Abstract
Background
Cancer-related fatigue (CRF) is an extremely common and long-term condition that affects the physical and mental health of oncology patients. While the treatment for CRF with western medicine and non-pharmacological therapy remains uncertain and challenging, traditional Chinese medicine (TCM) has become a trending option for the patients. Based on the findings from randomized controlled trials (RCTs), this study aims to identify and evaluate the evidence about the efficacy and safety of TCM for CRF.
Methods
A systematic literature search was conducted according to the PRISMA literature research guidelines. Seven electronic databases including PubMed, the Cochrane Library, Embase, Web of Science, Scopus, China National Knowledge Infrastructure (CNKI) and Wanfang database were searched to identify RCTs which investigated TCM in the treatment of CRF published since inception to December 2022. RCTs comparing TCM with no treatment, placebo, or pharmacological interventions were considered eligible for this review. The Consolidated Standards of Reporting Trials Statement extensions for Chinese herbal medicine Formulas (CONSORT-CHM) and the Cochrane Collaboration’s Risk of Bias tool were used in this review to evaluate the quality and the risk of bias of all included trials.
Results
A total of 82 RCTs were included in this review, regardless of whether they were published in English or Chinese. After data extraction and results evaluation, 78 trials demonstrated overall efficacy in using TCM for CRF patients compared with the control group, in which 33 trials showed that the efficacy rate was statistically significant (p < 0.05 or p < 0.01). TCM was also shown to be beneficial in improving the scores of relevant scales (e.g., PFS, QoL, TCM syndrome score, other fatigue scales etc.) or physical tests indicators (e.g., cytokines, blood test etc.). The most common herbs found in Chinese medicine were Astragali Radix, Ginseng Radix and Codonopsis Radix. Some TCM products, such as Kangai Injection, Buzhong Yiqi Decoction and Shenqi Fuzheng Injection could provide a reference for medication in this review. A range of non-serious, reversible adverse effects associated with the use of TCM was also reported. However, the result of evaluation showed that none of the trials fully met all the CONSORT-CHM criteria, the quality of included trials was generally poor and the risk of bias was mostly uncertain.
Conclusion
There is some evidence supporting the efficacy and safety of TCM in managing CRF in this systematic review. However, no clear conclusion can be made due to the inadequate reporting of efficacy and adverse reactions. In view of some concerns about the existing evidence after the evaluation, it is essential to standardize the comprehensive identification and efficacy measurement standards, improve the quality of RCTs and conduct more multicomponent therapies to provide an updated reference for CRF patients medication in the future.
The protocol of this systematic review has been registered on PROSPERO (CRD42023413625). [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023413625].
Similar content being viewed by others
Background
Cancer-related fatigue (CRF) refers to a persistent, subjective sense of tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning [1]. Underlying cancer pathology, treatment toxicity, physiological abnormalities resulting from complications (including anemia, infection, nausea etc.), or psychological factors can all be predisposing factors for CRF [2]. At present, CRF is extremely common imposing long-term impacts on the physical and mental health of patients, with some reports estimating a prevalence up to 60% [3, 4].
Currently, there is not a standard approach to CRF treatment. Western medicine therapies and non-pharmacological therapies have been commonly employed to help alleviate the fatigue experienced by oncology patients [2, 5]. The recommended western medicines include hematopoietic drugs, antidepressants, psychostimulants, analgesic, etc. [2]. Some patients reportedly benefit from exercise, acupuncture, massage, nutrition, sleep, education or other cognitive–behavioral interventions [5,6,7,8]. Nevertheless, the clinical use of these therapies has been considered controversial due to a lack of adequate systematic assessment and strong evidence.
Apart from western medicines and non-pharmacological therapies, traditional Chinese medicines (TCM) have attracted extensive attention for patients in managing CRF during their fight against cancer [9]. TCM has a long history and is famous worldwide for its theories. According to the clinical symptoms, CRF is recognized as a deficiency pattern [10], including both qi and blood deficiency, disharmony of yin and yang, hypofunction of liver, kidney, spleen or other organs [11]. Common herbs used in TCM such as Astragalus, Turmeric, Ginseng have been shown to be beneficial in relieving CRF and pain as well as improving immune system function [9]. Decoctions of TCM used for CRF often contain complex and multiple pharmacological activities at various targets. For instance, Buzhong Yiqi Decoction contains at least eight herbs, and its therapeutic effects could be attributed to the processes such as interfering with tumor cell proliferation, inducing tumor cell apoptosis and correcting tumor cell drug resistance, and eventually improving quality of life (QoL) [12, 13]. To many oncology patients, TCM has become an option in the treatment of CRF due to its potential efficacy, but the potential of adverse reactions and toxicity should also be taken into consideration [14].
Recently, a growing body of clinical trials is emerging to investigate the efficacy and safety of TCM in treating CRF. A systematic review in 2014 reported that combining Chinese herbal medicines with chemotherapy or supportive care was more beneficial to improving QoL of CRF patients when compared with the treatment using chemotherapy or supportive care alone [10]. Another review, which analyzed 11 clinical trials that evaluated fatigue severity, QoL, activities of daily life and incidence of adverse events among lung cancer patients, found that, as compared to the use of conventional medicines only, combining herbal medicines with conventional medicines showed additional effectiveness and safety for CRF [15]. However, the number of the randomized controlled trial (RCTs) considered in these systematic reviews is still limited. In order to improve the evidence-base about using TCM for CRF, it is necessary to critically assess the emerging evidence from the RCTs. Therefore, this study aims to evaluate the efficacy and safety of TCM reported in RCTs. It is anticipated that the findings will be useful to inform the management of CRF for the patients and clinicians.
Methods
This study was a systematic literature review conducted and reported in compliance with the updated Preferred Reporting Items for Systematic Reviews and Meta analyses (PRISMA) guidelines [16]. The Consolidated Standards of Reporting Trials Statement extensions for Chinese herbal medicine Formulas (CONSORT-CHM) [17] and the Cochrane Collaboration’s Risk of Bias tool [18] were used in this review to evaluate the reporting quality and the risk of bias of all included trials. The protocol of this systematic review has been registered on PROSPERO (CRD42023413625). [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023413625].
Types of studies
Randomized controlled trials which investigated the use of TCM in CRF regardless of blinding, status, date or language of publication were considered eligible for this review. Open-label experiment and observational studies were excluded.
Types of RCTs participants
Participants of any age, gender or ethnic origin with a diagnosis of CRF, or the presence of fatigue after chemotherapy were eligible for the studies. RCTs in which participants had other diseases resulting in fatigue symptoms, or non-drugs treatments were excluded.
Types of interventions
The interventions in the included RCTs for the treatment of CRF were divided into two categories, primary interventions and behavioral interventions. In particular, the primary interventions usually included TCM, western medicine (WM), TCM plus western medicine (TCM + WM), placebo or no treatment; behavioral interventions were set for chemotherapy (CH), other treatment (OT) and chemotherapy plus other treatment (CH + OT). Other treatment (OT) referred to the conventional treatment, symptomatic treatment, supportive treatment, nutritional therapy or other non-drug treatment for cancer patients after chemotherapy.
Primary interventions:
-
TCM
-
Western medicine (WM)
-
TCM plus western medicine (TCM + WM)
-
Placebo
-
No treatment
Behavioral interventions:
-
Chemotherapy (CH)
-
Other treatment (OT)
-
Chemotherapy plus other treatment (CH + OT)
Types of outcomes
Both efficacy and safety of the TCM investigated in the included RCTs were analyzed. As such, the primary and the secondary outcomes of managing CRF with the use of TCM were as follows:
Primary outcomes
The primary outcome measures under consideration included changes in Piper Fatigue Scale (PFS) and the scales of QoL. On the one hand, PFS consists of twenty-two items and four subscales in total, six items of behavioral/severity, five items of affective meaning, five items of sensory and six items of cognitive/mood [19, 20]. Each item is scored from 0 (no fatigue) to 10 (most severe fatigue). Higher total scores indicate more severe fatigue.
On the other hand, QoL is defined as: “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns” from the World Health Organization (WHO) [21, 22]. As a significant indicator in medical and health research, QoL is mainly applied to patients with specific cancer and long-term diseases [23]. For example, the scales of KPS (Karnofsky Performance Status), QLQ-C30 (EORTC Quality of Life Questionnaires), GQoLI-74 (Generic Quality of Life Inventory-74), SF-36 (36-item Short-Form), FACT-F (Functional Assessment of Cancer Therapy–Fatigue), FACT-G (Functional Assessment of Cancer Therapy–General), FACIT-F (Functional Assessment of Chronic Illness Therapy–Fatigue), FACT-ES (Functional Assessment of Chronic illness Therapy–Endocrine Symptoms), PSQI (Pittsburgh sleep quality index), QoL1 (Quality of Life Questionnaire); QoL2 (Quality of Life Questionnaire and Quality of life score (QoL) for cancer patients (Chinese version draft) were used to evaluate the QoL when TCM was used for treating CRF in this review.
Secondary outcomes
The secondary outcome measures were TCM syndrome score, fatigue scales, cytokines tests, blood tests, other scales and indicators:
-
1.
TCM syndrome score: According to the Chinese Medicine Clinical Research of New Drugs Guiding Principles [11], TCM syndrome scores are mainly used to evaluate symptoms such as fatigue, shortness of breath, lazy speech, irritability, insomnia, and weakness of the waist and knees, divided into three levels, for mild ( +), moderate (+ +), and severe (+ + +), and the relative scores are 1 point, 2 points, and 3 points respectively. The higher the score, the more severe the symptoms.
-
2.
Fatigue scales: BFI (Brief Fatigue Inventory), CFS (Cancer Fatigue Scale), CFS* (Chalder Fatigue Scale), MFSI-SF (Multidimensional Fatigue Symptom Inventory–Short Form), FSI (Fatigue Symptom Inventory), BFI-C (Chinese Version of Brief Fatigue Inventory), TOI-F (Trial Outcome Index–Fatigue), VAS-F (Visual Analogue Scale of Global Fatigue), NGFRS (NCCN Guidelines Fatigue Rating Scale)
-
3.
