Abstract
Despite continued advances in prevention and treatment strategies, cardiovascular diseases (CVDs) remain the leading cause of death worldwide, and more effective therapeutic methods are urgently needed. Polygonatum is a traditional Chinese herbal medicine with a variety of pharmacological applications and biological activities, such as antioxidant activity, anti-inflammation, antibacterial effect, immune-enhancing effect, glucose regulation, lipid-lowering and anti-atherosclerotic effects, treatment of diabetes and anticancer effect. There has also been more and more evidence to support the cardioprotective effect of Polygonatum in recent years. However, up to now, there has been a lack of comprehensive studies on the active ingredients and their pharmacotoxicological effects related to cardiovascular diseases. Therefore, the main active components of Polygonatum (including Polysaccharides, Flavonoids, Saponins) and their biological activities were firstly reviewed in this paper. Furthermore, we summarized the pharmacological effects of Polygonatum’s active components in preventing and treating CVDs, and its relevant toxicological investigations. Finally, we emphasize the potential of Polygonatum in the prevention and treatment of CVDs.
Similar content being viewed by others
Background
Despite considerable progresses in prevention and treatment, cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality globally, seriously threatening to human health [1, 2]. The incidence of CVDs has been increasing in recent years and is predicted to rise to 23.6 million by 2030 [3,4,5]. The traditional Chinese medicine (TCM) have been applied in the prevention and treatment of CVDs with a long history, according to the therapeutic methods and concepts of promoting blood circulation, dissipating blood stasis, detoxifying, dredging collaterals and tonifying qi [6, 7]. Thousands of years of clinical practice have confirmed the effectiveness of Chinese herbal medicine in treating CVDs [8].
Polygonatum, a traditional Chinese herbal medicine, belongs to the genus Polygonatum in the plant family Liliaceae. The medicinal portions of Polygonatum are the dried rhizomes of Polygonatum kingianum, Polygonatum sibiricum, and Polygonatum cyrtonthema. As a natural product resource of the same origin as medicine and food, Polygonatum was originally published in Mingyi Bielu. Polygonatum is considered to have the effects of tonifying qi and nourishing yin, fortifying spleen, moistening lung, and benefiting kidney, according to the Chinese Pharmacopoeia [9].
Polygonatum contains a variety of active chemical ingredients, such as polysaccharides, steroidal saponins, flavonoids, triterpenoid saponins, alkaloids, lignans, coumarins, fatty acids, and aliphatic long-chain compounds [10, 11]. Among the multiple ingredients, Polygonatum sibiricum polysaccharides (PSPs) are abundant and hold significant medicinal value. Extensive research has been conducted on PSPs, which are recognized as a highly significant chemical ingredient of Polygonatum. A large amount of evidence has demonstrated that Polygonatum and its active ingredients have various pharmacological effects, such as antioxidant properties [12], anti-aging effects [13], immunomodulatory effects [14], antibacterial and anti-inflammatory activities [15, 16]. In addition, studies have also proved its efficacy in the prevention and treatment of cardiovascular diseases (CVDs) [17, 18], Alzheimer's disease [19, 20], diabetes [21], cancer [22], etc.
Numerous studies in vitro and in vivo have shed light on the potential benefits of Polygonatum in CVDs [23, 24]. Evidence suggests that Polygonatum may exert anti-atherosclerotic effects, protect cardiomyocytes, and attenuate myocardial fibrosis, which are achieved through the inhibition of oxidative stress, modulation of inflammatory processes, and regulation of lipid metabolism pathways [18, 25]. PSPs, the active ingredients in Polygonatum, has been reported to inhibit oxidative stress to mitigate D-galactose(D-gal)-induced cardiac damage and aging [18]. Polygonatum and its active ingredients also have anti-atherosclerotic effects in Apolipoprotein E (ApoE) gene knocked-out mice by inhibiting Toll-like receptor 4 (TLR4)-mediated activation of nuclear factor kappa-B (NF-κB) [25].
The diverse medicinal uses of Polygonatum have attracted significant attention, and researchers have focused their attention on its potential bioactive ingredients and pharmacological mechanisms. However, few researches have addressed the phytochemical composition of Polygonatum and its pharmacological properties in CVDs in recent years. This paper reviews the protective effects of Polygonatum in CVDs and its active ingredients in order to promote the pharmacological research, development, and utilization of Polygonatum.
Main chemical constituents of Polygonatum and their biological activities
Polygonatum possesses a diverse array of chemical constituents, and researchers have now isolated and identified its composition as polysaccharides, flavonoids, saponins, alkaloids, lignans, and many other types of chemical constituents from its main medicinal parts. It is actually true that Polygonatum has different origins, varieties, and chemical compositions.
Polysaccharides
Polysaccharides are the predominant chemical constituents in Polygonatum, comprising a variety of monosaccharides. In a study conducted by Zhao et al. [14], the polysaccharide compounds extracted from Polygonatum were found to be primarily composed of fructose and glucose. After optimizing the hydrolysis and analytical methods for PSPs, Zhao et al. concluded that PSPs are mainly composed of fructose, galactose, and galacturonic acid, as well as small amounts of glucose, arabinose, rhamnose, and xylose [26]. Mannose is also considered to be one of the ingredients of PSPs due to the hydrolysis of fructose into mannose and glucose under strong acidic conditions. Hu et al. used ultra-high-performance liquid chromatography quadrupole trap tandem mass spectrometry to analyze the PSPs after hydrolysis under strongly acidic conditions, which included monosaccharides such as glucose, mannose, rhamnose, galactose, ribose, and arabinose [27]. The identified compounds of PSPs are listed in Table 1.
Fructose, with the chemical formula C6H12O6, is the predominant monosaccharide found in Polygonatum. It is an isomer of glucose but exhibits a faster metabolic rate [28]. The liver is considered the primary site of fructose metabolism, although Gonzalez et al. suggested that the intestine may also play a significant role in fructose metabolism [29]. Fructose metabolism is closely associated with adipogenesis, and its metabolites can activate adipogenic transcription factors [30]. Mannose, with the chemical formula C6H12O6, is abundant in the fluids and tissues of the human body. Mannose is absorbed and metabolized by the intestines through glycolysis and the tricarboxylic acid cycle [31,32,33]. Glucose, with the chemical formula C6H12O6, is the most easily absorbed monosaccharide by the body and serves as a direct source of energy [34]. It can easily cross the blood–brain barrier and provides energy to the brain, playing a crucial role in various biological processes [35].
The polysaccharides in Polygonatum have anti-inflammatory, antioxidant, and antitumor effects [36, 37], among which glucose has an antagonistic effect on cellular reactive oxygen species levels [38].
Flavonoids
Flavonoid compounds are secondary metabolites of Polygonatum. Scientists have extracted several classes of flavonoid compounds from Polygonatum, which are categorized according to their chemical structures into the classes of homoisoflavones, isoflavones, flavonoids, chalcones, and dihydroflavonoids.
Wang et al. isolated 15 flavonoids from the rhizomes of Polygonatum cyrtonema by repeated column chromatography and preparative high-performance liquid chromatography techniques, including homoisoflavones and dihydroflavonoids [39]. In another study, Yu et al. employed high-performance liquid chromatography mass spectrometry (HPLC–MS) to isolate seven flavonoids from Polygonatum sibiricum, which mainly consisted of homoisoflavones, isoflavones, and flavonoids [40]. Additionally, Jiang et al. further identified four homoisoflavones, two dihydroflavones, two chalcones, and four isoflavones from Polygonatum sibiricum and Polygonatum kingianum [41]. The flavonoid compounds found in Polygonatum are listed in Table 2.
Polygonatum is also rich in other flavonoids, such as rutin, quercetin, isorhamnetin, kaempferol, and baicalein [19, 42,43,44]. Upon ingestion, rutin is hydrolyzed in the body and converted to quercetin, which is further metabolized to isorhamnetin after absorption in the intestines [45]. Quercetin undergoes hepatic metabolism to form glucuronic acid, methyl, and sulfate conjugates, which are eventually excreted through the kidneys [46]. Isorhamnetin, predominantly excreted through the kidneys, is found in the bloodstream and urine in the conjugated form with glucuronic acid [47]. Kaempferol is metabolized in the liver as glucuronic acid, methyl, and sulfate metabolites [46]. Baicalein undergoes methylation in the human body and is metabolized to oroxylin A, which is then converted to the final metabolites: baicalin, oroxylin A-7-O-b-D-glucuronide, and 5,7-dihydroxy-6-O-b-D-glucuronide, and is finally excreted in the urine [48].
