Abstract
Purpose
Fatigue is a distressing symptom in head & neck cancer patients before during and at the end of curative therapy. Pharmacologic and not pharmacologic treatments have been proposed with scarce or no evidence of efficacy. The aim of the study is to evaluate the efficacy of American ginseng in respect to placebo in reducing fatigue in patients treated for head and neck cancer with curative intent.
Methods
Thirty-two patients who had completed oncological treatment for a primary Head & neck tumor for at least 1 year and had a global fatigue score > 4 by means of Brief Fatigue Inventory (BFI) were randomized to receive 1000 mg of American ginseng or placebo per day for 8 weeks with the aim to assess their efficacy. Changes in fatigue scores in the 2 subgroups of patients before and after the treatment with American ginseng or placebo, were assessed by the BFI at baseline and at the end of week 8.
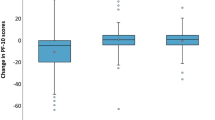
Results
The mean of the mean values of the BFI measured at 8 weeks (end of treatment) was 4.6 in the Ginseng arm and 3.4 in the Placebo arm (p = ns). Mean comparison showed a tendency to statistical significance only for the single item on interference with general activity (p = 0.06), with better performance for placebo. The mean of the differences between baseline values and 8 weeks values was not significantly different between treatment arms considering the entire questionnaire.
Conclusion
The present data shows that American ginseng has insufficient evidence to be recommended for Cancer Related Fatigue (CRF) in post treatment HNC survivors.


Similar content being viewed by others
References
Ahlberg K, Ekman T, Gaston-Johansson F et al (2003) Assessment and management of cancer-related fatigue in adults. Lancet 362:640–650
American Joint Committee on Cancer (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Arring NM, Barton DL, Brooks T, Zick SM (2019) Integrative therapies for cancer-related fatigue. Cancer J 25:349–356
Attele AS, Wu JA, Yuan CS (1999) Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58:1685–1693
Barton DL, Liu H, Dakhil SR, Linquist B, Sloan JA et al (2013) Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 105:1230–1238
Bossi P, Di Pede P, Guglielmo M, Granata R et al (2019) Prevalence of fatigue in head and neck cancer survivors. Ann Otol Rhinol Laryngol 128:413–419
Bower JE, Bak K, Berger A et al (2014) Screening assessment and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology Clinical Practice Guideline Adaptation. J Clin Oncol 32:1840–1850
Brown WA (1998) The placebo effect. Sci Am 278:90–95
Bruera E, Valero V, Driver L, Shen L, Willey J, Zhang T, Palmer JL (2006) Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol 1(24):2073–2078
Bruera E, El Osta B, Valero V, Driver LC, Pei BL, Shen L, Poulter VA, Palmer JL (2007) Donepezil for cancer-related fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol 10(25):3475–3481
Catania G, Bell C, Ottonelli S, Marchetti M, Bryce J, Grossi A, Costantini M (2013) Cancer-related fatigue in Italian cancer patients: validation of the Italian version of the Brief Fatigue Inventory (BFI). Support Care Cancer 21:413–419
Cella D, Davis K, Breitbart W, Curt G (2001) Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol 19:3385–3391
Chang YD, Smith J, Portman D et al (2018) Single institute experience with methylphenidate and American Ginseng in cancer-related fatigue. Am J Hosp Palliat Care 35:144–150
Chaukar DA, Walvekar RR, Das AK et al (2009) Quality of life in head and neck cancer survivors: a cross-sectional survey. Am J Otolaryngol 30:176–180
Chen X (1996) Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol 23:728–732
Colloca L, Barsky AJ (2020) Placebo and Nocebo effects. N Engl J Med 6(382):554–561
Cui JF, Eneroth P, Bruhn JG (1999) Gynostemma pentaphyllum: identification of major sapogenins and differentiation from Panax species. Eur J Pharm 8:187–191
De la Cruz M, Hui D, Parsons HA, Bruera E (2010) Placebo and nocebo effects in randomized double-blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer 1(116):766–774
Duncan GG, Epstein JB, Tu D et al (2005) Quality of life, mucositis, and xerostomia from radiotherapy for head and neck cancers: a report from the NCIC CTG HN2 randomized trial of an antimicrobial lozenge to prevent mucositis. Head Neck 27(5):421–428
Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, Weis J, Jordan K & Ripamonti C.I (2020) On behalf of the ESMO Guidelines Committee. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol S0923–7534(20):36077–4
Gillis CN (1997) Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol 54:1–8
Gunn GB, Mendoza TB, Garden AS et al (2011) Patient-reported fatigue in head and neck cancer survivors. J Clin Oncol 29(15 suppl):5523
Gunn GB, Hansen CC, Garden AS, Fuller CD et al (2015) Favorable patient reported outcomes following IMRT for early carcinomas of the tonsillar fossa: results from a symptom assessment study. Radiother Oncol. 117:132–138
Hickok JT, Roscoe JA, Morrow GR et al (2005) Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer 104:1772–1778
Higginson I, Armes J, Krishnasamy M (2004) Fatigue in cancer. Oxford University Press, Oxford
Huang KC (1999) The pharmacology of Chinese herbs. CRC Press, Boca Raton
Jereczek-Fossa BA, Santoro L, Alterio D et al (2007) Fatigue during head-and-neck radiotherapy: prospective study on 117 consecutive patients. Int J Radiat Oncol Biol Phys 68:403–415
Kim HS, Kim MK, Lee M, Kwon BS, Suh DH, Song YS (2017) Effect of red ginseng on genotoxicity and health-related quality of life after adjuvant chemotherapy in patients with epithelial ovarian cancer: a randomized, double blind, placebo-controlled trial. Nutrients 9:9
Mehanna HM, Morton RP (2006) Does quality of life predict long-term survival in patients with head and neck cancer? Arch Otolaryngol Head Neck Surg 132:27–31
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85:1186–1196
Mitchell SA, Beck SL, Edward Hood L, Moore K, Tanner ER (2007) Putting evidence into practice: evidence-based interventions of fatigue during and following cancer and its treatment. Clin J Oncol Nurs 11:99–113
Oskam IM, Verdonck-de Leeuw IM, Aaronson NK et al (2010) Quality of life as predictor of survival: a prospective study on patients treated with combined surgery and radiotherapy for advanced oral and oropharyngeal cancer. Radiother Oncol 97:258–262
Osthus AA, Aarstad AK, Olofsson J et al (2011) Health-related quality of life scores in long-term head and neck cancer survivors predict subsequent survival: a prospective cohort study. Clin Otolaryngol 36:361–368
Pourmohamadi K, Ahmadzadeh A, Latifi M (2018) Investigating the effects of oral ginseng on the cancer-related fatigue and quality of life in patients with non-metastatic cancer. Int J Hematol Oncol Stem Cell Res 12:313–317
Qi LW, Wang CZ, Yuan CS (2011) Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep 28:467–495
Weimer K, Colloca L, Enck P (2015) Placebo effects in psychiatry: mediators and moderators. Lancet Psychiatry 2:246–257
Yennurajalingam S, Tannir NM, Williams JL et al (2017) A double-blind, randomized, placebo-controlled trial of Panax Ginseng for cancer-related fatigue in patients with advanced cancer. J Natl Compr Canc Netw 15:1111–1120
Yuan CS, Wu JA, Lowell T, Gu M (1998) Gut and brain effects of American ginseng root on brainstem neuronal activities in rats. Am J Chin Med 26:47–55
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Licitra reports grants and personal fees from Astrazeneca, Boehringer Ingelheim, Merck-Serono, Eisai, MSD, Novartis, Roche; personal fees from Bayer, BMS, Debiopharm, Sobi, Ipsen, GSK, Doxa Pharma srl, Incyte Biosciences Italy srl, Amgen, Nanobiotics Sa, outside the submitted work. Dr. Bossi reports personal fees from Merck, Sanofi, Merck Sharp & Dohme, Sun Pharma, Angelini, AstraZeneca, Bristol-Myers Squibb, Helsinn, GSK, Kyowa Hakko Kirin, Roche, outside the submitted work. All the remaining authors declare no conflict of interest. Authors declare they have full control of all primary data and agree to allow the journal to review the data if requested.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guglielmo, M., Di Pede, P., Alfieri, S. et al. A randomized, double-blind, placebo controlled, phase II study to evaluate the efficacy of ginseng in reducing fatigue in patients treated for head and neck cancer. J Cancer Res Clin Oncol 146, 2479–2487 (2020). https://doi.org/10.1007/s00432-020-03300-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03300-z