Other scales: HADS (Hospital Anxiety and Depression Scale), POMS (Profile of Mood States), HAMA (Hamilton Anxiety Scale), SDS (Self-Rating Depression Scale), SS (satisfaction survey), CS (Comfort Survey), PSS (Perceived stress scale), GIC (the Global Impression of Change), LASA (Linear Analogue Self Assessment Scale), BDI (Beck Inventory Depression Scale), GCSG (Gastric Cancer Symptom Grading Scale), LCSG (Lung Cancer Symptom Grading Scale)
-
4.
Cytokines tests: CD3+, CD4+, CD8+, CD3+CD4+, CD3+CD8+, CD4+/CD8+, CD16+56+, TNF-α, IFN-γ, IL-1, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, NK cell, IgG, IgA, IgM, TGF-β.
-
5.
Blood tests: hemoglobin (HGB), albumin (Alb), white blood cell (WBC), total protein (TP), platelet (PLT), cortisol (COR), red blood cells (RBC), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), hematocrit (HCT), degree of myelosuppression (DM)
-
6.
Others: weight, cardiac function, liver function, kidney function, blood viscosity, urinalysis, Recombinant Human Granulocyte Colony Stimulating Factor (rhG-CSF) dosage, physical and chemical indicators, WHO Response Evaluation Criteria in Solid Tumors (RECIST), improve cancer cachexia-related symptoms, standards for the diagnosis and treatment of common malignant tumors in China.
Search strategies
This systematic review was conducted according to the PRISMA literature research guidelines [24]. Seven electronic databases including PubMed, the Cochrane Library, Embase, Web of Science (WOS), Scopus, China National Knowledge Infrastructure (CNKI) and Wanfang database were considered to identify RCTs which evaluated TCM in treating CRF from database inception to December 2022. The three primary search terms were “CRF”, “TCM” and “RCTs”.
As shown in Table 1, to ensure an effective search, Medical Subject Headings (MeSH) terms were used to develop a comprehensive search strategy. Common phrases and keywords related to the three terms (CRF, TCM, RCTs) were combined with OR. The results from each concept were combined with AND. A detailed description of each search strategy is provided in Additional file 1.
Exclusion criteria and screening
This review only included studies that reported the results of RCTs investigating the efficacy and/or safety of TCM in CRF. Studies subjected to exclusion were: (1) review, meta-analysis, protocol; (2) non-randomized trial; (3) pharmacodynamics or pharmacology studies; (4) animal experiments; (5) studies on other non-drugs therapy (such as acupuncture, qigong, music, and behavior); (6) studies on other diseases or fatigue caused by other diseases.
Data extraction and analysis
All references were categorized and archived in Endnote X9, data was recorded and organized using the EXCEL 2013. As listed in Table 2, relevant data from all eligible studies were extracted according to a standard extraction form, which included basic information of studies, methods, interventions, participants, outcomes, and overall findings.
Assessment of reporting quality
Two of the authors (JY, YL) independently assessed the included eligible studies based on 25-item of CONSORT-CHM 2017 statement [17]. This statement provided a grading system devised for each criterion that was used to determine the quality of every clinical trial of TCM interventions. According to the degree of conformity, the assessment results for each item were determined as non-existent, partially present and fully compliant.
Assessment of risk of bias
Cochrane Collaboration’s Risk of Bias [18] tool was used to evaluate the quality of each studies by two authors (JY, YL). The judgment was based on the definition of the recommendation by the Cochrane Handbook for Systematic Reviews of Interventions, and the assessment results for each item were grouped into one of the following three categories: “low risk of bias”, “unclear risk of bias”, and “high risk of bias”. Further explanation about the risk assessment was shown in Additional file 2: Table S1.
Results
Search results
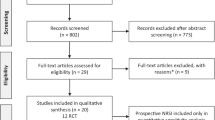
The screening process conducted in accordance with the PRISMA guidelines is summarized in the flow diagram as shown in Fig. 1. A total of 3338 records were initially yielded from the seven electronic databases and related sources. After removing 1068 duplicates, 2270 relevant articles were retained for screening. After title and abstract screening, 2120 records were excluded due to various reasons: study or publication type (review or meta-analysis or protocol articles or others (n = 203)), focus on conditions other than CRF (n = 1390), focus on symptoms of fatigue developed from other diseases (n = 391), focus on other treatments such as food, acupuncture, moxibustion or others (n = 132), non-TCM treatment (n = 2), and animal trials (n = 2). A total of 150 articles were retained for the subsequent full-text screening and 69 records were further excluded due to the following reasons: open-label study (n = 2), focus on conditions other than CRF (n = 53), self-control study (n = 4), single-center study (n = 1), study proposal for CRF (n = 8) and non-RCT (n = 1). Eventually, 81 eligible articles published in Chinese databases (n = 70) or English databases (n = 11) were included in this review.
Description of studies
Since one of the included studies reported two RCTs [25], this systematic review comprised 82 trials from 81 publications. In particular, 71 studies were published in Chinese and only 10 studies were published in English [25,26,27,28,29,30,31,32,33,34]. Except for some trials conducted in Korea (n = 3) [28, 33, 34], Brazil (n = 3) [25, 29], USA (n = 2) [27, 31], France (n = 1) [26], Italy(n = 1) [32], the remaining 72 trials were conducted in China. More details about the studies are shown in Table 3.
Participants
A total of 7547 participants with CRF were recruited in the RCTs included in this review. After 312 participants dropped out or withdrew before the intervention was initiated, the remaining participants in 82 trials were allocated into the test groups (n = 3801) and the control or comparison groups (n = 3434). Excluding eight trials which did not report the number of males and females [25, 26, 38, 43, 54, 67, 104], 3399 males and 3195 females* (the number of males and females had some errors in one study [62]) were eventually included. Out of 82 RCTs, only 53 trials specifically reported the mean age and standard deviation of the participants in test group and control group, 20 trials reported the mean, median or range of age in two groups, seven trials reported the overall age characteristics of participants, and two trials did not report any information of age.
The top three categories of cancer in 82 trials were lung (participants: n = 2671; trials: n = 46), bowel (participants: n = 1532; trials: n = 34) and breast (participants: n = 1329; trials: n = 34), followed by stomach, liver, ovarian, cervical, esophageal, kidney, ureteral, pancreatic, gallbladder, nasopharyngeal carcinoma, head and neck or other categories. Regarding places of recruitment, 73 trials recruited participants from hospitals, one from newspaper or hospital advertisements [28], one from community cancer centers [31], one from center (not specified) [33], and six trials in five studies did not mention the places of recruitment [25,26,27, 76, 88].
Diagnosis and included criteria
Information about the diagnosis and inclusion criteria of the CRF participants in this review is provided in Additional file 2: Table S2. Twenty-eight out of 82 trials regarded The International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) as a preferred reference for CRF diagnostic criteria. Nineteen trials selected the relevant standards of Chinese medicine diagnostic criteria according to the Chinese Medicine Clinical Research of New Drugs Guiding Principles [11], Chinese Medicine Diagnosis [106], Chinese Medicine Internal Science [107], Practical Traditional Chinese Medicine Tumor Manual [108] or others.
Other scales such as PFS, KPS, ECOG, BFI, QLQ-C30, CFS*, NGFRS, VAS, a 11-point fatigue scale and a question about fatigue level were used as the tool to determine hte inclusion criteria for CRF participants in 52 trials. In particular, the score of KPS was set between 40 to 80 in 34 trials included in this review. Seven trials used ECOG with the score less than two. Seventy-two trials used tumor diagnosis and 10 trials had no information regarding the tumor diagnosis. One trial mentioned the level of HGB > 80 g /L [58], one trial limited the subjects with appropriate liver function (AST, ALT ≤ 2.5 X ULN), kidney function (Cr ≤ 1.5 X ULN) and the level of Hb ≥ 9 g/dL [33]. Three trials mentioned fatigue for at least one month [27, 31, 34].
In terms of life expectancy, 31 trials did not provide any information whereas 51 trials discussed different life expectancy periods, with more than 30 days in one trial, more 1–3 months in 33 trials, more than 4 months in one trial, more than or equal to 6 months in 15 trials and more than 9 months in one trial.
Intervention
All the ingredients from each TCM preparation investigated in the included studies are summarized in Table 3. The TCM preparations tested in the included studies might be given in the form of injections (n = 34), decoctions (n = 31), capsules (n = 6), granules (n = 3), pills (n = 2), tablets (n = 1), oral solutions (n = 2), powder (n = 1), and decoction and injections (n = 1). One TCM preparation included did not provide information on formulation. Compared to the injections, most of the decoctions had no quality standards of the TCM in included RCTs. The names of the five most commonly studied TCM were Kangai Injection (n = 16) (康艾注射液), Buzhong Yiqi Decoction (n = 9) (補中益氣湯), Shenqi Fuzheng Injection (n = 6) (參芪扶正注射液), Guarana (Paullinia cupana) (n = 4) and Shenfu Injection (n = 3) (參附注射液).
At least 100 Chinese medicinal herbs were involved in the included trials for treating CRF and the 20 most common herbs were Astragali Radix(黃芪) (n = 48), Ginseng Radix(人參) (n = 34), Codonopsis Radix(黨參) (n = 30), Atractylodis Macrocephalae Rhizoma(白朮) (n = 30), Glycyrrhizae Radix(甘草) (n = 25), Poria (茯苓) (n = 21), Angelicae Sinensis Radix (當歸) (n = 21), Sophorae Flavescentis Radix (苦參) (n = 17), Citri Reticulatae Pericarpium (陳皮) (n = 15), Bupleuri Radi (柴胡) (n = 12), Cimicifugae Rhizoma (升麻) (n = 11), Ophiopogonis Radix (麥冬) (n = 8), Rehmanniae Radix (地黃) (n = 8), Dioscoreae Rhizoma (山藥) (n = 7), Coicis Semen (薏苡仁) (n = 7), Lycii Fructus (枸杞) (n = 7), Ziziphus Jujuba Mill (大棗) (n = 6), Paeoniae Radix Alba (白芍) (n = 6), Pinelliae Rhizoma (半夏) (n = 6) and Amomi Fructus (砂仁) (n = 5).