Flavonoid compounds found in Polygonatum exhibit various beneficial effects, including antitumor, antihyperglycemic, anti-inflammatory, and antioxidant properties [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. Specifically, kaempferol has been shown to decrease the expression of transcription factors involved in lipid formation [44], while baicalein enhances the activity of the Adenosine 5ʹ-monophosphate (AMP)-activated protein kinase/nuclear factor erythroid 2-related factor/heme oxygenase-1 (AMPK/Nrf2/HO-1) signaling pathway, thereby inhibiting ferroptosis in chondrocytes [64].
Saponins
The saponin compounds found in Polygonatum can be divided into two categories: steroidal saponins and triterpenoid saponins. A total of 86 steroidal saponins and 12 triterpenoid saponins have been identified and isolated from Polygonatum. The specific saponin compounds are listed in Table 3.
Zhang et al. conducted ethanol extraction and isolated ten kinds of steroidal saponins from Polygonatum kingianum [76]. Song et al. isolated nine saponins, all steroidal saponins, from Polygonatum sibiricum by methanol extraction [77]. Among these, sibiricoside A is a specific steroidal saponin that is predominantly distributed in the stomach, small intestine, kidney, liver, and other tissues of rats, and is mainly excreted through feces [78]. Yu et al. isolated 13 saponins from Polygonatum kingianum by acetone extraction [79]. Among which, ginsenoside Rb1 exhibits anti-inflammatory, antioxidant, and anti-apoptotic effects [80, 81], and is absorbed in the human gut after oral administration, undergoing metabolism by intestinal flora to produce more active metabolites [82]. Methanol and ethanol have strong polarity, which are easier to extract steroid saponins with the same polarity. On the contrary, acetone with weak polarity, is more suitable for extracting triterpene saponins.
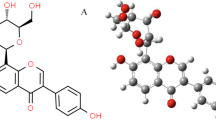
Dioscin, with the chemical formula C45H72O16, is the principal active compound among the class of Polygonatum saponins and has been extensively studied. Its structural formula is shown in Fig. 1. Dioscin has limited bioavailability, and it was discovered to be absorbed slowly in the intestinal tract of rats, with only a small amount being metabolized into diosgeninogen and primarily excreted in feces. Dioscin has been found to possess anti-inflammatory, antioxidant, and antitumor properties [83,84,85,86,87], especially has effectiveness in inhibiting adriamycin-induced oxidative damage in the myocardium [88].
Others
Polygonatum contains trace amounts of alkaloids, and 18 alkaloids have been identified. Based on their chemical structures, these alkaloids can be categorized as amides, indolizines, phenylpropanoids, quinolines, and purines [98]. Sun et al. isolated two alkaloids, namely 3-hydroxymethyl-5,6,7,8-tetrahydroindolizin-8-one(1) and 3-ethoxymethyl-5,6,7,8-tetrahydroindolizin-8-one(2) from Polygonatum [99], and their specific structures are depicted in Fig. 2a. Wang et al. isolated two alkaloids, kinganone(3) and 3-ethoxy-methyl-5,6,7,8-tetrahydro-8-indolizinone(4) from the rhizomes of Polygonatum [100], and their specific structures are illustrated in Fig. 2b. These two alkaloids exhibit weak antibacterial and antifungal activities. Additionally, Virk et al. isolated Quinine(5) from Polygonatum [101], a quinoline alkaloid with anti-malarial activity [102,103,104], and its structure is presented in Fig. 2c.
Lignans are present in minimal amounts in Polygonatum and have been identified to include syringaresinols, liriodendrin, pinoresinols, and furanones [98]. Lignans are natural compounds formed by the polymerization of two molecules of phenylpropanoid derivatives. They can be absorbed and metabolized in the intestinal tract, leading to the production of polyphenolic compounds [105]. Lignans have been shown to possess antitumor effects [106,107,108]. Osmakov et al. found that most lignans exhibit both anti-inflammatory and antioxidant properties [109].
Application and pharmacological mechanism of Polygonatum and its active ingredients in CVDs
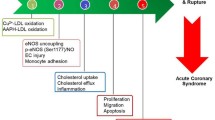
The pharmacological mechanisms underlying the preventive and therapeutic effects of Polygonatum and its active ingredients in CVDs are multifaceted, primarily encompassing anti-oxidative stress in cardiomyocytes, lipid regulation, anti-atherosclerosis, anti-inflammation, and anti-myocardial fibrosis. Moreover, Polygonatum and its active ingredients exhibit distinct advantages in preventing pharmacological cardiotoxicity. The subsequent sections will comprehensively analyze and discuss these aspects (Fig. 3, Table 4).
Pharmacological mechanism of Polygonatum for prevention and treatment of CVDs Nrf2, nuclear factor erythroid 2-related factor; HO-1, heme oxygenase-1; ROS, reactive oxygen species; NLRP3, nod-like receptor protein 3; TLR4, Toll-like receptor4; MyD88, myeloid differentiation factor88; NF-κB, nuclear factor kappa-B; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; TGF-β, transforming growth factor beta; JAK, Janus kinase; STAT3, signal transducer and activator of transcription 3; DOX, doxorubicin; MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; NO, nitric oxide; IL-6, interleukin-6; IL-1β, interleukin-1 beta; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein; Bcl-2, B-cell lymphoma-2; Bax, BCL2-associated X
Anti-oxidative stress
Oxidative stress plays a significant role in the pathogenesis of various cardiovascular diseases [110, 111]. It arises from the excessive accumulation of free radicals or reactive oxygen species (ROS) [112]. Under normal conditions, ROS levels can be kept in balance by a complex antioxidant system. During disease progression, oxidative stress injury is induced when antioxidant enzymes, such as catalase, superoxide dismutase (SOD), and glutathione/glutathione synthase (GSH/GSS), are unable to neutralize the overproduced ROS [113]. These oxygen-free radicals exceed the ability of endothelial cells to protect themselves and, through a variety of mechanisms, lead to endothelial cell dysfunction, a process that occurs in most cardiovascular diseases [110, 114, 115]. In contrast to other tissues, the myocardium is more susceptible to oxidative injury due to its high oxidative metabolism and low antioxidant defenses [116, 117]. Notably, several studies have shown that Polygonatum can exert antioxidant effects by mediating multiple oxidative stress signaling pathways, making it a natural antioxidant [118, 119].
In vitro study
PSPs were found to have scavenging effects on 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydroxyl radicals, superoxide anion radicals, and special scavenging effects on ferrous iron chelating ability, indicating that PSPs may be a potential antioxidant [118]. Li et al. [12] utilized a hypoxia inducer to execute a cardiomyocyte hypoxia model in rat H9c2 cardiomyocytes and then applied Polygonatum kingianum’s effective ingredients, saponins Gracillin and flavonoids Liquiritigenin. The results demonstrated that the intervention of 20 μM Gracillin significantly reduced the expression of malondialdehyde (MDA) and lactate dehydrogenase (LDH), while increasing the expression level of superoxide dismutase (SOD). Similarly, liquiritigenin exhibited similar effects following the intervention. Teng [120] extracted crude P. Cyrtonema and steam-processed P. cyrtonema from Polygonatum cyrtonema in vitro using different methods, and the results showed that both polysaccharides had free radical scavenging activity in vitro. Ha et al. [121] extracted seven steroidal saponins from Polygonatum, which were further found to significantly inhibit nitric oxide activity in lipopolysaccharide-activated cells.
In vivo study
In animal experiments, Li et al. discovered that the extract of Polygonatum kingianum could effectively prolong the survival time of hypoxic SD rats, increase the expression levels of SOD, glutathione peroxidase (GSH-Px), and catalase (CAT), and decrease the expression levels of MDA, LDH, and creatine kinase (CK) in the myocardial tissues of SD rats. The anti-hypoxic effect of Polygonatum kingianum may be related to the scavenging of excess free radicals and inhibition of lipid peroxidation [12]. In an in vivo antioxidant assay, PSPs at doses of 200 and 400 mg/kg/d were found to significantly reduce ROS and MDA and increase SOD levels in a mouse model of cardiac senescence induced by D-Gal (500 mg/kg/d), indicating that PSPs are able to protect myocardial tissues by exerting antioxidant capacity [18].
In addition, it was shown that PSPs attenuated myocardial fibrotic injury in autoimmune myocarditis (AM) rats, demonstrating that PSPs decreased myocardial tissue MDA content and serum levels of tumor necrosis factor-α (TNF-α), Interleukin-6 (IL-6), and transforming growth factor beta1 (TGF-β1), and increased myocardial tissue Janus kinase phosphorylation /Janus kinase(p-JAK/JAK), STAT3 phosphorylation/signal transducer and activator of transcription 3 (p-STAT3/STAT3), SOD content and levels, suggesting that PSPs inhibit oxidative stress and inflammatory responses in AM rats through activation of the JAK/STAT3 signaling pathway [122]. In a mouse model of oxidative damage, treatment with PSPs significantly reversed histopathological alterations, decreased ROS accumulation, increased antioxidant enzyme activities, and promoted nuclear translocation of Nrf2 by decreasing Keap-1 expression and increasing HO-1 expression. It is suggested that this antioxidant activity of Polygonatum is closely related to the activation of the NRF2/HO-1 pathway [120, 123].
