, Ito J, Tokiguchi S, Furusawa T. Atrophy of bilateral extraocular muscles. CT and clinical features of seven patients. J Neuroophthalmol. 1996;16:286–8.
Kupersmith MJ, Ying G. Ocular motor dysfunction and ptosis in ocular myasthenia gravis: effects of treatment. Br J Ophthalmol. 2005;89:1330–4.
Oosterhuis HJ. Observations of the natural history of myasthenia gravis and the effect of thymectomy. Ann N Y Acad Sci. 1981;377:678–90.
Caldwell JR, Furst DE. The efficacy and safety of low-dose corticosteroids for rheumatoid arthritis. Semin Arthritis Rheum. 1991;21:1–11.
Pascuzzi RM, Coslett HB, Johns TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol. 1984;15:291–8.
• Benatar M, Sanders DB, Wolfe GI, McDermott MP, Tawil R. Design of the efficacy of prednisone in the treatment of ocular myasthenia (EPITOME) trial. Ann N Y Acad Sci. 2012;1275:17–22. This article outlines the protocol of the first RCT (currently in progress) of corticosteroids in the treatment of ocular myasthenia.
Benatar M, Sanders DB, Burns TM, Cutter GR, Guptill JT, Baggi F, et al. Recommendations for myasthenia gravis clinical trials. Muscle Nerve. 2012;45:909–17.
Tackenberg B, Hemmer B, Oertel WH, Sommer N. Immunosuppressive treatment of ocular myasthenia gravis. BioDrugs. 2001;15:369–78.
• Kumar V, Kaminski HJ. Treatment of myasthenia gravis. Curr Neurol Neurosci Rep. 2011;11:89–96. This recent review summarizes the treatment and mechanisms of action of pharmaceutical treatment for MG.
Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis. 2013;20:433–40.
Pedersen EG, Pottegård A, Hallas J, Friis S, Hansen K, Jensen PEH, et al. Use of azathioprine for non-thymoma myasthenia and risk of cancer: a nationwide case-control study in Denmark. Eur J Neurol. 2013;20:942–8.
Heckmann JM, Rawoot A, Bateman K, Renison R, Badri M. A single-blinded trial of methotrexate versus azathioprine as steroid-sparing agents in generalized myasthenia gravis. BMC Neurol. 2011;11:97.
Ciafaloni E, Massey JM, Tucker-Lipscomb B, Sanders DB. Mycophenolate mofetil for myasthenia gravis: an open-label pilot study. Neurology. 2001;56:97–9.
Chaudhry V, Cornblath DR, Griffin JW, O'Brien R, Drachman DB. Mycophenolate mofetil: a safe and promising immunosuppressant in neuromuscular diseases. Neurology. 2001;56:94–6.
Meriggioli MN, Ciafaloni E, Al-Hayk KA, Rowin J, Tucker-Lipscomb B, Massey JM, et al. Mycophenolate mofetil for myasthenia gravis: an analysis of efficacy, safety, and tolerability. Neurology. 2003;61:1438–40.
Hehir MK, Burns TM, Alpers J, Conaway MR, Sawa M, Sanders DB. Mycophenolate mofetil in AChR-antibody-positive myasthenia gravis: outcomes in 102 patients. Muscle Nerve. 2010;41:593–8.
Sanders DB, Hart IK, Mantegazza R, Shukla SS, Siddiqi ZA, De Baets MHV, et al. An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology. 2008;71:400–6.
Muscle Study Group. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008;71:394–9.
Tindall RS, Rollins JA, Phillips JT, Greenlee RG, Wells L, Belendiuk G. Preliminary results of a double-blind, randomized, placebo-controlled trial of cyclosporine in myasthenia gravis. N Engl J Med. 1987;316:719–24.
Tindall RS, Phillips JT, Rollins JA, Wells L, Hall K. A clinical therapeutic trial of cyclosporine in myasthenia gravis. Ann N Y Acad Sci. 1993;681:539–51.
Yoshikawa H, Kiuchi T, Saida T, Takamori M. Randomised, double-blind, placebo-controlled study of tacrolimus in myasthenia gravis. J Neurol Neurosurg Psychiatr. 2011;82:970–7.
Benatar M, Sanders D. The importance of studying history: lessons learnt from a trial of tacrolimus in myasthenia gravis. J Neurol Neurosurg Psychiatr. 2011;82:945.