Control and comparison
In test group, the primary interventions were designed for TCM (n = 80) and TCM plus western medicine (n = 2). Thirty-seven trials used other treatment, 15 trials considered chemotherapy and 17 trials provided chemotherapy combined with other treatment as behavioral interventions. The primary interventions in control groups included placebo (n = 10), TCM (n = 2), western medicine (n = 3) and no treatment (n = 1). Thirty-nine trials used other treatment, 17 trials considered chemotherapy and 17 trials provided chemotherapy combined with other treatment as behavioral interventions. All the comparisons were described in Table 4 below.
The intervention durations
The duration of the intervention ranged from 10 days to 16 weeks. The intervention durations were shorter than or equal to 1 month in 59 trials, between 1–2 months in 12 trials, between 2–3 months in 7 trials, more than 3 months in one trial, two cycles of treatment in 1 trial and four cycles of treatment in 1 trial. One trial did not provide any information regarding intervention duration.
Outcomes
As shown in Table 5, the reported outcomes of the 82 trials included the primary outcomes (PFS and the scales of QoL) and the secondary outcomes (TCM syndrome score, Fatigue scales, other scales, cytokines test, blood test and others).
Primary outcome
-
PFS was considered a measuring tool for CRF and was selected by more than half of the trials (n = 46).
-
In addition, scales of QoL were selected in 51 trials, and 9 trials employed more than two scales. The most common scales included KPS (n = 27) and QLQ-C30 (n = 17), other were PSQI (n = 3), SF-36 (n = 3), FACIT-F (n = 3), GQoLI-74 (n = 2), FACT-F (n = 2), FACT-G (n = 1), FACT-ES (n = 1), Quality of Life Questionnaire (n = 2), but 31 trials did not provide any information.
Secondary outcome
-
Twenty-seven trials selected TCM syndrome score.
-
Fatigue scales included BFI or BFI-C (n = 17), CFS (n = 5), BFI combine CFS (n = 1), BFI combine MFSI-SF (n = 1), BFI combine CFS* (n = 4), FSI (n = 3), FSI combine MFSI-SF (n = 1), NCCN GFRS (n = 1), VAS-F combine TOI-F (n = 1).
-
Other less commonly used scales were HADS (n = 5), GIC combine LASA (n = 1) and HADS combine SDS (n = 1). The scales of BDI, CS, GCSG, LCSG, POMS, PSS, SS were only used once.
-
For the cytokines test, 15 trials reported the results of CD3+, CD4+, CD8+, CD3+CD4+, CD3+CD8+, CD4+/CD8+, CD16+56+, TNF-α, IFN-γ, IL-1, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, NK cell, IgG, IgA, IgM and TGF-β.
-
Out of the 11 trials that reported the results of blood test, 5 trials conducted HGB test, WBC combined PLT were reported in three trials, COR and DM were separately reported in two trials, and the remaining parameters including Alb, TP, RBC, MCH, MCHC, MCV, HCT were only reported once. Seventy-one trials did not provide any information regarding blood test results.
-
Other outcomes included RECIST (n = 5), weight (n = 2), cardiac function (n = 2), liver function (n = 2), kidney function (n = 2), rhG-CSF dosage (n = 2), blood viscosity (n = 1), urinalysis (n = 1), physical and chemical indicators (n = 1), improvement in cancer cachexia-related symptoms (n = 1), standards for the diagnosis and treatment of common malignant tumors in China (n = 1).
Efficacy
The results of the included trials showed that 78 trials reported an overall efficacy on the use of TCM in treating CRF whereas only 4 trials showed no effect.
Out of the 34 trials which demonstrated the efficacy rate in test groups as compared to control groups, 28 trials showed statistically significant difference (p < 0.05), 5 trials showed statistical differences at a two-sided P-value less than 0.01, while one trial showed the negative difference (p > 0.05). In particular, the efficacy rate in test groups ranged from 15.9 to 100%, 11 trials were more than 90%, 20 trials were over 50% and less than 90% and 3 trials were below 50%. The efficacy rate in control groups ranged from 6.96 to 80% with 15 trials over 90% and 19 trials below 50%. Based on the different classification of TCM dosage forms, the efficacy rate of test groups considering TCM injections ranged from 15.9 to 92.31% while for TCM decoctions in 15 trials, the range was 70.00 to 100.00%.
The three most used TCM products, such as Kangai Injection (n = 16), Buzhong Yiqi Decoction (n = 9) and Shenqi Fuzheng Injection (n = 6), all show overall efficacy in this review. In 16 trials, Kangai Injection not only effectively improved the scores of PFS, BFI, FSI, QLQ, HAMA, SDS and other scales, but also improved the level of IL-1β [49, 51, 62, 78,79,80,81,82,83,84, 89, 92,93,94,95,96]. The interventions in the test group included TCM plus OT (5 trials) [51, 79, 80, 82, 84], TCM plus OT plus CH (5 trials) [83, 89, 94,95,96], TCM plus CH (5 trials) [49, 62, 81, 92, 93] and only TCM (1 trial) [78]. A total of nine studies reported significant differences in the test group and the control group. The efficacy rate of the test group ranged from 15.90 to 85.71%, while the efficacy rate of the control group was lower than 55%.
Among 9 included RCTs, Buzhong Yiqi Decoction was not only used in China [38, 43, 45, 55, 58, 67, 76, 91], but also widely considered as Bojungikki-Tang in traditional Korean medicine [28], and the results all showed that this decoction had a good curative effect in the treatment of CRF. Two studies reported the efficacy rate of between the control group and the test group, with the efficacy rate as high as 90% in the test group [55, 76].
In 6 included trials studying Shenqi Fuzheng Injection [36, 53, 60, 70,71,72], PFS was used to measure the change of fatigue level and the results of one trial showed that physical fatigue might be improved, while other findings suggested that the four fatigue levels of PFS were improved in the test group. Four studies reported the efficacy rates after treatment with this injection, ranging from 67.7 to 92.31% in the test group and 52.9 to 68.6% in the control group [36, 53, 60, 71].
One trial found that Zini Yiqiyangxue Recipe (自擬益氣養血方) was fully effective in treating patients with colorectal cancer fatigue, with the overall efficacy rate of 100% in the test group as compared to 48% in control group (p < 0.01). After the intervention, it was reported that 9 cases were obviously effective and 16 cases were effective in the test treatment group, and nearly half of the control group were ineffective (13/25) [73].
The efficacy of American ginseng (Panax quinquefolius) and Guarana (Paullinia cupana) should be carefully considered in this review. Two trials that considered American ginseng as a TCM intervention had mixed results in terms of overall efficacy [27, 32]. One trial with American ginseng alone improved fatigue and QoL [27], but the other trial combining American ginseng with other treatments did not significantly improve BFI scores [32]. For Guarana, only 1 of 4 trials in this review supported its use for fatigue relief [29], and the other three trials did not show an overall efficacy of the treatment [25, 26]. In particular, a study reported two randomized, double-blind trials involving standardized dry-purified extract of Paullinia cupana (PC-18). One trial compared PC-18 (37.5 mg orally twice daily) with placebo, while another trial compared either 7.5 mg or 12.5 mg of PC-18 with placebo, and the results of both trials showed no statistical difference between the two groups [25]. Similarly, a clinical trial of 36 breast cancer patients receiving adjuvant radiation therapy did not show a significant difference between the Guarana group (75 mg orally per day) and placebo with the scores of CFS*, BFI or BDI [26]. However, one trial showed that Guarana (50 mg orally twice daily) significantly improved FACIT-F, FACT-ES, and BFI scores compared to placebo on day 21 and day 49 (p < 0.01) [29].
Safety
Among the 82 trials included, only 3 trials reported no adverse reactions [42, 52, 74], Forty-six trials did not provide any information about adverse reactions. One trial reported no serious adverse effects but did not reported any general adverse reactions [59]. For the other 32 trials (39.02%) that reported the adverse effects, 5 trials showed no obvious adverse reactions [26, 41, 51, 56, 77], and 27 trials reported specific symptoms with the number of cases. Non-serious adverse effects mainly included gastrointestinal discomfort (such as diarrhea, nausea, vomiting or constipate), myelosuppression (leukopenia, thrombocytopenia, or erythrocytopenia) and other mild complaints including anxiety, insomnia, fever, dizziness, rash, etc. The various forms of TCM preparations tested also drew further attention to the safety considering that, among the included trials that reported adverse reactions, 12.2% (10/82) of the trials used TCM injections while 6.09% (5/82) used TCM decoctions.
Only 5 trials reported several serious adverse reactions (Common Terminology Criteria for Adverse Events level ≥ 3) during TCM treatment in the test group [25, 27, 31, 33, 96]. The most frequently encountered serious adverse symptoms were nausea (or vomiting) (n = 28 cases), neutropenia (n = 28 cases), leukopenia (n = 21 cases), hair loss (n = 8 cases) and insomnia (6 cases). Due to TCM and chemotherapy being considered interventions at the same time, a trial using Kangai Injection showed more serious side effects [96]. In four other trials, Korean red ginseng (KRG) [33], American ginseng [27], Paullinia cupana [25] and Wisconsin Ginseng [31] were used alone with a few serious side effects. Overall, no clear conclusions can be made about the safety of TCM due to inadequate reporting on adverse reactions in trials included in this systematic review.