Lipid and glucose regulation, and anti-atherosclerosis
Hyperglycemia and dyslipidemia are widely recognized as significant contributors to cardiovascular disease [124], specifically in the pathogenesis of atherosclerosis [125]. Research has demonstrated that abnormalities in lipoproteins play a critical role in driving the development of atherosclerotic CVDs [126]. Consequently, the identification of effective drugs and therapeutic targets for early intervention in atherosclerosis holds great importance in reducing mortality. Several studies have highlighted that Polygonatum and its active ingredients, such as polysaccharides, flavonoids and saponins, have hypoglycemic and lipid-lowering activities, and have shown better efficacy in the treatment of atherosclerosis [127, 128].
In vitro study
PSPs was reported to reduce lipid accumulation and diminish the expression level of inflammatory factors in insulin-resistant cell models by regulating microRNA-140-5p/interleukin-1 receptor-associated kinase 3 (miR-340-3p/IRAK3), which plays a crucial role in inhibiting atherogenesis [129]. Different from the lipid-lowering effect of PSPs, other studies have revealed that steroid saponins and homoisoflavones extracted from Polygonatum have potential hypoglycemic effects [130,131,132], and the latter has been found to be able to inhibit the formation of advanced glycosylation end products [131]. Recent studies demonstrated that homoisoflavones were also effective glucose transporter 2 (GLUT 2) inhibitors [133], providing a novel mechanism for the hypoglycemic properties of Polygonatum.
In vivo study
Zhu et al. [134] investigated the role of PSPs in atherosclerosis by feeding adult golden hamsters an atherosclerotic diet containing 1.5 mL of olive oil, 8 mg (3,200,000 IU) of vitamin D2, and 40 mg of cholesterol for 60 consecutive days to construct an atherosclerosis model. Subsequent intervention with PSPs for 60 days showed significant amelioration in serum lipid profile, lipoproteins, and endothelial dysfunction parameters, as well as normalization of the morphology of the aorta and myocardial tissues compared with the model group. Ye et al. [25] constructed a ApoE−/− high-fat mouse model and intervened with PSPs. Compared to the control group, PSPs significantly suppressed the expression of serum lipids including Low-Density Lipoprotein Cholesterol (LDL-C), total cholesterol (TC), and triglycerides, as well as cell adhesion molecules including vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). Histomorphometric analysis revealed that PSPs reduced aortic lipid accumulation and mitigated aortic intimal hyperplasia and inflammatory cell infiltration. These effects may be attributed to the inhibition of the TLR4/MyD88/NF-κB signaling pathway. Yang et al. [135] found similar effects of PSPs in an atherosclerosis model in rabbits. In addition, Polygonatum alleviated lipid metabolism disorders in rats by mediating a network of miRNAs pivoting on the miR-484-Bacteroides/Roseburia axis to prevent hyperlipidemia and atherosclerosis [136].
Consistent with the results of in vitro studies, in vivo experiments also confirmed the hypoglycemic properties of saponins in Polygonatum. It can significantly alleviate insulin resistance of diabetic mice [137]. It was also reported that the combination of Polygonatum sibiricum saponin and probiotics had a better hypoglycemic effect on diabetic mice, bringing more benefits [138]. The reduction of serum glucose could prevent the occurrence of CVDs such as atherosclerosis to a certain extent [139].
Anti-inflammation and anti-myocardial fibrosis
Inflammation and myocardial fibrosis are recognized as critical factors in the development of cardiovascular disease, ultimately leading to the onset of heart failure [140,141,142,143]. On the one hand, during myocardial injury, irrespective of the initiating cause, aseptic myocardial inflammation occurs, which in turn initiates an elevation in the levels of circulating inflammatory factors [144]. On the other hand, the extracellular matrix (ECM) provides a framework for the cardiac myocytes, thereby ensuring the structural and functional integrity of the heart [145]. However, excessive accumulation of ECM in the cardiac interstitium leads to fibrous remodeling of the myocardium, causing contractile dysfunction of the myocardium [146, 147]. In injured myocardial tissues, inflammation and fibrosis often coexist, with substantial infiltration of inflammatory factors observed in the fibrotic myocardium [148]. Studies have demonstrated that Polygonatum exerts its effects by attenuating inflammatory response and fibrosis through the modulation of multiple signaling pathways, including the TLR4-MyD88-NF-kappa B and AMPK-Nrf2 pathways [16, 149,150,151].
In vitro study
Zhao et al. [152] identified 27 active components through chemical studies of Polygonatum sibiricum Red. Led. Thirteen of these compounds were subsequently investigated for their anti-inflammatory activities, and the results showed that among the 13 compounds, compound β-carboline significantly inhibited the expression of Nitric Oxide (NO), TNF-α, IL-6, and interleukin-1 beta (IL-1β) in lipopolysaccharide (LPS)-treated RAW264.7 macrophages and suppressed the activation of NF-κB, suggesting that compound β-carboline has effective anti-inflammation activities. Lei constructed an H/R model in H9c2 cardiomyocyte in vitro and found that after PSPs intervention, the cell survival rate was significantly increased, the content of inflammatory cytokines and the expression of NF-κB protein were significantly decreased, the expression of inhibitor κB (I-κB) protein was increased, and the level of mRNA expression of TLR4 and MyD88 was significantly decreased, as compared with that of the model group. It is suggested that PSPs may protect H9c2 cardiomyocytes from H/R injury by inhibiting the TLR4-MyD88-NF-κB pathway [153].
In vivo study
Yin et al. constructed an autoimmune myocarditis model in SD rats and applied PSPs as an intervention. The results showed that PSPs reduced the levels of TNF-α, IL-6, and TGF-β1, and attenuated myocardial pathological injury and fibrosis in rats. These effects may be attributed to the activation of the JAK/STAT3 signaling pathway [122]. In a study conducted by Zhang et al. [154] using diabetic cardiomyopathic rats, PSPs were administered as an intervention, resulting in a significant improvement in myocardial tissue fibrosis. The mechanism underlying this improvement may be associated with the mediation of the TGF-β1/Smads signaling pathway. Similarly, other researchers have obtained similar conclusions in a rat model of diabetic cardiomyopathy, demonstrating that PSPs can restore the morphology of myocardial tissues and attenuate myocardial fibrosis. These effects may be mediated through the inhibition of the Nod-like receptor protein 3 (NLRP3) inflammasome [155, 156]. In myocardial infarction rats, PSPs were found to improve myocardial injury, reduce inflammatory response, and aid in the repair of ischemic myocardium in acute myocardial infarction rats. The mechanism underlying these effects may be associated with the modulation of NF-κB-mediated inflammatory response [157]. Furthermore, Hirai et al. [158] demonstrated that the methanolic extract of Polygonatum exhibited a cardiotonic effect. They observed an increase in the pressure in the left atrium of rats following the administration of the extract. Additionally, the extract effectively inhibited the activity of cyclic adenosine monophosphate (cAMP). The cardiotonic effect was found to be induced by the activation of sympathetic nerves and stimulation of β-adrenergic receptors.
Amelioration of drug cardiotoxicity
Drug cardiotoxicity refers to the adverse effects of drugs that result in myocardial injury, arrhythmias, abnormalities in cardiac function (systolic or diastolic), cardiac hypertrophy, and in severe cases, heart failure [159]. This issue has become particularly common in the field of oncologic cardiology, where cancer treatments can have detrimental effects on the heart [160,161,162]. Typical indicators of cardiotoxicity include decreased left ventricular ejection fraction, reduced overall longitudinal strain compared to baseline, and elevated levels of myocardial injury markers such as cardiac troponin T (cTnT), Brain Natriuretic Peptide (BNP), and N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) [163]. Cardiotoxicity associated with traditional cancer treatments, such as those caused by chemotherapeutic agents, targeted agents, and the emerging interest in immune checkpoint inhibitor cardiomyopathy, lacks therapeutic agents, and the prevention and treatment strategies have significant room for optimization [162].
Cardiotoxicity is a crucial factor that limits the use of certain drugs [164, 165]. For instance, some chemotherapeutic drugs like anthracyclines carry the risk of dose-dependent cardiotoxicity, increasing the likelihood of heart failure and restricting their clinical application [166]. The mechanism underlying chemotherapy drug-induced cardiotoxicity is highly complex and involves mitochondrial damage, oxidative stress, and cell death. It is a multifactorial interaction of various factors contributing to the development and progression of cardiotoxicity [167,168,169]. Some previous studies have shown that among some natural products and herbal extracts, tanshinone IIA, salicylic acid, ginsenoside Rg3, ginkgolide B, curcumin, resveratrol, and hexane/ethanol extract of licorice can inhibit ROS production by decreasing mitochondrial lipid peroxidation in cardiomyocytes, inhibiting cardiomyocyte apoptosis, increasing antioxidant activity, and inhibiting inflammation, thus antagonize chemotherapy-induced structural damage in cardiomyocytes [170,171,172].