Yagi Y, Sanjo N, Yokota T, Mizusawa H. Tacrolimus monotherapy: a promising option for ocular myasthenia gravis. Eur Neurol. 2013;69:344–5.
Gummert JF, Ikonen T, Morris RE. Newer immunosuppressive drugs: a review. J Am Soc Nephrol. 1999;10:1366–80.
Schneider-Gold C, Hartung H-P, Gold R. Mycophenolate mofetil and tacrolimus: new therapeutic options in neuroimmunological diseases. Muscle Nerve. 2006;34:284–91.
• Mineo TC, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. J Thorac Cardiovasc Surg. 2013;145:1319–24. This study showed that patients with ocular myasthenia treated with extended thymectomy achieved a more rapid remission than after non-surgical treatment. Significantly better outcomes resulted when thymectomy was performed within 6 months of onset of symptoms.
Hatton PD, Diehl JT, Daly BD, Rheinlander HF, Johnson H, Schrader JB, et al. Transsternal radical thymectomy for myasthenia gravis: a 15-year review. Ann Thorac Surg. 1989;47:838–40.
• Liu Z, Feng H, Yeung S-CJ, Zheng Z, Liu W, Ma J, et al. Extended transsternal thymectomy for the treatment of ocular myasthenia gravis. Ann Thorac Surg. 2011;92:1993–9. This study showed that extended transsternal thymectomy in OMG was safe and effective, particularly in patients with short duration of illness.
• Marulli G, Schiavon M, Perissinotto E, Bugana A, Di Chiara F, Rebusso A, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg. 2013;145:730–5. This article reported improved outcome in patients with ocular myasthenia treated with thymectomy.
Nakamura H, Taniguchi Y, Suzuki Y, Tanaka Y, Ishiguro K, Fukuda M, et al. Delayed remission after thymectomy for myasthenia gravis of the purely ocular type. J Thorac Cardiovasc Surg. 1996;112:371–5.
Schumm F, Wiethölter H, Fateh-Moghadam A, Dichgans J. Thymectomy in myasthenia with pure ocular symptoms. J Neurol Neurosurg Psychiatr. 1985;48:332–7.
Huang C-S, Hsu H-S, Huang B-S, Lee H-C, Kao K-P, Hsu W-H, et al. Factors influencing the outcome of transsternal thymectomy for myasthenia gravis. Acta Neurol Scand. 2005;112:108–14.
Masaoka A, Yamakawa Y, Niwa H, Fukai I, Kondo S, Kobayashi M, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg. 1996;62:853–9.
Papatestas AE, Pozner J, Genkins G, Kornfeld P, Matta RJ. Prognosis in occult thymomas in myasthenia gravis following transcervical thymectomy. Arch Surg. 1987;122:1352–6.
Roberts PF, Venuta F, Rendina E, De Giacomo T, Coloni GF, Follette DM, et al. Thymectomy in the treatment of ocular myasthenia gravis. J Thorac Cardiovasc Surg. 2001;122:562–8.
Shrager JB, Nathan D, Brinster CJ, Yousuf O, Spence A, Chen Z, et al. Outcomes after 151 extended transcervical thymectomies for myasthenia gravis. Ann Thorac Surg. 2006;82:1863–9.
Takanami I, Abiko T, Koizumi S. Therapeutic outcomes in thymectomied patients with myasthenia gravis. Ann Thorac Cardiovasc Surg. 2009;15:373–7.
Sonett JR, Jaretzki A. Thymectomy for nonthymomatous myasthenia gravis: a critical analysis. Ann N Y Acad Sci. 2008;1132:315–28.
Chicaiza-Becerra LA, Garcia-Molina M, Gamboa O, Castañeda-Orjuela C. The cost-effectiveness of open or thoracoscopic thymectomy compared to medical treatment in managing Myasthenia gravis without thymomas. Rev Salud Publica (Bogota). 2012;14:260–70.
Newsom-Davis J, Cutter G, Wolfe GI, Kaminski HJ, Jaretzki A, Minisman G, et al. Status of the thymectomy trial for nonthymomatous myasthenia gravis patients receiving prednisone. Ann N Y Acad Sci. 2008;1132:344–7.
Evoli A, Tonali PA, Padua L, Monaco ML, Scuderi F, Batocchi AP, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003;126:2304–11.
McConville J, Farrugia ME, Beeson D, Kishore U, Metcalfe R, Newsom-Davis J, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55:580–4.
Oh SJ. Muscle-specific receptor tyrosine kinase antibody positive myasthenia gravis current status. J Clin Neurol. 2009;5:53–64.