CONSORT-CHM
The summary of the CONSORT-CHM quality assessment results of the 82 RCTs is shown in Additional file 2: Table S3. None of the trials fully met all the CONSORT-CHM criteria. The items of randomization, allocation, implementation, blinding, ancillary analysis, harms, limitations, registration, protocol, funding were mostly lacking relevant information. For the random sequences, 46 trials did not provide any information, 9 trials partially and 27 trials fully reported information about randomization, in which 21 trials used random number tables, 4 trials used computer-generated randomization, one trial considered central randomization, and one used the method of sealed envelope. Only 4 trials specifically introduced allocation in detail. Four trials referred to the design of clinical trial implementation, for whom generated the random assignment sequence, enrolled the participants, and assigned the participants to the intervention groups. Seventy-five trials had no information on blinding. Only seven trials mentioned the blinding methods, but lacked a detailed introduction. Six trials used ancillary analysis, including correlation analysis (n = 2), cross experiment (n = 3), and subgroup analysis (n = 1). For the harms, 36 trials reported some information while 47 trials did not. Among the 16 trials that reported limitations of RCTs, only 7 trials specifically mentioned disadvantages in designing experiments whereas the remaining 9 trials lacked a detailed description on limitations. There were only 3 articles reporting details of registration, protocol, and funding.
Risk of bias
The six domains of the risks of bias evaluation for included trials are shown in the following and the summary of the risk of bias assessment is shown in Fig. 2. Detailed evaluation of the risk of bias of eac included study is provided in the Additional file 2: Fig. S1a, S1b.
Random sequence generation (selection bias)
-
Twenty-six trials were at a low risk of bias due to the application of random sequence generation, 9 trials were deemed having a high risk of bias based on admission order, hospital number, medical record number or other order [37, 39, 40, 49, 67, 72, 75, 95, 103]. The remaining 47 trials had no specific information.
Allocation concealment (selection bias)
-
Four trials reported detailed information were regarded as low risk of bias [27, 29, 33, 34], and 78 trials were regarded as unclear risk of bias.
Blinding of participants and personnel (performance bias)
-
Five trials reported using double-blinded design were regarded as low risk [27, 29, 32,33,34] and one trials were at a high risk of bias for blinding patients only [37]. The remaining 76 trials did not mention the blinding of participants and personnel.
Blinding of outcome assessment (detection bias)
-
Three articles mentioned blinding of outcome measurers [29, 33, 34]. One trial did not blind the outcome measurers and was therefore regarded as high risk of bias [37]. Seventy-eight trials had unclear information.
Incomplete outcome data (attrition bias)
-
All trials did not provide detailed information.
Selective reporting (reporting bias)
-
Since results of some scale points were not presented in a trial, it was regarded as having high risk of bias. The remaining 81 trials were evaluated at an unclear risk of bias.
Other bias
-
There was no baseline population characteristics in a trial [54]. One trial had a problem with the statistics of the number of male and female [62], six trials did not mention the inclusion and exclusion criteria for participants [67, 70, 80, 85, 88, 92] and result statistics of one trial was wrong [100].
Discussion
This systematic literature review included 82 RCTs which assessed the efficacy and safety of TCM for the treatment of CRF. Regarding efficacy, 78 studies reported an overall efficacy of TCM in treating CRF compared with the control group regardless of whether behavioral interventions were involved, in which 33 trials demonstrated that the efficacy rate was statistically significant (p < 0.05 or p < 0.01). Regarding the safety of TCM under investigation, non-serious or reversible adverse effects associated with the use of TCM for CRF had been reported. Among the included studies, TCM which demonstrated significant benefits on the management of CRF could be either herbs (including Astragali Radix (黃芪), Ginseng Radix (人參) and Codonopsis Radix (黨參)) or finished products (including Kangai Injection (康艾注射液), Buzhong Yiqi Decoction (補中益氣湯) and Shenqi Fuzheng Injection (參芪扶正注射液)). The benefits of TCM were reported in terms of improvements in the scores of the fatigue scales as well as physical indicators (e.g., cytokines level, blood tests). Overall, the findings of this review suggested that some TCM were effective in the management of CRF and relatively safe to use. However, the positive findings of the current review should be interpreted with cautions due to the concerns about the quality of the included RCTs. The implications of the review findings about TCM for CRF on clinical practice and the methodology of RCTs will be discussed in further details in the following.
Efficacy of TCM herbs for CRF
Among the included studies in this review, more than 100 herbs, whether used as single herb alone or as a combination of herbs, were under investigation for their potential role in treating CRF. The three most common herbs which demonstrated benefits on CRF were Astragali Radix (黃芪) (n = 48), Ginseng Radix (人參) (n = 34) and Codonopsis Radix (黨參) (n = 30). From the perspective of TCM theory, these three herbs are the representative herbs of tonifying qi [109]. Qi is the driving force of biological activity in the human body and plays a key role in body metabolism and immunity, which may help explain the mechanism of TCM in treating fatigue [15]. For cancer patients, radiation therapy and chemotherapy are regarded as heat toxicity, which may lead to spleen-qi deficiency and accumulation of dampness. Therefore, the herbs which promote and nourish the balance between yin and qi are often recommended in the treatment for CRF [110].
In terms of the pharmacological mechanism, the two major pathological reactions of CRF are inflammatory immune and metabolic disorders [111]. Similarly, these 3 herbs have demonstrated certain immunological regulatory and anti-tumor activities and the effect of reducing the adverse toxicity of chemotherapy or radiotherapy in the clinical treatment [112,113,114]. For example, Astragaloside IV, as an active extract of Astragali Radix, could inhibit the growth and proliferation of tumor cells and induce apoptosis of tumor cells [115], and promote immunity by regulating levels of IL-1β, IL-6, and TNF-α cytokines [116]. Ginsenosides, the main active agents in Panax ginseng, could help boost immunity by altering the levels of various cytokines [117, 118]. It was also reported that Ginseng Radix was associated with reduced inflammatory processes and released of cortisol through the stress axis and therefore could benefit CRF [119]. Codonopsis Radix had the characteristics of Gan and Ping, tonifying the spleen and lung, nourishing blood, stimulating fluid, and strengthening body’s resistance [120]. As reported, it had a beneficial effect on improving the clinical symptoms and QoL of cancer patients [113]. Toxicological studies had been conducted on various extracts of Codonopsis Radix, and few significant toxicity and side effects had been observed or recorded [121].
Efficacy of TCM products for CRF
In addition to some herbs, a variety of TCM products were investigated for their role in the treatment of CRF. Among the TCM products which showed efficacy, the most researched products were Kangai Injection (康艾注射液), followed by Buzhong Yiqi Decoction (補中益氣湯) and Shenqi Fuzheng Injection (參芪扶正注射液). These products composed of the three herbs mentioned above (Astragali Radix, Ginseng Radix and Codonopsis Radix), and their benefits could be partly explained with the functions of tonifying qi and supporting righteousness, enhancing the body's immunity and improving fatigue.
Kangai Injection, a famous TCM injection, mainly consists of Astragali Radix, Ginseng Radix and Sophorae Flavescentis Radix. Network pharmacological indicated that some of the active extracts, such as oxymatrine, ginsenoside, and kaempferol, could regulate the pharmacological activity of TP53, TNF, VEGFA, EGFR or other key targets, and also mediate cancer, TNF, HIF-1, PI3K-Akt and some signaling pathways [122, 123]. It was also reported that this product could enhance the body's immune system without causing damage to the body's normal cells [124]. The product could also improve the clinical effectiveness of lung, colorectal, breast or other cancer patients and was reportedly beneficial to the QoL and physical condition of the patients [115, 125, 126] without causing any significant adverse reactions [127].
Buzhong Yiqi Decoction (also known as Hochuekki-to or TJ-41 in Japanese, or Bojungikki-Tang in Korean) mainly composes of eight herbs including Astragali Radix, Atractylodis Macrocephalae Rhizoma, Ginseng Radix, Glycyrrhizae Radix, Angelicae Sinensis Radix, Bupleuri Radi, Citri Reticulatae Pericarpium and Cimicifugae Rhizoma. As a traditional Chinese decoction, it mainly played the role of regulating the spleen and stomach and ascending qi and yang [112, 128, 129]. In recent years, some studies have shown that Buzhong Yiqi Decoction had strong immunomodulatory and anticancer effects [130, 131]. Based on clinical experiences, this decoction could also prevent immunosuppression caused by surgical stress, improve the QoL of patients with CRF, and reduce side effects caused by radiotherapy or chemotherapy such as leukopenia [113].
Shenqi Fuzheng Injection mainly composes of Astragali Radix and Codonopsis Radix [132] and has been approved by China's FDA since the 1990s. The main function of this product is to tonify qi and support righteousness mostly for symptoms caused by deficiency of the lung and spleen. It can also be considered as an adjuvant therapy of lung cancer, breast cancer and colorectal cancer [133,134,135,136]. Improvement in the QoL and immunity of cancer chemotherapy patients and reduced adverse reactions during the treatment of cancer associated with the use of this preparation had been previously reported [112, 133, 134, 137].
Similarly, this systematic review showed that these three TCM products might have a positive effect on CRF by improving some fatigue scale scores, cytokines level and blood tests compared with control group, and most patients showed no obvious adverse reaction after medication. Some findings also supported the anti-fatigue efficacy of these products in CRF patients [128, 138]. According to the findings of this review, Kangai Injection, Buzhong Yiqi Decoction and Shenqi Fuzheng Injection are the TCM prescriptions worth considered for their role and potential benefits in treating CRF.
Safety of TCM for CRF
Apart from efficacy, the evaluation of the safety of TCM was another aspect of this review. Although the current evidence that some herbs improve the fatigue in cancer patients is strong, some herbs, even when used alone, should be considered with caution, such as American ginseng (Panax quinquefolius) and Guarana (Paullinia cupana). Third-degree adverse reactions with American ginseng have been reported in rates ranging from 1 to 5% for agitation, insomnia, nausea, and vomiting [139]. For Guarana, depending on the different intervention doses and outcome measures, this herb might be effective or ineffective in alleviating fatigue symptoms [140]. Currently, the available evidence cannot accurately confirm the efficacy and safety of these two herbs as a TCM treatment for CRF. In addition, incomplete processing or long-term usage of large doses of TCM will also aggravate the harm [141]. For example, excessive use of certain herb for invigorating qi could damage the vitality of the body, produce dryness and heat, and cause symptoms of “Shang huo” in TCM theory [142]. The risk of drug-herb interactions should also be taken into consideration, and the combination of herbs and some prescription medicines (such as central stimulants and antipsychotics) should be used with caution or even avoided. [142,143,144].