Polygonatum has been found to exhibit a protective effect against pharmacological cardiotoxicity. Zhu et al. [17] administered PSPs to rats at doses of 100, 200, and 400 mg/kg for 5 days, followed by an intraperitoneal injection of adriamycin on day 6 to induce acute heart failure (AHF). The results showed that compared to the model group, the administration of PSPs significantly improved cardiac function. Additionally, the levels of serum myocardial injury markers cTnT and creatine kinase-MB (CK-MB) were significantly decreased. PSPs also elevated the levels of myocardial Na+-K+-ATPase, Ca2+-Mg2+-ATPase, succinate dehydrogenase, and B-cell lymphoma-2 (Bcl-2) protein expression. Moreover, PSPs reduced the levels of TNF-α, IL-6, MDA, and NO, as well as the protein expression of myocardial BCL2-Associated X (Bax) and cleaved caspase-3, indicating a significant reduction in cardiomyocyte apoptosis. These findings suggest that PSPs can prevent adriamycin-induced AHF and the mechanism may be attributed to their anti-oxidative stress, anti-inflammatory, and anti-cardiomyocyte apoptosis properties. Dioscin is the predominant active ingredient in Polygonatum saponins. Zhao et al. [88] investigated the efficacy and mechanism of dioscin against drug cardiotoxicity, another active ingredient found in Polygonatum. They established in vivo and in vitro models of doxorubicin (DOX)-induced myocardial injury, and the results demonstrated that dioscin increased the viability of H9c2 cells, reduced the expression levels of CK and LDH, and improved histopathological changes and cardiac function in rats and mice exposed to DOX. Furthermore, dioscin exhibited significant inhibition of myocardial oxidative damage both in vitro and in vivo. In addition, dioscin activated the Nrf2 and Sirt2 signaling pathways, leading to the modulation of HO-1, Quinone Oxidoreductase 1 (NQO1), glutathione-S-transferase (Gst), glutamate cysteine ligase modifier subunit (GCLM), Keap1, and Forkhead box O3 (FOXO3a) expression. Moreover, it decreased the expression level of miR-140-5p. These findings suggest that dioscin mitigated DOX-induced cardiotoxicity by regulating miR-140-5p-mediated myocardial oxidative stress. In a word, the active ingredient found in Polygonatum shows great potential for application in the field of cardiotoxicity, which can be developed and utilized as a potential candidate for future drug development.
Discussion
Polygonatum, as a traditional herbal medicine, has a long history of medicinal use and precise clinical efficacy and has a broad development prospect. Nowadays, thanks to the rapid development of separation means and structure identification techniques, people have carried out in-depth studies on the active ingredients of Polygonatum, optimized and identified its extraction process and chemical composition, and further clarified the pharmacokinetic characteristics of Polygonatum’s to promote the application and promotion of the drug. Polysaccharides, flavonoids, and saponins are the three main constituents studied most in Polygonatum, while research reports on the other trace constituents are not deep enough. In addition, the biological activities of the various chemical ingredients of Polygonatum need to be further developed, which is of great significance in exploring its pharmacological effects.
In this review, we systematically summarized the pharmacological effects and underlying mechanisms of Polygonatum and its main active ingredients (Polysaccharides, Flavonoids, Saponins) in the prevention and treatment of CVDs. Polygonatum possesses significant antioxidant activity and can play a cardioprotective role by activating NRF2/HO-1 and JAK/STAT3 signaling pathways [120,121,122,123]. Polygonatum has also been shown to attenuate myocardial fibrosis and inflammation and restore the morphology and function of myocardial tissue by regulating AMPK-Nrf2, NF-κB, TGF-β1/Smads, and NLRP3 inflammasome signaling pathway [152,153,154,155,156,157]. In addition, Polygonatum also has significant efficacy in lipid regulation and anti-atherosclerosis, which can prevent and control the occurrence of atherosclerosis by regulating metabolic abnormality, reducing lipid accumulation, inhibiting hyperglycemia, and reducing vascular damage, so as to intervene in the risk factors of CVDs [129,130,131,132,133,134,135,136,137,138,139]. Atherosclerosis is closely related to hyperlipidemia, inflammation, and oxidative stress, so it is clear that the pharmacological effects exerted by Polygonatum are intertwined.
Impressively, Polygonatum is demonstrated to have protective effects against cardiotoxicity induced by anticancer therapy and other compounds [17, 88], which is a unique highlight of Polygonatum in preventing and treating CVDs. In the future, it is necessary to further elucidate the underlying pharmacological mechanism to promote the application of Polygonatum in the field of Cardio-Oncology. In summary, the existing studies suggest that Polygonatum may be a potential therapeutic agent for CVDs, and further clarification of the pharmacological mechanisms of Polygonatum and its monomer ingredients or active sites are necessary, so as to provide a more rigorous and systematic basis for clinical applications.
Toxicological studies of drugs are an important part of the process of clinical use. Existing studies have shown that Polygonatum can reduce its volatile components after concoction, which plays a certain role in reducing toxicity. Some of the acute and chronic toxicity studies done on animals have also shown that Polygonatum has very low toxicity and did not cause serious toxicity or death in animals, and no genotoxicity was found either. However, there is still a great lack of high-quality studies on the clinical application, toxicity and side effects of Polygonatum, so it is difficult to determine the possible chronic accumulation caused by long-term use of Polygonatum. Further studies are still needed to determine the optimal therapeutic dose, safe dosing range, and recommended duration of Polygonatum. This suggests that the toxicologic evaluation of Polygonatum must be fully considered in future studies in the pharmacologic study of Polygonatum. Therefore, further studies are needed to establish an accurate, rapid, reliable, and sensitive modern method of analyzing toxic components to mitigate toxicity to ensure its safe use.
Conclusions and prospects
In recent years, significant progress has been made in the research on the application of Polygonatum and its active ingredients in cardiovascular diseases. Based on the available evidence, the active ingredients of Polygonatum, including polysaccharides, flavonoids, and saponins, have shown potential cardioprotective effects through various mechanisms such as anti-oxidative stress, anti-inflammation, anti-fibrosis, lipid regulation, and anti-atherosclerosis. Moreover, Polygonatum exhibits a broad therapeutic scope for drug-induced cardiotoxicity. Despite these promising findings, there are several limitations in the current research on Polygonatum. Firstly, many pharmacological mechanisms of Polygonatum in the prevention and treatment of cardiovascular diseases remain unclear, and the existing studies are relatively isolated, emphasizing the need for more extensive and in-depth research in the future. Secondly, most pharmacological studies have been conducted at the animal and cellular levels, with limited evaluation of its clinical applications and a lack of high-quality evidence-based medical evidence. Therefore, further studies are necessary to validate the potential cardioprotective effects of Polygonatum in patients with cardiovascular diseases. Polygonatum, as a traditional Chinese medicine with a long history of clinical use, requires more rigorous investigations into its active ingredients, pharmacological mechanisms, pharmacokinetics, and toxicology. These studies are crucial for the wider adoption and modernization of Chinese medicine.
Availability of data and materials
Not applicable.