Sanders DB, El-Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003;60:1978–80.
Zhou L, McConville J, Chaudhry V, Adams RN, Skolasky RL, Vincent A, et al. Clinical comparison of muscle-specific tyrosine kinase (MuSK) antibody-positive and -negative myasthenic patients. Muscle Nerve. 2004;30:55–60.
Vincent A, Leite MI. Neuromuscular junction autoimmune disease: muscle specific kinase antibodies and treatments for myasthenia gravis. Curr Opin Neurol. 2005;18:519–25.
Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. 2011;44:36–40.
Bau V, Hanisch F, Hain B. Zierz S [Ocular involvement in MuSK antibody-positive myasthenia gravis]. Klin Monbl Augenheilkd. 2006;223:81–3 [in German].
Bennett DLH, Mills KR, Riordan-Eva P, Barnes PRJ, Rose MR. Anti-MuSK antibodies in a case of ocular myasthenia gravis. J Neurol Neurosurg Psychiatr. 2006;77:564–5.
Caress JB, Hunt CH, Batish SD. Anti-MuSK myasthenia gravis presenting with purely ocular findings. Arch Neurol. 2005;62:1002–3.
Chan JW, Orrison WW. Ocular myasthenia: a rare presentation with MuSK antibody and bilateral extraocular muscle atrophy. Br J Ophthalmol. 2007;91:842–3.
Hanisch F, Eger K, Zierz S. MuSK-antibody positive pure ocular myasthenia gravis. J Neurol. 2006;253:659–60.
Hosaka A, Takuma H, Ohta K, Tamaoka A. An ocular form of myasthenia gravis with a high titer of anti-MuSK antibodies during a long-term follow-up. Intern Med. 2012;51:3077–9.
Zouvelou V, Papathanasiou A, Koros C, Rentzos M, Zambelis T, Stamboulis E. Pure ocular anti-MuSK myasthenia under no immunosuppressive treatment. Muscle Nerve. 2013;48:464.
Wong SH, Virgo JD, Plant G. Risks and benefits of corticosteroid therapy in ocular myasthenia gravis: a retrospective study. Abstracts of the European Neuro-Ophthalmology Society (EUNOS) 11th meeting. Neuro-ophthalmology. 2013;37(S1):107–8.
Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, et al. IgG1 antibodies to acetylcholine receptors in “seronegative” myasthenia gravis. Brain. 2008;131:1940–52.
• Jacob S, Viegas S, Leite MI, Webster R, Cossins J, Kennett R, et al. Presence and pathogenic relevance of antibodies to clustered acetylcholine receptor in ocular and generalized myasthenia gravis. Arch Neurol. 2012;69:994–1001. This article shows that 50% of previously seronegative ocular myasthenia patients had complement-fixing IgG1-clustered AChR-antibodies, and demonstrated that their pathogenicity and the mechanisms seem similar to those of patients with typical AChR Abs.
• Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol. 2011;69:418–22. This article is the first to show that a proportion of seronegative MG patients have antibodies to LRP4, one of the essential proteins at the neuromuscular junction.
Pevzner A, Schoser B, Peters K, Cosma N-C, Karakatsani A, Schalke B, et al. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol. 2012;259:427–35.
Zhang B, Tzartos JS, Belimezi M, Ragheb S, Bealmear B, Lewis RA, et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. 2012;69:445–51.
Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A, et al. Auto-antibodies to the receptor tyrosine kinase MuSk in patients with myasthenia gravis without acetycholine receptor antibodies. Nat Med. 2001;7(3)365–358.
Acknowledgements
Saif Huda is supported by a Fellowship from the Watney Trust/Myasthenia Gravis Association and the Oxford NIHR Biomedical Research Centre. Dr Wong & Dr Plant have received bursaries from the Myasthenia Gravis Association for their work on Ocular Myasthrnia.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Sui H. Wong and Gordon T. Plant declare that they have no conflict of interest. Angela Vincent and the University of Oxford hold patents and receive royalties and payments for autoantibody tests including MuSK antibodies.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuroophthalmology
Rights and permissions
About this article
Cite this article
Wong, S.H., Huda, S., Vincent, A. et al. Ocular Myasthenia Gravis: Controversies and Updates. Curr Neurol Neurosci Rep 14, 421 (2014). https://doi.org/10.1007/s11910-013-0421-9
Published:
DOI: https://doi.org/10.1007/s11910-013-0421-9