However, compared to other TCM preparations, the risk of adverse effects associated with the use of injections was more concerning [145]. Adverse drug reactions (ADR) related to TCM injections accounted for more than 50% of the total ADR reported in the use of TCM [146]. Common adverse reactions included rash, itching, chills, fever, abdominal pain, etc. Allergic reactions and shock could also occur in some severe cases. Bacterial endotoxin (or pyrogen), abnormal toxicity, visible foreign matter, insoluble particles, residual solvents, and other substances were considered as safety indicators for detection. Besides, the composition of TCM injection is complex and diverse, such as multiple extracts or unknown chemicals from plant/animal sources, which increase the difficulty of scientific quality monitoring and control [146]. Continuous research effort to evaluate the efficacy and safety of TCM injections and to standardize the quality control system is prominent for promoting evidence-based clinical use of TCM injection in CRF.
Improvement of RCTs design
Due to the insufficient evidence and high or unclear risk of bias of included RCTs, recommending TCM for CRF patients is a topic worthy of consideration. Further analysis of trials included in this review identified common limitations and provided relevant insights for designing and improving the quality of RCTs.
Firstly, the overall quality of RCTs studies included in this review was generally poor, a finding commonly reported in previous systematic reviews involving TCM for other conditions [147,148,149,150]. Among 82 included trials, none of them were completely in compliance with CONSORT-CHM guideline. Based on the Cochrane risk-of-bias tool, two major considerations for risk of bias were randomization generation and blinding. However, in this review, only 27 (32.93%) trials fully reported the mechanism of randomization generated via random sequence, other trials only addressed randomization using phrases including “randomization into two groups” or “in order of admission”. At the same time, only 7 (8.54%) trials mentioned blinding procedures in this review. Some research indicated that efficacy rate was overestimated by 17% in RCTs with no blinding as compared to those with blinding. Unclear or inadequate allocation concealment overstated the effect by 30–41% as compared to those with allocation fully hidden [151]. Therefore, it is meaningful and necessary to fully report the details of randomization and blinding by referring to the CONSORT-CHM statement and Cochrane guidelines.
Secondly, the limited sample size of included RCTs is also a concern. In this review, only 3 trials (3.66%) had more than 200 participants in the clinical studies [27, 33, 63] and 14 trials (17.07%) had a sample size between 100 and 200. Most of the trials had a limited number of subjects with sample size of less than 100 in 65 trials (79.27%), and three studies with less than 20 patients assigned to the test and control groups [26, 32, 89]. If the sample size is too small in clinical trials, it is difficult to observe related occurrence and progression of the disease outcomes. On the contrary, a sample size which is too large will lead to a waste of time, resource, and money [152, 153]. When designing the study, expected sample size should be indicated, either based on accurate calculations or practical limitations. According to the CONSORT statement, it is essential to calculate and report the sample size in all published RCTs. Sample size calculation does not only allow researchers to identify the endpoint, but also inform readers about some possible adaptation to actual practice, such as performing further analysis or advance trial termination [152]. Therefore, the quality and validity of studies will be greatly improved if all important details of sample size characteristics and calculations are considered and presented.
Thirdly, most of trials included in this review treated scale score changes as an outcome indicator to estimate the efficacy of using TCM. More than half of trials considered PFS (47/82) and QoL scales (51/82). Some other types of scales, including TCM syndrome score and fatigue scales, were also widely used. Currently, researchers aim to investigate more evidence for CRF, and a large number of fatigue scales are gradually appearing in the current research to assess psychological, physiological, endocrine, metabolic and other indicators of patients [154, 155]. But there is still no single authoritative evaluation tool and diagnostic method [156]. Due to the complex pathogenesis and multiple correlation factors of CRF, it is difficult for related scales to comprehensively assess the patient's symptoms, disease duration and intensity [157]. Therefore, in this review, repeatability and reliability of test results cannot be fully guaranteed in included trials with incomplete reporting. Based on the CONSORT statement and Cochrane guidelines, the scale items reported should be as specific as possible to eliminate any concerns about incomplete result data or selective reporting bias.
Limitations
There were several limitations in this review. Firstly, we only focused on RCTs, and excluded other study design and intervention approaches, resulting in limited consideration of the evidence for the treatment of CRF. Secondly, this review analyzed data from primary sources and the quality of trials was found to be low upon evaluation. There was some risk of bias in trials investigating the evidence on the efficacy and safety of TCM for CRF. Thirdly, no accurate diagnostic criteria for CRF patients were considered as multiple and incomplete diagnostic criteria were reported in the included RCTs. Finally, in TCM, there is often more than one ingredient to exert the therapeutic effect. The mutual induction or inhibition of various ingredients will also have an impact on the final curative effect. In our study, however, we only examined a few representative herbs and lacked an in-depth exploration of the inner relationship among other ingredients, which could result in bias when suggesting the herbs and products. Therefore, further studies need to improve on the quality of evidence, to establish the network analysis between relevant herb-ingredient-pharmacological activity of TCM for CRF, and to emphasize more on the efficacy of TCM.
Improvement for further research
Based on further analysis of RCTs included in this review, common limitations and improvement were identified. The following summarizes insights into developments in the field of TCM treatment of CRF. (1) In order to standardize the use of TCM in the diagnosis and treatment of CRF, unified comprehensive diagnosis and efficacy measurement standards should be established; (2) multicenter, large-sample RCTs of CRF should be performed to minimize bias caused by age and sex differences and to ensure the validity of the results; and (3) clinical studies of multicomponent therapies should be conducted to enrich the TCM studies of CRF and at the same time verify whether it has a comparative advantage over a single components in terms of safety and reliability.
Conclusion
Based on this review, the included TCM approaches have shown to be beneficial in the management and treatment of CRF. However, no recommended conclusion on the efficacy and safety of TCM in the treatment of CRF patients could be drawn as there were some concerns over the quality and bias of relevant RCTs. It is fundamental to standardize the diagnosis and treatment of CRF, improve RCTs protocol and conduct more clinical studies to present convincing evidence and provide an updated reference for disease medication in the future.
Availability of data and materials
All data are fully available without restriction.
Abbreviations
- CRF:
-
Cancer-related fatigue
- TCM:
-
Traditional Chinese medicine
- RCTs:
-
Randomized controlled trials
- CNKI:
-
China National Knowledge Infrastructure
- WOS:
-
Web of science
- PRISMA:
-
Preferred reporting items for systematic reviews and meta analyses
- CONSORT-CHM:
-
Consolidated standards of reporting trials statement extensions for Chinese herbal medicine formulas
- WM:
-
Western medicine
- CH:
-
Chemotherapy
- OT:
-
Other treatment
- CH + OT:
-
Chemotherapy plus other treatment
- MeSH:
-
Medical subject headings
- ICD-10:
-
The International Statistical Classification of Diseases and Related Health Problems 10th Revision
- KRG:
-
Korean Red Ginseng
- ADR:
-
Adverse drug reactions
- PFS:
-
Piper fatigue scale
- QoL:
-
Quality of life
- WHO:
-
World Health Organization
- KPS:
-
Karnofsky performance status
- QLQ-C30:
-
EORTC quality of life questionnaires
- GQoLI-74:
-
Generic quality of life inventory-74
- SF-36:
-
36-Item short-form
- FACT-F:
-
Functional assessment of cancer therapy–fatigue
- FACT-G:
-
Functional assessment of cancer therapy–general
- FACIT-F:
-
Functional assessment of chronic illness therapy–fatigue
- FACT-ES:
-
Functional assessment of chronic illness therapy–endocrine symptoms
- PSQI:
-
Pittsburgh sleep quality index
- QoL1 :
-
Quality of life questionnaire
- QoL2 :
-
Quality of Life Questionnaire and Quality of life score (QoL) for cancer patients (Chinese version draft)
- BFI:
-
Brief fatigue inventory
- CFS:
-
Cancer fatigue scale
- CFS*:
-
Chalder fatigue scale
- MFSI-SF:
-
Multidimensional fatigue symptom inventory–short form
- FSI:
-
Fatigue symptom inventory
- BFI-C:
-
Chinese version of brief fatigue inventory
- TOI-F:
-
Trial outcome index–fatigue
- VAS-F:
-
Visual analogue scale of global fatigue
- NGFRS:
-
NCCN guidelines fatigue rating scale
- HADS:
-
Hospital anxiety and depression scale
- POMS:
-
Profile of mood states
- HAMA:
-
Hamilton anxiety scale
- SDS:
-
Self-rating depression scale
- SS:
-
Satisfaction survey
- CS:
-
Comfort survey
- PSS:
-
Perceived stress scale
- GIC:
-
The global impression of change
- LASA:
-
Linear analogue self assessment scale
- BDI:
-
Beck inventory depression scale
- GCSG:
-
Gastric cancer symptom grading scale
- LCSG:
-
Lung cancer symptom grading scale
- HGB:
-
Hemoglobin
- Alb:
-
Albumin
- WBC:
-
White blood cell
- TP:
-
Total protein
- PLT:
-
Platelet
- COR:
-
Cortisol
- RBC:
-
Red blood cells
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- MCV:
-
Mean corpuscular volume
- HCT:
-
Hematocrit
- DM:
-
Degree of myelosuppression
- rhG-CSF:
-
Recombinant human granulocyte colony stimulating factor
- RECIST:
-
Response evaluation criteria in solid tumors
- AST:
-
Enzymes aspartate transaminase
- ALT:
-
Alanine transaminase
References
Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13(8):1012–39.
Morrow GR, Shelke AR, Roscoe JA, et al. Management of cancer-related fatigue. Cancer Invest. 2005;23(3):229–39.
Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(S1):22–34.
Cella D, Davis K, Breitbart W, et al. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19(14):3385–91.
Campos MPO, Hassan BJ, Riechelmann R, et al. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22(6):1273–9.
Dimeo FC. Effects of exercise on cancer-related fatigue. Cancer. 2001;92(6 Suppl):1689–93.