Abbreviations
- AHF:
-
Acute heart failure
- AM:
-
Autoimmune myocardioptis
- AMPK:
-
Adenosine 5ʹ-monophosphate (AMP)-activated protein kinase
- ApoE:
-
Apolipoprotein E
- ATP:
-
Adenosine triphosphate
- Bax:
-
BCL2-Associated X
- Bcl-2:
-
B-cell lymphoma-2
- BNP:
-
Brain Natriuretic Peptide
- cAMP:
-
Cyclic adenosine monophosphate
- CAT:
-
Catalase
- CK:
-
Creatine kinase
- CK-MB:
-
Creatine kinase-MB
- cTnT:
-
Cardiac troponin T
- CVDs:
-
Cardiovascular diseases
- D-gal:
-
D-galactose
- DOX:
-
Doxorubicin
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ECM:
-
Extracellular matrix
- FOXO3a:
-
Forkhead box O3
- GCLM:
-
Glutamatecysteine ligase modifier subunit
- GLUT 2:
-
Glucose transporter
- GSH:
-
Glutathione
- GSH-Px:
-
Glutathione peroxidase
- GSS:
-
Glutathione synthetase
- Gst:
-
Glutathione-S-transferase
- H/R:
-
Hypoxia/reoxygenation
- HO-1:
-
Heme oxygenase-1
- HPLC–MS:
-
High Performance Liquid Chromatography-Mass Spectrometry
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL-1β:
-
Interleukin-1 beta
- IL-6:
-
Interleukin-6
- IRAK3:
-
Interleukin-1 receptor-associated kinase 3
- I-κB:
-
Inhibitor κB
- Keap1:
-
Kelch-1ike ECH-associated protein l
- LDH:
-
Lactate dehydrogenase
- LDL-C:
-
Low-Density Lipoprotein Cholesterol
- Lp(a):
-
Lipoprotein(a)
- LPS:
-
Lipopolysaccharide
- MDA:
-
Malondialdehyde
- miR-140-5p:
-
MicroRNA-140-5p
- miR-340-3p:
-
MicroRNA-340-3p
- MyD88:
-
Myeloid differentiation factor88
- NF-κB:
-
Nuclear factor kappa-B
- NLRP3:
-
Nod-like receptor protein 3
- NO:
-
Nitric Oxide
- NQO1:
-
NADPH: Quinone Oxidoreductase 1
- Nrf2:
-
Nuclear factor erythroid 2-related factor
- NT-proBNP:
-
N-Terminal Pro-Brain Natriuretic Peptide
- p-JAK/JAK:
-
Janus kinase
- PSP:
-
Polygonatum sibiricum Polysaccharides
- p-STAT3/STAT3:
-
STAT3 phosphorylation/Signal Transducer And Activator Of Transcription 3
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TC:
-
Total cholesterol
- TGF-β1:
-
Transforming growth factor-beta1
- TLR4:
-
Toll-like receptor4
- TNF-α:
-
Tumor necrosis factor-α
- VCAM-1:
-
Vascular cell adhesion molecule-1
References
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25.
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18-209.
Simon-Yarza T, Bataille I, Letourneur D. Cardiovascular bio-engineering: current State of the Art. J Cardiovasc Transl Res. 2017;10(2):180–93.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American heart association. Circulation. 2014;129(3):e28-292.
Gaidai O, Cao Y, Loginov S. Global cardiovascular diseases death rate prediction. Curr Probl Cardiol. 2023;48(5):101622.
Yang Y, Shao M, Cheng W, Yao J, Ma L, Wang Y, et al. A pharmacological review of tanshinones, naturally occurring monomers from Salvia miltiorrhiza for the treatment of cardiovascular diseases. Oxid Med Cell Longev. 2023;2023:3801908.
Li D, Li Y, Yang S, Yu Z, Xing Y, Wu M. Mechanism and potential target of blood-activating Chinese botanical drugs combined with anti-platelet drugs: prevention and treatment of atherosclerotic cardiovascular diseases. Front Pharmacol. 2022;13:811422.
Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. 2017;6(24):2952–66.
Luo L, Qiu Y, Gong L, Wang W, Wen R. A review of Polygonatum Mill. Genus: Its taxonomy, chemical constituents, and Pharmacological effect due to processing changes. Molecules. 2022;27(15):4821.
Li XL, Ma RH, Zhang F, Ni ZJ, Thakur K, Wang S, et al. Evolutionary research trend of Polygonatum species: a comprehensive account of their transformation from traditional medicines to functional foods. Crit Rev Food Sci Nutr. 2023;63(19):3803–20.
Gong H, Gan X, Li Y, Chen J, Xu Y, Shi S, Li T, Li B, Wang H, Wang S. Review on the genus Polygonatum polysaccharides: extraction, purification, structural characteristics and bioactivities. Int J Biol Macromol. 2023;229:909–30.
Li X, Mei M, Pu X, Chen X, Li X, Meng F, et al. Protective effect and mechanism of Polygonatum kingianum against hypoxia-induced injury. Heliyon. 2023;93:e14353.
Zhang X, Ni L, Hu S, Yue B, Chen X, Yuan D, et al. Polygonatum sibiricum ameliorated cognitive impairment of naturally aging rats through BDNF-TrkB signaling pathway. J Food Biochem. 2022;46(12):e14510.
Zhao P, Zhou H, Zhao C, Li X, Wang Y, Wang Y, et al. Purification, characterization and immunomodulatory activity of fructans from Polygonatum odoratum and P. cyrtonema. Carbohydr Polym. 2019;214:44–52.
He L, Yan B, Yao C, Chen X, Li L, Wu Y, et al. Oligosaccharides from Polygonatum Cyrtonema Hua: structural characterization and treatment of LPS-induced peritonitis in mice. Carbohyd Polym. 2021;255:117392.
Shen F, Song Z, Xie P, Li L, Wang B, Peng D, et al. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J Ethnopharmacol. 2021;275:114164.
Zhu X, Wu W, Chen X, Yang F, Zhang J, Hou J. Protective effects of Polygonatum sibiricum polysaccharide on acute heart failure in rats 1. Acta Cir Bras. 2018;33(10):868–78.
Ma W, Wei S, Peng W, Sun T, Huang J, Yu R, et al. Antioxidant effect of Polygonatum sibiricum polysaccharides in D-galactose-induced heart aging mice. Biomed Res Int. 2021;2021:6688855.
Wang F, Chen H, Hu Y, Chen L, Liu Y. Integrated comparative metabolomics and network pharmacology approach to uncover the key active ingredients of Polygonati rhizoma and their therapeutic potential for the treatment of Alzheimer’s disease. Front Pharmacol. 2022;13:934947.
Luo S, Zhang X, Huang S, Feng X, Zhang X, Xiang D. A monomeric polysaccharide from Polygonatum sibiricum improves cognitive functions in a model of Alzheimer’s disease by reshaping the gut microbiota. Int J Biol Macromol. 2022;213:404–15.
Liu S, Jia QJ, Peng YQ, Feng TH, Hu ST, Dong JE, et al. Advances in mechanism research on Polygonatum in prevention and treatment of diabetes. Front Pharmacol. 2022;13:758501.
Long T, Liu Z, Shang J, Zhou X, Yu S, Tian H, et al. Polygonatum sibiricum polysaccharides play anti-cancer effect through TLR4-MAPK/NF-kappaB signaling pathways. Int J Biol Macromol. 2018;111:813–21.
Xie P, Chen L, Wang J, Wang X, Yang S, Zhu G. Polysaccharides from Polygonatum cyrtonema Hua prevent post-traumatic stress disorder behaviors in mice: mechanisms from the perspective of synaptic injury, oxidative stress, and neuroinflammation. J Ethnopharmacol. 2023;319(Pt1):117165.
Su J, Wang Y, Yan M, He Z, Zhou Y, Xu J, et al. The beneficial effects of Polygonatum sibiricum Red. superfine powder on metabolic hypertensive rats via gut-derived LPS/TLR4 pathway inhibition. Phytomedicine. 2022;106:154404.
Ye G, Zhao Y, Zhu J, Zhang Z, Wang Q, Jiang X, et al. Synergistic effect of Polydatin and Polygonatum sibiricum polysaccharides in combating atherosclerosis via suppressing TLR4-mediated NF-kappaB activation in ApoE-deficient mice. Evid Based Complement Alternat Med. 2022;2022:3885153.
Zhao P, Li X, Wang Y, Zhang X, Jia H, Guo L, et al. Comparative studies on characterization, saccharide mapping and antiglycation activity of polysaccharides from different Polygonatum ssp. J Pharm Biomed Anal. 2020;186:113243.
Hu J, Cheng H, Xu J, Liu J, Xing L, Shi S, et al. Determination and analysis of monosaccharides in Polygonatum cyrtonema Hua polysaccharides from different areas by ultra-high-performance liquid chromatography quadrupole trap tandem mass spectrometry. J Sep Sci. 2021;44(18):3506–15.
Liu L, Li T, Liao Y, Wang Y, Gao Y, Hu H, et al. Triose kinase controls the Lipogenic potential of fructose and dietary tolerance. Cell Metab. 2020;32(4):605-18.e7.
Gonzalez JT, Betts JA. Dietary fructose metabolism by splanchnic organs: size matters. Cell Metab. 2018;27(3):483–5.
Herman MA, Samuel VT. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab. 2016;27(10):719–30.
Sharma V, Ichikawa M, Freeze HH. Mannose metabolism: more than meets the eye. Biochem Biophys Res Commun. 2014;453(2):220–8.
Saito Y, Kinoshita M, Yamada A, Kawano S, Liu HS, Kamimura S, et al. Mannose and phosphomannose isomerase regulate energy metabolism under glucose starvation in leukemia. Cancer Sci. 2021;112(12):4944–56.
Slade PG, Caspary RG, Nargund S, Huang CJ. Mannose metabolism in recombinant CHO cells and its effect on IgG glycosylation. Biotechnol Bioeng. 2016;113(7):1468–80.
Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36(10):587–97.
Adeva-Andany MM, Perez-Felpete N, Fernandez-Fernandez C, Donapetry-Garcia C, Pazos-Garcia C. Liver glucose metabolism in humans. Biosci Rep. 2016;36(6):e00416.
