Abstract
Osteoarthritis (OA) is a common chronic degenerative joint disease in clinical practice with a high prevalence, especially in the elderly. Traditional Chinese Medicine (TCM) believes that OA belongs to the category of “Bi syndrome” and the “bone Bi syndrome”. The etiology and pathogenesis lie in the deficiency of the liver and kidney, the deficiency of Qi and blood, and external exposure to wind, cold, and dampness. Epimedium is a yang-reinforcing herb in TCM, which can tonify the liver and kidney, strengthen muscles and bones, dispel wind, cold and dampness, and can treat both the symptoms and the root cause of “bone Bi syndrome”. In addition, Epimedium contains a large number of ingredients. Through modern science and technology, more than 270 compounds have been found in Epimedium, among which flavonoids are the main active ingredients. Therefore, our study will review the effects and mechanisms of genus Epimedium in treating OA from two aspects: (1) Introduction of Epimedium and its main active ingredients; (2) Effects of Epimedium and its active ingredients in treating OA and relevant signaling pathways, in order to provide more ideas for OA treatment.
Similar content being viewed by others
Background
Osteoarthritis (OA) is a chronic degenerative disease with a significantly increased prevalence with age. OA can lead to disability, severely affecting patients’ life quality. Results from a Global Burden of Disease 2010 Study as early as 2010 showed that hip and knee OA ranked 11th out of 291 diseases causing disability and 38th out of disability-adjusted life years [1]. During 2013–2015, 54.4 million adults in the United States had arthritis, of those, 23.7 million (43.5%) were limited in their daily activities due to arthritis [2]. Besides, the high prevalence and disability rates have added to the burden on families and the entire healthcare system. According to statistics, the annual medical cost of knee OA exceeds $27 billion, and total knee arthroplasties-related expenditures have exceeded $11 billion annually [3]. China is one of the world’s most populous countries and is in the stage of rapid aging. Many scholars believe that OA will become one of the major problems of the Chinese health system [4]. Therefore, there is an urgent need for more research to be devoted to OA pathology and treatment.
Currently, the treatment methods of OA can be divided into non-drug intervention, drug treatment and surgical treatment [5]. Non-drug intervention mainly focuses on education, exercise and weight loss. Drug treatment includes the use of anti-inflammatory drugs, such as oral or topical non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors, intra-articular injections of corticosteroids or hyaluronic acid, and the use of pain relievers such as duloxetine or opioids. Surgical treatment is the ultimate option for patients with advanced OA or persistent pain [5]. However, the benefit/risk ratio of these commonly used methods may not be high [6]. In Kloppenburg’s study, he listed a number of studies on current drugs commonly used in the clinic to treat OA [6]. These clinical results indicate that their effects on pain and functional improvements in OA patients are relatively small, especially in terms of long-term effects and pain relief, and intra-articular injection with corticosteroids may lead to cartilage loss. In addition, some drugs have an increased risk of adverse events such as gastrointestinal ulcers, bleeding, hyperglycemia, and cardiovascular disease [5, 6].
After thousands of years of development and inheritance, Traditional Chinese Medicine (TCM) has formed a medical system with unique theories and methods. During the COVID-19 epidemic, TCM has played an essential role in fighting against the virus [7, 8]. In the treatment of OA, TCM also has many insights and great potential.
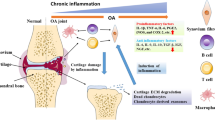
OA is classified as “Bi syndrome” and “bone Bi syndrome” in TCM [9]. The primary pathogenesis is the deficiency of Qi, blood, Ying, and Wei, and external exposure to wind, cold and dampness, resulting in the stagnation of Qi, blood and meridians, and then causing pain [9]. Epimedium, as shown in Fig. 1, is a Yang tonic herb in TCM, and the central medicinal part is dried leaves. Its effects are good at tonifying the kidney and Yang, strengthening the muscles and bones, and dispelling wind and dampness, which means that Epimedium can treat both the symptoms and the root cause of “bone Bi syndrome”. Therefore, our article will review Epimedium and its main active ingredients, and their roles and mechanisms in OA treatment, in order to provide more ideas for OA study.