Du XT, Tian WP, Liu B, et al. Prevention and treatment of acupuncture for cancer-related fatigue caused by chemotherapy of intestinal cancer: a randomized controlled trial. World J Acupuncture - Moxibustion. 2021;31(2):83–8.
David A, Hausner D, Frenkel M. Cancer-related fatigue—is there a role for complementary and integrative medicine? Curr Oncol Rep. 2021;23(12):145.
Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemoor radio-therapy for cancer. BioSci Trends. 2010;4(6):297–307.
Su CX, Wang LQ, Grant SJ, et al. Chinese herbal medicine for cancer-related fatigue: a systematic review of randomized clinical trials. Complement Ther Med. 2014;22(3):567–79.
Zheng XY. Chinese medicine clinical research of new drugs guiding principles. 2002; 29:208-216.
Ge LL, Cao F. Modern application of Buzhong Yiqi decoction in the treatment of tumor. J Shandong Univ Tradit Chin Med. 2018;42(04):367–9.
Yang L, Li TT, Chu YT, et al. Traditional Chinese medical comprehensive therapy for cancer-related fatigue. Chin J Integr Med. 2016;22(1):67–72.
Huang ZK, Zhang Q, Fan YH, et al. Effect of traditional Chinese medicine injection on cancer-related fatigue: a meta-analysis based on existing evidence. Evid Based Complement Alternat Med. 2020;2020:2456873.
Kwon CY, Lee B, Kong M, et al. Effectiveness and safety of herbal medicine for cancer-related fatigue in lung cancer survivors: a systematic review and meta-analysis. Phytother Res. 2021;35(2):751–70.
Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:160.
Cheng CW, Wu TX, Shang HC, et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med. 2017;167(2):112–21.
Higgins JPT, Thomas J, Chandler J et.al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane2020. https://training.cochrane.org/handbook.
Piper BF, Dibble SL, Dodd MJ et.al (2021). Piper Fatigue Scale—Revised. Oncol Nurs Forum.
Piper BF, Dibble SL, Dodd MJ, et al. The revised piper fatigue scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–84.
WHOQOL: Measuring Quality of Life. World Health Organization. https://www.who.int/tools/whoQoL.
The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc Sci Med. 1995;41(10):1403–1409
Haraldstad K, Wahl A, Andenæs R, et al. A systematic review of quality of life research in medicine and health sciences. Qual Life Res. 2019;28(10):2641–50.
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350: g7647.
Sette CVM, de Alcântara Ribas BB, Schoueri JHM, et al. Purified dry Paullinia cupana (PC-18) extract for chemotherapy-induced fatigue: results of two double-blind randomized clinical trials. J Diet Suppl. 2018;15(5):673–83.
da Costa MV, Trufelli DC, Santos J, et al. Effectiveness of guaraná (Paullinia cupana) for postradiation fatigue and depression: results of a pilot double-blind randomized study. J Altern Complement Med. 2009;15(4):431–3.
Barton DL, Soori GS, Bauer BA, et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer. 2010;18(2):179–87.
Jeong JS, Ryu BH, Kim JS, et al. Bojungikki-tang for cancer-related fatigue: a pilot randomized clinical trial. Integr Cancer Ther. 2010;9(4):331–8.
de Oliveira Campos MP, Riechelmann R, Martins LC, et al. Guarana (Paullinia cupana) improves fatigue in breast cancer patients undergoing systemic chemotherapy. J Altern Complement Med. 2011;17(6):505–12.
Zhao H, Zhang Q, Zhao L, et al. Spore powder of Ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: a pilot clinical trial. Evid Based Complement Alternat Med. 2012;2012: 809614.
Barton DL, Liu H, Dakhil SR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013;105(16):1230–8.
Guglielmo M, Di Pede P, Alfieri S, et al. A randomized, double-blind, placebo controlled, phase II study to evaluate the efficacy of ginseng in reducing fatigue in patients treated for head and neck cancer. J Cancer Res Clin Oncol. 2020;146(10):2479–87.
Kim JW, Han SW, Cho JY, et al. Korean red ginseng for cancer-related fatigue in colorectal cancer patients with chemotherapy: a randomised phase III trial. Eur J Cancer. 2020;130:51–62.
Lee JY, Kim EH, Yoon JH, et al. Traditional herbal medicine, Sipjeondaebo-Tang, for cancer-related fatigue: a randomized, placebo-controlled, preliminary study. Integr Cancer Ther. 2021;20:15347354211040830.
Gu GS, Cui SJ, Li CB, et al. A clinical study on treating cancer-related fatigue in patients with gastrointestinal tumors with the Buzhong Yishen decoction. Clin J Chin Med. 2021;13(22):108–11.
Li X, Cui YY, Dong Q, et al. Clinical study on Shenqi Fuzheng injection in the treatment of cancer-related fatigue. World J Integr Tradit West Med. 2020;15(11):1967–71.
Lin SF, Zhao R, Tao L, et al. Clinical observation on treating cancer-related fatigue with the XiaoPi decoction. Clin J Chin Med. 2020;12(25):85–7.
Hu H, Wu F. Analysis of the effect of Buzhong Yiqi decoction combined with nursing intervention on the quality of life of patients with cancer-related fatigue after breast cancer surgery. Chin Rural Health. 2020;12(24):87.
Wang L, Xie ZH, Wu XM, et al. Clinical observation of TCM syndrome differentiation on treatment of cancer-related fatigue in patients with breast cancer. Chin J Tradit Chin Med Pharm. 2016;31(12):5375–8.
Cao YY. Clinical study on Jiawei Sijunzi recipe in treatment of cancer related fatigue after chemotherapy. Liaoning J Tradit Chin Med. 2020;47(08):112–4.
OuYang ZW, Wu YH. Clinical study of Jianpi YangRong decoction in the treatment of postoperative cancer-related fatigue of primary liver cancer. Chin Foreign Med Res. 2022;20(11):4–8.
Zhang J, Yin YJ, Meng XF. Clinical observation of Sizibuxu decoction for tumor-related fatigue after chemotherapy. World Latest Med Inf. 2018;18(47):258.
Zhu GD, Liu F, Zhu ZG, et al. Clinical observation on Buzhong Yiqi decoction treating cancer-related fatigue during chemotherapy interval of gastric cancer. Mod Hosp. 2016;16(08):1169–71.
Wang X, You J. Treating cancer-related fatigue after chemotherapy in lung cancer patients by Yiqi Jianpi Recipe: a clinical study. Chin J Integr Tradit West Med. 2015;35(09):1069–73.
Chen LC. Clinical observation on 30 cases of postoperative cancer-related fatigue treated by nursing intervention combined with oral administration of traditional Chinese medicine. Guiding J Tradit Chin Med Pharm. 2011;17(08):89–90.
Yang GB, Liu YF. Clinical observation of Shuyu pill in treatment of cancer-related fatigue. Hubei J Tradit Chin Med. 2015;37(04):8–10.
Kong XY, Huang J, Wang L, et al. Clinical observation of Fuzheng Yiliu decoction in treating chemotherapy-related fatigue in patients with advanced non-small cell lung cancer. J N Pharm. 2016;13(06):85–6.
Su YY, Chen F, Xiang J, et al. Chaiqi Sanhua decoction treating 28 cases of cancer-related fatigue in the rehabilitation period of breast cancer. Zhejiang J Tradit Chin Med. 2022;57(10):742–3.
Jiang R. Clinical observation of Kangai injection combined with chemotherapy in the treatment of digestive tract tumor cancer-related fatigue. The Med Forum. 2022;26(28):63–5.
Luo XX, Wang Q. 43 cases of cancer-related fatigue of lung and spleen deficiency treated by Peitu Shengjin method. Zhejiang J Tradit Chin Med. 2021;56(09):644–5.
Wang XT, Chen XJ, Wang LJ, et al. Clinical observation of Kangai injection in the treatment of cancer-related fatigue. Yunnan J Tradit Chin Med Materia Med. 2021;42(03):28–31.
He SQ, Wang RX, Yang T, et al. Clinical observation of invigorating spleen and tonifying kidney in the treatment of intestinal cancer-related fatigue. Clin J Tradit Chin Med. 2020;32(01):128–30.
Luo JB, He FZ, Chen HX, et al. Observation on clinical efficacy of Shenqi Fuzheng injection in the treatment of gastrointestinal cancer and cancer-related fatigue of lung cancer. Chin Pract Med. 2019;14(12):3–5.
Liu WW. Clinical observation of Guipi decoction in treating digestive tract cancer related-fatigue. Electron J Gen Stomatol. 2018;5(36):187–8.
Lin ZR, Pan P. Clinical observation on Buzhong Yiqi decoction in treating carcinogenic fatigue. Guangming J Chin Med. 2018;33(14):2039–41.
Li ZM. Randomized prospective study of Jianpi Yishen method combined with glucocorticoid in the treatment of cancer-related fatigue in elderly patients with advanced colorectal cancer. Liaoning J Tradit Chin Med. 2016;43(12):2592–4.
Song YY, Xu P, Yang CG, et al. Effects of Yangzheng Xiaoji capsule on cancer-related fatigue patients. J Tianjin Univ of Tradit Chin Med. 2016;35(04):238–41.
Liu YY, Han T, Zhang SH, et al. Clinical observation of Buzhong Yiqi decoction in treating cancer-related fatigue in patients with advanced tumors. Clin Misdiagn Misther. 2016;29(S1):88–91.
Li ZM. Effect and mechanism of Jianpi Yishen method on improving cancer-related fatigue in patients with advanced colorectal cancer. Chin J Exp Tradit Med Formulas. 2016;22(09):148–52.
Zhang Q, Lu CM, Fang WY. Clinical observation on integrative traditional Chinese and western medicine in treating cancer-related fatigue in advanced lung cancer. Shanxi J Tradit Chin Med. 2016;32(01):20–2125.
Liang CX, Zuo YF, Chen MY, et al. Clinical research on the effect of Shengxuebao mixture on cancer related fatigue. J Qiqihar Med Univ. 2016;37(01):8–10.