Gonzalez PS, O’Prey J, Cardaci S, Barthet VJA, Sakamaki JI, Beaumatin F, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature. 2018;563(7733):719–23.
Wang J, Jalali Motlagh N, Wang C, Wojtkiewicz GR, Schmidt S, Chau C, et al. D-mannose suppresses oxidative response and blocks phagocytosis in experimental neuroinflammation. Proc Natl Acad Sci U S A. 2021;118(44):e2107663118.
Wang CZY, Li F, Wei Y. Conserved roles of glucose in suppressing reactive oxygen species-induced cell death and animal survival. Aging. 2019;11(15):5726–43.
Wang W, Dabu X, He J, Yang H, Yang S, Chen J, et al. Polygonatone H, a new homoisoflavanone with cytotoxicity from Polygonatum Cyrtonema Hua. Nat Prod Res. 2018;33(12):1727–33.
Yu YM, Ma XY, Zhang TJ, Tang C. Rapid characterization on components of Polygonatum Sibiricum based on HPLC-MS technology. Lishizhen Med Mate Med Res. 2016;27(4):794–6.
Jiang CX, Zhang TJ, Chen CQ, Xiao XK, Liu LCX. Research progress in Polygonati Rhizoma and predictive analysis on Q-marker. Chin Tradit Herb Drugs. 2017;48(1):1–16.
Mu C, Sheng Y, Wang Q, Amin A, Li X, Xie Y. Potential compound from herbal food of Rhizoma Polygonati for treatment of COVID-19 analyzed by network pharmacology: Viral and cancer signaling mechanisms. J Funct Foods. 2021;77:104149.
Guo KL, Liu JP, Zhao CB, Shi YH, Wang B, Quan LN, et al. Enzymatic-ultrasonic assisted extraction of total flavonoids from Shaanxi Polygonatum sibiricum and in vitro evaluation of their anti-oxidant and anti-A549 proliferation activities. Nat Prod Res Dev. 2022;34:630–8.
Park UH, Jeong JC, Jang JS, Sung MR, Youn HS, Lee SJ, et al. Negative regulation of adipogenesis by kaempferol, a component of Rhizoma Polygonati falcatum in 3T3-L1 cells. Biol Pharm Bull. 2012;35(9):1525–33.
Manach C, Morand C, Demigne C, Texier O, Regerat F, Remesy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409(1):12–6.
Dabeek WM, Marra MV. Dietary quercetin and Kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. 2019;11(10):2288.
Graefe EU, Wittig J, Mueller S, Riethling AK, Uehleke B, Drewelow B, et al. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001;41(5):492–9.
Zhang R, Cui Y, Wang Y, Tian X, Zheng L, Cong H, et al. Catechol-O-methyltransferase and UDP-glucuronosyltransferases in the metabolism of baicalein in different species. Eur J Drug Metab Pharmacokinet. 2017;42(6):981–92.
Fernandes AA, Novelli EL, Okoshi K, Okoshi MP, Di Muzio BP, Guimaraes JF, et al. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed Pharmacother. 2010;64(3):214–9.
Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed Pharmacother. 2017;96:305–12.
Ghanbari-Movahed M, Mondal A, Farzaei MH, Bishayee A. Quercetin- and rutin-based nano-formulations for cancer treatment: a systematic review of improved efficacy and molecular mechanisms. Phytomedicine. 2022;97:153909.
Gonzalez-Arceo M, Gomez-Lopez I, Carr-Ugarte H, Eseberri I, Gonzalez M, Cano MP, et al. Anti-obesity effects of Isorhamnetin and Isorhamnetin conjugates. Int J Mol Sci. 2022;24(1):299.
Li S, Li J, Pan R, Cheng J, Cui Q, Chen J, et al. Sodium rutin extends lifespan and health span in mice including positive impacts on liver health. Br J Pharmacol. 2022;179(9):1825–38.
Li Y, Fan B, Pu N, Ran X, Lian T, Cai Y, et al. Isorhamnetin suppresses human gastric cancer cell proliferation through mitochondria-dependent apoptosis. Molecules. 2022;27(16):5191.
Devi KP, Malar DS, Nabavi SF, Sureda A, Xiao J, Nabavi SM, et al. Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res. 2015;99:1–10.
Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, Imran A, et al. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24(12):2277.
Wang X, Yang Y, An Y, Fang G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed Pharmacother. 2019;117:109086.
Dong X, Zhou S, Nao J. Kaempferol as a therapeutic agent in Alzheimer’s disease: evidence from preclinical studies. Ageing Res Rev. 2023;87:101910.
Patel RV, Mistry BM, Shinde SK, Syed R, Singh V, Shin HS. Therapeutic potential of quercetin as a cardiovascular agent. Eur J Med Chem. 2018;155:889–904.
Yan W, Ma X, Zhao X, Zhang S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther. 2018;12:3961–72.
Tuli HS, Aggarwal V, Kaur J, Aggarwal D, Parashar G, Parashar NC, et al. Baicalein: a metabolite with promising antineoplastic activity. Life Sci. 2020;259:118183.
Li YY, Wang XJ, Su YL, Wang Q, Huang SW, Pan ZF, et al. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacol Sin. 2021;43(6):1495–507.
Di Petrillo A, Orru G, Fais A, Fantini MC. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res. 2022;36(1):266–78.
Wan Y, Shen K, Yu H, Fan W. Baicalein limits osteoarthritis development by inhibiting chondrocyte ferroptosis. Free Radic Biol Med. 2023;196:108–20.
Yu HS, Zhang J, Kang LP, Han LF, Zou P, Zhao Y, et al. Three new saponins from the fresh rhizomes of Polygonatum kingianum. Chem Pharm Bull. 2009;57(1):1–4.
Gan LS, Chen JJ, Shi MF, Zhou CX. A New Homoisoflavanone from the Rhizomes of Polygonatum Cyrtonema. Nat Product Commun. 2013;8(5):1934578X300800.
Xu J, Liu L, Yang S, Kuang Y. Chemical constituents from aerial part of Polygonatum cyrtonema. Chin Tradit Herb Drugs. 2016;47(20):3569–72.
Ren H, Deng Y, Zhang J, Ye X, Xia L, Liu M, et al. Research progress on processing history evolution, chemical components and pharmacological effects of Polygonati Rhizoma. Zhongguo Zhong Yao Za Zhi. 2020;45(17):4163–82.
De Wang YF, Mu TH, Chen JJ, Luo SD. Studies on chemical constituents from the root of Polygonatum kingianum. Zhongguo Zhong Yao Za Zhi. 2003;28(6):525–7.
Gao Y, Qi CL, Zhang L, Yue XM, Wang H. Studies on the chemical constituents of fresh Polygonatum sibiricum. Pharm Clin Res. 2015;23(4):365–7.
Wang J, Lu CS, Liu DY, Xu YT, Zhu Y, Wu HH. Constituents from Polygonatum sibiricum and their inhibitions on the formation of advanced glycosylation end products. J Asian Nat Prod Res. 2016;18(7):697–704.
Li X, Lai GF, Wang YF, Zhang BG, Luo SD. Study on the chemical composition of dian huangjin. Chin Tradit Herb Drugs. 2008;39(6):825–8.
Chen H, Feng S, Sun YJ, Hao Z, Feng WS, Zheng XK. Research progress on chemical composition and pharmacological activity of three medicinal Polygonati Rhizoma. Chin Tradit Herb Drugs. 2015;46(15):2329–38.
Chen H, Li YJ, Li XF, Sun YJ, Li HW, Su FY, et al. Homoisoflavanones with estrogenic activity from the rhizomes of Polygonatum sibiricum. J Asian Nat Prod Res. 2018;20(1):92–100.
Wang WX, Zhang X, Dabu XLT, He J, Yang SC, Chen JW, et al. Analysis of chemical constituents from Polygonatum cyrtonema after “Nine-Steam-Nine-Bask” processing. Phytochem Lett. 2019;29:35–40.
Zhang J, Ma BP, Kang LP, Yu HS, Yang Y, Yan XZ, et al. Furostanol saponins from the fresh rhizomes of Polygonatum kingianum. Chem Pharm Bull. 2006;54(7):931–5.
Son KH, Do JC, Kang SS. Steroidal saponins from the rhizomes of Polygonatum sibiricum. J Nat Prod. 1990;53(2):333–9.
Zhang YF, Liu LJ, Xu F, Shang MY, Liu GX, Cai SQ. Investigation of the in vivo metabolism of sibirioside a and angoroside C in rats by HPLC-ESI-IT-TOF-MS(n). Molecules. 2018;23(10):2702.
Yu HS, Ma BP, Song XB, Kang LP, Zhang T, Fu J, et al. Two new steroidal saponins from the processed Polygonatum kingianum. Helv Chim Acta. 2010;93(6):1086–92.
Zhou P, Xie W, He S, Sun Y, Meng X, Sun G, et al. Ginsenoside Rb1 as an anti-diabetic agent and Its underlying mechanism analysis. Cells. 2019;8(3):204.
Jiang L, Yin X, Chen YH, Chen Y, Jiang W, Zheng H, et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics. 2021;11(4):1703–20.
Kim HK. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J Ginseng Res. 2013;37(4):451–6.
Tao X, Xu L, Yin L, Han X, Qi Y, Xu Y, et al. Dioscin induces prostate cancer cell apoptosis through activation of estrogen receptor-beta. Cell Death Dis. 2017;8(8):e2989.
Li XL, Ma RH, Ni ZJ, Thakur K, Cespedes-Acuna CL, Wang S, et al. Dioscin inhibits human endometrial carcinoma proliferation via G0/G1 cell cycle arrest and mitochondrial-dependent signaling pathway. Food Chem Toxicol. 2021;148:111941.
Jiang W, Lin M, Wang Z. Dioscin: a new potential inhibitor of Skp2 for cancer therapy. EBioMedicine. 2020;51:102593.
Wu MM, Wang QM, Huang BY, Mai CT, Wang CL, Wang TT, et al. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol Res. 2021;172:105796.
Song S, Chu L, Liang H, Chen J, Liang J, Huang Z, et al. Protective effects of dioscin against doxorubicin-induced hepatotoxicity via regulation of Sirt1/FOXO1/NF-kappab signal. Front Pharmacol. 2019;10:1030.
Zhao L, Tao X, Qi Y, Xu L, Yin L, Peng J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018;16:189–98.
Zhang HY, Hu WC, Ma GX, Zhu NL, Sun XB, Wu HF, et al. A new steroidal saponin from Polygonatum sibiricum. J Asian Nat Prod Res. 2018;20(6):586–92.
Li XC, Yang CR, Ichikawa M, Matsuura H, Kasai R, Yamasaki K. Steroid saponins from Polygonatum kingianum. Phytochemistry. 1992;31(10):3559–63.
Ahn MJ, Kim CY, Yoon KD, Ryu MY, Cheong JH, Chin YW, et al. Steroidal saponins from the rhizomes of Polygonatum sibiricum. J Nat Prod. 2006;69(3):360–4.
Yu HS, Ma BP, Kang LP, Zhang T, Jiang FJ, Zhang J, et al. Saponins from the processed rhizomes of Polygonatum kingianum. Chem Pharm Bull. 2009;57(9):1011–4.
Ma K, Huang XF, Kong LY. Steroidal saponins from Polygonatum cyrtonema. Chem Nat Compd. 2013;49(5):888–91.
Zeng T, Tang YR, Li B, Tasneem S, Yuan HW, Jia YZ, et al. Chemical characterization of constituents from Polygonatum cyrtonema Hua and their cytotoxic and antioxidant evaluation. Nat Prod Res. 2020;34(17):2482–9.
Tang C, Yu YM, Qi QL, Wu XD, Wang J, Tang SA. Steroidal saponins from the rhizome of Polygonatum sibiricum. J Asian Nat Prod Res. 2019;21(3):197–206.
Xu DP, Hu CY, Zhang Y. Two new steroidal saponins from the rhizome of Polygonatum sibiricum. J Asian Nat Prod Res. 2009;11(1):1–6.
Hu CY, Xu DP, Wu YM, Ou SY. Triterpenoid saponins from the rhizome of Polygonatum sibiricum. J Asian Nat Prod Res. 2010;12(9):801–8.
Zhao L, Xu C, Zhou W, Li Y, Xie Y, Hu H, et al. Polygonati Rhizoma with the homology of medicine and food: A review of ethnopharmacology, botany, phytochemistry, pharmacology and applications. J Ethnopharmacol. 2023;309:116296.
Sun LR, Li X, Wang SX. Two new alkaloids from the rhizome of Polygonatum sibiricum. J Asian Nat Prod Res. 2005;7(2):127–30.
Wang YF, Lu CH, Lai GF, Cao JX, Luo SD. A new indolizinone from Polygonatum kingianum. Planta Med. 2003;69(11):1066–8.
Virk JK, Kumar S, Singh R, Tripathi AC, Saraf SK, Gupta V, et al. Isolation and characterization of quinine from Polygonatum verticillatum: a new marker approach to identify substitution and adulteration. J Adv Pharm Technol Res. 2016;7(4):153–8.
Tisnerat C, Dassonville-Klimpt A, Gosselet F, Sonnet P. Antimalarial drug discovery: from quinine to the most recent promising clinical drug candidates. Curr Med Chem. 2022;29(19):3326–65.
Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144.
Jones RA, Panda SS, Hall CD. Quinine conjugates and quinine analogues as potential antimalarial agents. Eur J Med Chem. 2015;97:335–55.
Senizza A, Rocchetti G, Mosele JI, Patrone V, Callegari ML, Morelli L, et al. Lignans and gut microbiota: an interplay revealing potential health implications. Molecules. 2020;25:5709.
Webb AL, McCullough ML. Dietary lignans: potential role in cancer prevention. Nutr Cancer. 2005;51(2):117–31.
Teodor ED, Moroeanu V, Radu GL. Lignans from medicinal plants and their anticancer effect. Mini Rev Med Chem. 2020;20(12):1083–90.
Yan H, Shi Y, Wang J. Therapeutic potential of naturally occurring lignans as anticancer agents. Curr Top Med Chem. 2022;22(17):1393–405.
Osmakov DI, Kalinovskii AP, Belozerova OA, Andreev YA, Kozlov SA. Lignans as pharmacological agents in disorders related to oxidative stress and inflammation: chemical synthesis approaches and biological activities. Int J Mol Sci. 2022;23(11):6031.
Shaito A, Aramouni K, Assaf R, Parenti A, Orekhov A, Yazbi AE, et al. Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front Biosci. 2022;27(3):105.
Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. 2019;11(9):2090.
He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44(2):532–53.
Vetrani C, Costabile G, Di Marino L, Rivellese AA. Nutrition and oxidative stress: a systematic review of human studies. Int J Food Sci Nutr. 2013;64(3):312–26.
Legchenko E, Chouvarine P, Borchert P, Fernandez-Gonzalez A, Snay E, Meier M, et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci Transl Med. 2018;10(438):eaao0303.
Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2013;55:31–41.
Saito K, Kuroda A, Tanaka H, Yoshida A, Yoshida H, Ferrans VJ. Differential sensitivity of rat cardiac sarcolemma and mitochondria to damage induced by lipid peroxidation. J Electron Microsc. 1993;42(5):305–9.
Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, et al. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation. 2002;105(7):849–54.
Wang S, Li G, Zhang X, Wang Y, Qiang Y, Wang B, et al. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydr Polym. 2022;291:119524.
Cui X, Wang S, Cao H, Guo H, Li Y, Xu F, et al. A Review: the bioactivities and pharmacological applications of Polygonatum sibiricum polysaccharides. Molecules. 2018;23(5):1170.
Teng H, Zhang Y, Jin C, Wang T, Huang S, Li L, et al. Polysaccharides from steam-processed Polygonatum cyrtonema Hua protect against d-galactose-induced oxidative damage in mice by activation of Nrf2/HO-1 signaling. J Sci Food Agric. 2023;103(2):779–91.
Ha TTT, Dung NT, Tai BH, Van Kiem P. Polypunctosides E-K: seven new steroidal saponins from Polygonatum punctatum Royle ex Kunth and their nitric oxide production inhibitory activities. J Nat Med. 2023;77(1):238–49.
Yin X, Wang B, Li X, Zhu C, Chen P. Effects of Polygonatum sibiricum polysaccharide on JAK/STAT pathway and myocardial fibrosis in rats with autoimmune myocarditis. Int J Clin Exp Pathol. 2021;37(1):26–32.
Li J, Wang X, Zhou R, Cheng F, Tang X, Lao J, et al. Polygonatum cyrtonema Hua polysaccharides protect BV2 microglia relief oxidative stress and ferroptosis by regulating NRF2/HO-1 pathway. Molecules. 2022;27(20):7088.
Zhang S, Liu Y, Cao Y, Zhang S, Sun J, Wang Y, et al. Targeting the microenvironment of vulnerable atherosclerotic plaques: an emerging diagnosis and therapy strategy for atherosclerosis. Adv Mater. 2022;34(29):e2110660.
Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and the European atherosclerosis society (EAS). Eur Heart J. 2011;32(14):1769–818.
Melita H, Manolis AA, Manolis TA, Manolis AS. Lipoprotein(a) and cardiovascular disease: a missing link for premature atherosclerotic heart disease and/or residual risk. J Cardiovasc Pharmacol. 2022;79(1):e18-35.