Sun X. Clinical observation of Kangai injection in improving cancer-related fatigue in patients with digestive tract malignant tumors after chemotherapy. Heilongjiang Med J. 2015;28(05):1068–9.
Li HC, Li L. Efficacy observation of Shenfu injection on the cancer-related fatigue in lung cancer patients after chemotherapy. Chin Pharm. 2011;22(48):4570–1.
Huang S. Clinical observation of Kanglaite injection in treating cancer-related fatigue. Chin J Integr Tradit West Med Dig. 2001;03:184–7.
Cao X, Gan L, Wang YF, et al. Clinical observation of Sini plus Renshen decoction in treatment of fatigue caused by cancer. Chin Arch Tradit Chin Med. 2022;40(01):58–60.
Zhang M, Zhang ZM. Clinical observation on Jiawei Sijunzi decoction in alleviating qi and blood deficiency type cancer-related fatigue. J Sichuan of Tradit Chin Med. 2019;37(04):91–3.
Wang JL. Effect of Buzhong Yiqi decoction combined with nursing intervention on postoperative cancer-induced fatigue in breast cancer. Guangming J Chin Med. 2019;34(01):151–3.
Yao LF. Shengxian decoction in the treatment of lung and spleen deficiency type of non-small cell lung cancer related fatigue clinical curative effect. Chin J Cancer Prev Tr. 2019;26(S1):60–1.
Chen YQ, Yang CW, Chen ZJ, et al. An effective study on treating cancer-related fatigue of advanced gastric cancer of the Qixue Kuixu type with the Bazhen decoction plus the Shenqi Fuzheng injection. Clin J Chin Med. 2019;11(08):48–50.
Wang QY. Clinical study on Shenqi Fuzheng injection in the treatment of cancer-related fatigue in patients with gastric cancer undergoing chemotherapy. J Clin Med. 2018;5(47):127–8.
Guo HR, Liu LS, Sun JL, et al. Clinical efficacy and quality of life evaluation of Shenqi Fuzheng injection in the treatment of cancer related fatigue in patients with advanced lung cancer. J N Sichuan Med Coll. 2017;32(02):163–6.
Gu YC, Xu HB, Jiang GG, et al. Clinical observation on Shenqi Fuzheng injection in treating cancer-related fatigue. Chin J Integr Tradit West Med. 2009;29(04):363–5.
Liu Q, Fang MZ. Clinical observation of ‘Yiqi Yangxue formula’ in treating 25 cases of fatigue after chemotherapy for colorectal cancer. Jiangsu J Tradit Chin Med. 2014;46(02):48–9.
Li N, Chen XY, Li X, et al. Clinical observation in treating cancer-related fatigue with Fufang Ejiao Jiang. Chin J Tradit Chin Med Pharm. 2013;28(02):565–7.
Wang L, Xie ZH, Xiao H, et al. Clinical observation on Guishao Liujunzi decoction in the treatment of cancer-related fatigue of colorectal cancer patients. Chin Med Mod Distance Educ Chin. 2016;14(21):70–3.
Cai GY. Clinical effect analysis of Buzhong Yiqi decoction in the treatment of tumor-related fatigue. Inner Mongolia J Tradit Chin Med. 2016;35(07):12–3.
Ning BB, Li BH, Hao SL, et al. Clinical study on Jiawei Buzhong Yiqi decoction in the treatment of cancer-related fatigue with spleen deficiency after non-small cell lung cancer surgery. Lishizhen Med Materia Med Res. 2020;31(11):2685–8.
Yang X. Clinical observation of Kangai injection in treating cancer-related fatigue after chemotherapy for lung cancer. Electron J Clin Med Lit. 2020;7(50):138+147.
Shan DN, Jiang XB, Song FH, et al. Observation on the clinical effect of Kangai injection in the treatment of cancer-related fatigue after chemotherapy for lung cancer. Chin J Mod Drug Appl. 2020;14(02):128–9.
Wu XM. Clinical observation of Kangai injection in treating cancer-related fatigue after chemotherapy for lung cancer. The Med Forum. 2018;22(17):2347–8.
Shi F. Clinical effect of Kangai injection on cancer-related fatigue after chemotherapy for lung cancer. Med Equip. 2017;30(21):134–5.
Zhang Y, Yang SL, Zhang HR, et al. Clinical study on Kangai injection in treating cancer-related fatigue after chemotherapy for lung cancer. Asia-Pacific Tradit Med. 2017;13(07):143–4.
Wang J, Lu HD. Clinical research of Kangai injection combined with chemotherapy on cancer related fatigue of patients with non-small cell lung cancer. Hubei J Tradit Chin Med. 2015;37(05):5–6.
Lu JF. Clinical observation on Kangai injection in treating cancer-related fatigue in advanced malignant tumors. Med People. 2014;27(05):106–7.
Zhang C, Wang LX, Guo YT. Clinical effect of Guipi decoction on digestive tract cancer-related fatigue. Inner Mongolia J Tradit Chin Med. 2019;38(08):20–1.
Zhang QD, Yuan QL, Lyu K. Observation on clinical curative effect of Shenqi granules on patients with cancer-related fatigue after colorectal cancer surgery. Chongqing Med. 2017;46(25):3578–80.
Wu J, Song M, Xu Y, et al. Improving effect of Shenmai injection on cancer-related fatigue of lung cancer patients receiving palliative treatment. Med J Natl Defending Forces Southwest Chin. 2014;24(12):1306–8.
Feng YB. Effect of shenfu injection on cancer-related fatigue of patients with advanced non-small cell lung with single-agent docetaxel chemotherapy. Liaoning J Tradit Chin Med. 2014;41(11):2358–60.
Zhang H, Shan LZ, Zhang J, et al. Clinical observation on pancreatic cancer’s cancer-related fatigue with Kangai injection combined chemotherapy. Chin J Surg Integr Tradit West Med. 2012;18(05):438–40.
Li ZM. Effect of spleen invigorating and kidney nourishing therapy on cancer-related fatigue, syndrome integral and survival status of patients with advanced colorectal cancer undergoing FOLFOX6 chemotherapy. Chin Gen Pract. 2015;18(36):4492–5.
Yang CW, Zhu Z, Chen YQ, et al. Effects of the Buzhong Yiqi decoction on cancer related fatigue and quality of life in patients with advanced malignant tumor. Clin J Chin Med. 2018;10(07):74–6.
Ou BQ, Du LJ. Application effect of Kangai injection in cancer-related fatigue after chemotherapy for lung cancer. Shaanxi J Tradit Chin Med. 2016;37(07):846–7.
Zhao D. Effects of Kang’ai injection on cancer-related fatigue after lung cancer chemotherapy. J Shandong First Med Univ & Shandong Acad Med Sci. 2015;36(01):55–6.
Leng ZQ. Efficacy of Kangai injection in the treatment of cancer-related fatigue after chemotherapy for lung cancer. For All Health. 2015;9(01):121.
Jing NC, Cheng L, Wang J, et al. Efficacy of Kangai injection in the treatment of cancer-related fatigue after chemotherapy for lung cancer. Chin J Gerontol. 2010;30(22):3393–4.
Huang YN. Influence of Kangai injection on cancer-related fatigue of lung cancer in elderly patients receiving chemotherapy and its impact on the quality of life. Clin J Chin Med. 2012;4(09):1–3.
Wei XL. Effect of Shenmai injection on cancer fatigue in lung cancer patients receiving palliative care. Chin J Clin Ration Drug Use. 2016;9(32):43–4.
Wu J, Xu Y, Jiang ZH, et al. Effect of Shenmai injection combined with western medicine in treating lung cancer and cancer-induced fatigue. Shaanxi J Tradit Chin Med. 2014;35(10):1358–9.
Dai YL, Li HM, Zhang T, et al. Efficacy of compound Kushen injection for cancer-related fatigue in patients with lung cancer following chemotherapy. Eval Anal Drug-Use Hosp Chin. 2013;13(08):722–4.
Huang ZF, Wei JS, Yuan Y et.al (2013). Effect of Shenmai injection on quality of life of patients with cancer-related fatigue. 2013 Natl Annu Conf Tradit Chin Med Oncol, Beijing.
Wu XJ. Symptom scores and mechanism of Shenmai injection on cancer-related fatigue in patients. Liaoning J Tradit Chin Med. 2014;41(06):1171–3.
Liang J, Xu DS, Wei Y. Clinical observation of Shenmai injection treatment for cancer related fatigue. Chin J Exp Tradit Med Formulae. 2012;18(17):279–81.
Wei LL. Clinical observation of Kanglaite injection in the treatment of cancer-relayed fatigue in patients with cancer cachexia. Chin Health Vision. 2021;21:58.
Cui YX, Mi JW, Feng Y, et al. Huangqi Sijunzi decoction for treating cancer-related fatigue in breast cancer patients: a randomized trial and network pharmacology study. J South Med Univ. 2022;42(5):649–57.
Gu YC, Xu HB, Zhao MS. Clinical study on effect of Shenfu injection treating cancer-related fatigue of patients with advanced carcinoma. Chin J Chin Materia Med. 2010;35(07):915–8.
Ji SL, Cheng ZZ. Diagnostics of traditional Chinese medicine. Chin J Integr Trad Western Med. 2002;8:142.
Wu MH. Chinese medicine internal science. 2012;
Liu JX. Practical traditional Chinese medicine tumor manual. 1996;93–97
Dong J, Wang S, Gui Y, et al. Astragalus membranaceus (Huang Qi) for cancer-related fatigue: a protocol for systematic review and meta-analysis. Medicine. 2022;101(3): e28633.
Yang YF, Ross J. (2010). Theories and concepts in the composition of Chinese herbal formulas. Chin Herb Formul. Yang Yifan and Ross Jeremy. Edinburgh, Churchill Livingstone: 1-34.
Zhang C, Guo W, Yao XH, et al. Database mining and animal experiment-based validation of the efficacy and mechanism of Radix Astragali (Huangqi) and rhizoma atractylodis macrocephalae (Baizhu) as core drugs of traditional Chinese medicine in cancer-related fatigue. J Ethnopharmacol. 2022;285: 114892.
Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. BioSci Trends. 2010;4(6):297–307.
Qi F, Zhao L, Zhou A, et al. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. BioSci Trends. 2015;9(1):16–34.
Zhan MR, Li GH, Guo XC. Advances in studies on chemical constituents and pharmacological activities of codonopsis. Shangdong Chem Ind. 2021;50(19):79–82+84.
Xue JX, Zhu ZY, Bian WH, et al. The traditional Chinese medicine Kangai injection as an adjuvant method in combination with chemotherapy for the treatment of breast cancer in Chinese patients: a meta-analysis. Evid Based Complement Alternat Med. 2018;2018:6305645.
Li Y, Meng TT, Hao N, et al. Immune regulation mechanism of Astragaloside IV on RAW264.7 cells through activating the NF-κB/MAPK signaling pathway. Int Immunopharmacol. 2017;49:38–49.
Ratan ZA, Youn SH, Kwak YS, et al. Adaptogenic effects of Panax ginseng on modulation of immune functions. J Ginseng Res. 2021;45(1):32–40.
Riaz M, Rahman NU, Zia-Ul-Haq M, et al. Ginseng: a dietary supplement as immune-modulator in various diseases. Trends Food Sci Technol. 2019;83:12–30.
Mancuso C, Santangelo R. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem Toxicol. 2017;107:362–72.
Li LH, Chen CJ, Hu XY, et al. Research progress on chemical constituents and pharmacological effects of codonopsis pilosula. Acta Chin Med Pharmacol. 2023;51(03):112–5.
Gao SM, Liu JS, Wang M, et al. Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: a review. J Ethnopharmacol. 2018;219:50–70.
Zheng BB, Wang X, Wang Q, et al. Study on the molecular mechanism of Kangai injection in the treatment of gastric cancer based on network pharmacology and molecular docking. J Modern Oncol. 2022;30(11):2003–7.
Ke CH, Wang YW, Yan H, et al. Research on the mechanism of Kangai injection in treatment of breast cancer based on network pharmacology and molecular docking. Anhui Med Pharm J. 2023;27(01):24–9+215.
Zhang JJ, Chen XF. Clinical research progress and adverse reactions of Kang Ai injection. Chin Commun Doct. 2016;5:16–7.
Li H, Ji Y, Zhang S, et al. Kangai injection combined with platinum-based chemotherapy for the treatment of stage III/IV non-small cell lung cancer: a meta-analysis and systematic review of 35 randomized controlled trials. J Cancer. 2019;10(21):5283–98.
Huang S, Peng W, Mao D, et al. Kangai injection, a traditional Chinese medicine, improves efficacy and reduces toxicity of chemotherapy in advanced colorectal cancer patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:8423037.
Liu XQ, Zhou S. Clinical research progress of Kangai injection in the treatment of tumor. Herb Med. 2009;28(5):625–6.
Li GW, Ding JX, Zhang YN, et al. The clinical application and pharmacological mechanism of Bu-Zhong-Yi-Qi decoction for treating cancer-related fatigue: An overview. Biomed Pharmacother. 2022;156: 113969.
Zheng XF, Tian JS, Liu P, et al. Analysis of the restorative effect of Bu-zhong-yi-qi-tang in the spleen-qi deficiency rat model using 1H-NMR-based metabonomics. J Ethnopharmacol. 2014;151(2):912–20.
Kido T, Mori K, Daikuhara H, et al. The protective effect of hochu-ekki-to (TJ-41), a Japanese herbal medicine, against HSV-1 infection in mitomycin C-treated mice. Anticancer Res. 2000;20(6a):4109–13.
Kimura M, Sasada T, Kanai M, et al. Preventive effect of a traditional herbal medicine, Hochu-ekki-to, on immunosuppression induced by surgical stress. Surg Today. 2008;38(4):316–22.
Xiong Y, Zhao Q, Gu L, et al. Shenqi Fuzheng Injection reverses cisplatin resistance through mitofusin-2-mediated cell cycle arrest and apoptosis in A549/DDP Cells. Evid Based Complement Alternat Med. 2018;2018:8258246.
Dong J, Su SY, Wang MY, et al. Shenqi fuzheng, an injection concocted from Chinese medicinal herbs, combined with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review. J Exp Clin Cancer Res. 2010;29(1):137.
Xu R, Lin L, Li Y, et al. ShenQi FuZheng Injection combined with chemotherapy in the treatment of colorectal cancer: a meta-analysis. PLoS ONE. 2017;12(9): e0185254.
Wu JH, Xie BC, Wang QH, et al. Systematic review of Shenqi Fuzheng Injection combined with chemotherapy for treatment of breast cancer. Zhongguo Zhong Yao Za Zhi. 2019;44(3):589–96.
Gao W, Zhang K. Network meta-analysis of 8 types of traditional Chinese medicine injection combined with chemotherapy in colorectal cancer treatment. J Cancer Res Clin Oncol. 2023;149(12):9823–38.
Yang Y, Wang T, Liu X, et al. Immunoregulation of shenqi fuzheng injection combined with chemotherapy in cancer patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2017;2017:5121538.
Li H, Hou T, Sun S, et al. Efficacy of ginseng oral administration and ginseng injections on cancer-related fatigue: a meta-analysis. Medicine. 2022;101(46): e31363.
Sadeghian M, Rahmani S, Zendehdel M, et al. Ginseng and cancer-related fatigue: a systematic review of clinical trials. Nutr Cancer. 2021;73(8):1270–81.
de Araujo DP, Pereira PTVT, Fontes AJC, et al. The use of guarana (Paullinia cupana) as a dietary supplement for fatigue in cancer patients: a systematic review with a meta-analysis. Support Care Cancer. 2021;29(12):7171–82.
Huang HQ. Analysis of reasons and countermeasures for adverse reaction of Chinese herbal decoction. Chin J Clin Ration Drug Use. 2018;11(33):112–3.
Tan J, Zhang H, Zhang L, et al. Ginseng and “Shanghuo” (fireness): a comprehensive review from the viewpoints of TCM theory and modern science. Food Funct. 2023;14(8):3437–53.
Paik DJ, Lee CH. Review of cases of patient risk associated with ginseng abuse and misuse. J Ginseng Res. 2015;39(2):89–93.
Nocerino E, Amato M, Izzo AA. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71:S1–5.
Li H, Deng J, Deng L, et al. Safety profile of traditional Chinese herbal injection: an analysis of a spontaneous reporting system in China. Pharmacoepidemiol Drug Saf. 2019;28(7):1002–13.
Li H, Wang S, Yue Z, et al. Traditional Chinese herbal injection: current status and future perspectives. Fitoterapia. 2018;129:249–56.
Liang Z, Chen X, Shi J, Hu H, Xue Y, Ung COL. Efficacy and safety of traditional Chinese medicines for non-alcoholic fatty liver disease: a systematic literature review of randomized controlled trials. Chin Med. 2021;16(1):9.
Chen X, Shi J, Lai Y, Xue Y, Ung COL, Hu H. Systematic analysis of randomised controlled trials of Chinese herb medicine for non-alcoholic steatohepatitis (NASH): implications for future drug development and trial design. Chin Med. 2023;18(1):58.
Shi J, Hu H, Harnett J, Zheng X, Liang Z, Wang YT, Ung COL. An evaluation of randomized controlled trials on nutraceuticals containing traditional Chinese medicines for diabetes management: a systematic review. Chin Med. 2019;29(14):54.
Li L, Xie H, Wang L, Zhang A, Mou X, Lin Y, Ma H, Wang Y, Li J, Gao J, Wang CC, Leung PC, Fan X, Wu X. The efficacy and safety of combined chinese herbal medicine and western medicine therapy for COVID-19: a systematic review and meta-analysis. Chin Med. 2022;17(1):77.
Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12.
Noordzij M, Tripepi G, Dekker FW, et al. Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transplant. 2010;25(5):1388–93.
Gupta KK, Attri JP, Singh A, et al. Basic concepts for sample size calculation: critical step for any clinical trials! Saudi J Anaesth. 2016;10(3):328–31.
Xue XJ, Xu CP, Yang XY, et al. Research progress on assessment tools and indicators of cancer-related fatigue. Chin J Nurs. 2012;47(09):859–61.
Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health Qual Life Outcomes. 2007;5(1):12.
Wang XS, Woodruff JF. Cancer-related and treatment-related fatigue. Gynecol Oncol. 2015;136(3):446–52.
Yang H, Lu HZ, Li L, et al. Research progress of cancer fatigue evaluation tools. Chin Gen Pract Nurs. 2020;18(12):1444–7.
Acknowledgements
We are also grateful to Chair Prof. Yi-Tao Wang for his technical guidance and support.
Funding
The study was funded by the University of Macau (Reference number: SRG2021-00007-ICMS and MYRG2022-00229-ICMS).
Author information
Authors and Affiliations
Contributions
COLU, JY and YL conceived and designed the study. JY, YL, JS and XC were responsible for data management and analysis. COLU, JY, YL, JS and XC contributed to interpretation of study results. JY, YL and CIC drafted the manuscript. COLU, HH and CIC critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of authors consent to publication of this work in Chinese Medicine.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
A detailed description of each search strategy.
Additional file 2:
Table S1. Level of risks of bias. Table S2. Diagnosis and included criteria of the CRF participants in RCTs in this review. Table S3. Evaluation of included trial studies using the CONSORT-CHM statement. Figure S1a. Risk of bias summary: review authors' judgements about each risk of bias item for each included study (Study No. 01-42). Figure S1b. Risk of bias summary: review authors' judgements about each risk of bias item for each included study (Study No. 43-81).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, J., Li, Y., Chau, C.I. et al. Efficacy and safety of traditional Chinese medicine for cancer-related fatigue: a systematic literature review of randomized controlled trials. Chin Med 18, 142 (2023). https://doi.org/10.1186/s13020-023-00849-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-023-00849-y