Zhang Q, Liu J, Duan H, Li R, Peng W, Wu C. Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J Adv Res. 2021;34:43–63.
Jeon SM, Lee MK, Park YB, Park HM, Choi MS. Polygonatum rhizoma affects antioxidant defense systems without changing mRNA expression in diet-induced hypercholesterolemic rabbits. J Med Food. 2004;7(3):358–65.
Cai JL, Li XP, Zhu YL, Yi GQ, Wang W, Chen XY, et al. Polygonatum sibiricum polysaccharides (PSP) improve the palmitic acid (PA)-induced inhibition of survival, inflammation, and glucose uptake in skeletal muscle cells. Bioengineered. 2021;12(2):10147–59.
Deng Y, He K, Ye X, Chen X, Huang J, Li X, et al. Saponin rich fractions from Polygonatum odoratum (Mill.) Druce with more potential hypoglycemic effects. J Ethnopharmacol. 2012;141(1):228–33.
Dong W, Shi HB, Ma H, Miao YB, Liu TJ, Wang W. Homoisoflavanones from Polygonatum odoratum rhizomes inhibit advanced glycation end product formation. Arch Pharm Res. 2010;33(5):669–74.
Xu C, Xia B, Zhang Z, Lin Y, Li C, Lin L. Research progress in steroidal saponins from the genus Polygonatum: chemical components, biosynthetic pathways and pharmacological effects. Phytochemistry. 2023;213:113731.
Wang H, Fowler MI, Messenger DJ, Terry LA, Gu X, Zhou L. Homoisoflavonoids are potent glucose transporter 2 (GLUT2) inhibitors: a potential mechanism for the glucose-lowering properties of Polygonatum odoratum. J Agric Food Chem. 2018;66(12):3137–45.
Zhu X, Li Q, Lu F, Wang H, Yan S, Wang Q, et al. Antiatherosclerotic potential of Rhizoma Polygonati polysaccharide in hyperlipidemia-induced atherosclerotic hamsters. Drug Res. 2015;65(9):479–83.
Yang JX, Wu S, Huang XL, Hu XQ, Zhang Y. Hypolipidemic activity and antiatherosclerotic effect of polysaccharide of Polygonatum sibiricum in rabbit model and related cellular mechanisms. Evid Based Complement Alternat Med. 2015;2015:391065.
Dong J, Gu W, Yang X, Zeng L, Wang X, Mu J, et al. Crosstalk between Polygonatum kingianum, the miRNA, and gut microbiota in the regulation of lipid metabolism. Front Pharmacol. 2021;12:740528.
Luo J, Chai Y, Zhao M, Guo Q, Bao Y. Hypoglycemic effects and modulation of gut microbiota of diabetic mice by saponin from Polygonatum sibiricum. Food Funct. 2020;11(5):4327–38.
Luo J, Jiang L, Gao B, Chai Y, Bao Y. Comprehensive in silico analysis of the probiotics, and preparation of compound probiotics-Polygonatum sibiricum saponin with hypoglycemic properties. Food Chem. 2023;404(Pt A):134569.
Ma X, Liu Z, Ilyas I, Little PJ, Kamato D, Sahebka A. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci. 2021;17(8):2050–68.
Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, et al. Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol. 2019;114(3):19.
Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17(5):269–85.
Halade GV, Lee DH. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine. 2022;79:103992.
Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(11):1324–40.
Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015;6(1):187–214.
Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(1):15.
Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail. 2002;8(6 Suppl):S319–25.
Nagalingam RS, Chattopadhyaya S, Al-Hattab DS, Cheung DYC, Schwartz LY, Jana S, et al. Scleraxis and fibrosis in the pressure-overloaded heart. Eur Heart J. 2022;43(45):4739–50.
Rao M, Wang X, Guo G, Wang L, Chen S, Yin P, et al. Resolving the intertwining of inflammation and fibrosis in human heart failure at single-cell level. Basic Res Cardiol. 2021;116(1):55.
Cai J, Zhu Y, Zuo Y, Tong Q, Zhang Z, Yang L, et al. Polygonatum sibiricum polysaccharide alleviates inflammatory cytokines and promotes glucose uptake in high-glucose- and high-insulin-induced 3T3-L1 adipocytes by promoting Nrf2 expression. Mol Med Rep. 2019;20(4):3951–8.
Gan Q, Wang X, Cao M, Zheng S, Ma Y, Huang Q. NF-κB and AMPK-Nrf2 pathways support the protective effect of polysaccharides from Polygonatum cyrtonema Hua in lipopolysaccharide-induced acute lung injury. J Ethnopharmacol. 2022;291:115153.
Xiong S, Yang C, Pan D, Gong N. Effects of Polygonatum saponins on the EMT, complement system and PI3K/AKT/NF-kappaB signal pathway of HK-2 cells induced by TGF-beta1. Immunol J. 2022;38(2):157–63.
Zhao H, Wang QL, Hou SB, Chen G. Chemical constituents from the rhizomes of Polygonatum sibiricum Red. and anti-inflammatory activity in RAW264.7 macrophage cells. Nat Prod Res. 2019;33(16):2359–62.
Lei S, Wang L, Long Z, Shi H, Gao H, Zhu Y, et al. Inhibitory effect of Polygonatum sibiricum polysaccharides on release of inflammatory cytokines of anoxia/reoxygenation H9c2 myocardial cells through TLR4-MyD88-NF-kappaB signaling pathway. Chin Pharmacol Bulletin. 2017;33(2):255–60.
Zhang Z, Wang G, Chen T. Effect of Polygonatum sibiricum polysaccharides on myocardial fibrosis in rats with diabetes. China J Public Health. 2016;32(6):807–10.
Zhao CC, Zhang M, Peng JF, Ma YY, Zhao XN, Wen ZY, et al. Polygonatum polysaccharide attenuates inflammation through inhibiting NLRP3 inflammasome in diabetic cardiomyopathy rats. J Food Sci. 2023;35(1):10–8.
Chen T, Wang G, Fu T, Zhang Z. The protective effect of Polygonatum sibiricum polysaccharide on myocardial inflammation in type Idiabetic rats. Pharmacol Clin Chin Mater Med. 2015;31(4):86–90.
Li L, Long Z, Huang J, Ren Z, Lu S, Wang X. Effect of Polygonatum sibiricum polysaccharides on inflammatory raction mediated by NF-kappaB and myocardium tissue morphology in acute myocardial infarction rats. Chin Tradit Herb Drugs. 2015;46(18):2750–4.
Hirai N, Miura T, Moriyasu M, Ichimaru M, Nishiyama Y, Ogura K, et al. Cardiotonic activity of the rhizome of Polygonatum sibiricum in rats. Biol Pharm Bull. 1997;20(12):1271–3.
Varga ZV, Ferdinandy P, Liaudet L, Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015;309(9):H1453–67.
Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–8.
Lenneman CG, Sawyer DB. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res. 2016;118(6):1008–20.
Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17(8):474–502.
Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J. 2022;43(41):4229–361.
Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298(10):1189–95.
Ferdinandy P, Baczkó I, Bencsik P, Giricz Z, Görbe A, Pacher P, et al. Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications. Eur Heart J. 2019;40(22):1771–7.
Banke A, Fosbøl EL, Møller JE, Gislason GH, Andersen M, Bernsdorf M, et al. Long-term effect of epirubicin on incidence of heart failure in women with breast cancer: insight from a randomized clinical trial. Eur J Heart Fail. 2018;20(10):1447–53.
Wallace KB, Sardão VA, Oliveira PJ. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res. 2020;126(7):926–41.
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42.
Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672–80.
Yang X, Liu N, Li X, Yang Y, Wang X, Li L, et al. A Review on the effect of traditional Chinese medicine against anthracycline-induced cardiac toxicity. Front Pharmacol. 2018;9:444.
Yu J, Wang C, Kong Q, Wu X, Lu JJ, Chen X. Recent progress in doxorubicin-induced cardiotoxicity and protective potential of natural products. Phytomedicine. 2018;40:125–39.
Yarmohammadi F, Karbasforooshan H, Hayes AW, Karimi G. Inflammation suppression in doxorubicin-induced cardiotoxicity: natural compounds as therapeutic options. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(10):2003–11.
Acknowledgements
Not applicable.
Funding
This work was fnancially supported by the Chinese Medicine inheritance and innovation “thousand million” Talents Project (Qihuang Project) Qihuang Scholars (NO. RS086).
Author information
Authors and Affiliations
Contributions
XW and HC designed, organized, and supervised the study and helped to coordinate support and funding. HL, WW, and PM performed the research and drafted the manuscript. YK and XZ participated in the revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, H., Wang, W., Peng, M. et al. Pharmacological properties of Polygonatum and its active ingredients for the prevention and treatment of cardiovascular diseases. Chin Med 19, 1 (2024). https://doi.org/10.1186/s13020-023-00871-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-023-00871-0