Epimedium and its main active ingredients
The genus Epimedium (Yinyanghuo in Chinese) belongs to the family Berberidaceae Juss., and was initially documented in “Shen Nong Ben Cao Jing” [10]. At present, as many as 80 species of Epimedium have been discovered, most of which are endemic to China and mainly distributed in the southwest and central regions [11, 12]. There are also a few species in eastern, southern and central Asia and Europe. In the Chinese Pharmacopoeia (2020 edition), Epimedium brevicomu Maxim., Epimedium sagittatum (Sieb. et Zucc.) Maxim., Epimedium pubescens Maxim., and Epimedium koreanum Nakai were considered as the main medicinal species [13]. In fact, besides these species, species such as Epimedium wushanense T.S.Ying and Epimedium acuminatum Franch. are also widely used [14]. Due to the good medicinal properties of Epimedium, many proprietary Chinese medicines and health products that have been marketed contain Epimedium, of which Epimedium as the main medicine are shown in Table 1. They are used for the treatment of osteoporosis, reproductive system disorders, kidney yang deficiency symptoms, and other diseases. With the increasing research on Epimedium and its ingredients, medical scientists have also found that they have the potential to treat uveitis [15], cardiovascular disease [16], tumors [17, 18], influenza A [19], and neuroprotection [20, 21] through anti-inflammation [22], antioxidant [20], regulating cell proliferation, apoptosis and autophagy [16, 23], modulating immunity [24], and antiviral [19] mechanisms.
Epimedium contains a large number of ingredients. According to statistics, more than 270 ingredients have been identified from different species of Epimedium, including flavonoids, lignans, polysaccharides, alkaloids, and volatile oils [14, 25]. Among them, flavonoids account for more than half of the ingredients, which shows that flavonoids are the vital material basis for Epimedium to function [12]. Flavonoids refer to a series of compounds formed by the interconnection of two benzene rings through three carbon atoms, that is, the C6–C3–C6 structure, as shown in Fig. 2 [26, 27]. In Epimedium, prenylated flavonols and their glycosides are the most distinctive compounds, and the basis of Epimedium’s efficacy, such as icariin, epimedin A, epimedin B, epimedin C, and baohuoside I [25, 28]. Icariin and epimedins A–C are also specified in the Chinese Pharmacopoeia as quality control standards for crude and processed products of Epimedium [13]. The structures of these five main ingredients in Epimedium are shown in Fig. 2. However, the absorption permeability of these compounds is not high, so their bioavailability is actually very low [29, 30]. A higher number of glycosyl groups means higher solubility, but poorer permeability. Baohuoside I (monoglycoside), icariin (diglycoside) and epimedins A–C (triglycosides) have sequentially lower permeability [29, 31]. In different research models and different body fluids, these compounds undergo complex chemical transformations, and deglycosylation is a crucial process for metabolism and absorption in vivo [25, 30]. Epimedins A–C can convert to secondary glycosides and eventually to monoglycosides or aglycone. Icariin can deglycosylate to monoglycosides or aglycone, and baohuoside I can convert to aglycone [25, 31]. Baohuoside I and icaritin (aglycone) are the main metabolites of icariin, of which, icaritin is not soluble enough in water due to the absence of sugar substitution, which greatly limits its research and application [28, 29, 32]. Therefore, improving the bioavailability of these flavonoids is a prerequisite for better performance of their effects. Szabo et al. summarized some of the current methods to improve the bioavailability of icariin and its derivatives by searching a large number of literature [33]. These methods include phospholipid complex formation, micelle formation, etc. They also believed that the simultaneous application of multiple methods may increase the bioavailability of sparingly soluble flavonoids exponentially [33]. In the Processing of Chinese Materia Medica, the most commonly used processing method of Epimedium is to stir-fry it with suet oil [34]. Studies have shown that stir-frying with suet oil can transform polyglycosides to secondary glycosides in Epimedium, which is more conducive to absorption [29, 35]. Moreover, the addition of suet oil can further improve the bioavailability of Epimedium flavonoids through the formation of self-assembled micelles [30].
Besides flavonoids, lignans, polysaccharides and alkaloids are also important ingredients in Epimedium. These ingredients have been shown to have anti-inflammatory [36], antioxidant [37, 38], and immunomodulatory activities [39]. In addition, Epimedium polysaccharides have also been shown that can increase the solubility of icariin and baohuoside I in a concentration-dependent manner, and the solubilizing effect of acidic polysaccharides was more pronounced [40]. Magnoflorine is the main alkaloid contained in Epimedium [41]. Gao et al. determined the content of alkaloids in the leaves of 29 species of genus Epimedium, and found that the content of magnoflorine ranged from 0.003 to 2. 603%, with the highest content value being 867 times higher than the lowest content value [42]. Epimedium species with similar magnoflorine contents often have consanguinity or belong to the same taxon. Therefore, they believed that the content of magnoflorine can be used as an evidence to identify the subgenus relationship of Epimedium [42].
However, there are few studies of these ingredients in the treatment of OA. Through searching and summarizing a large number of studies, we found that the researches on Epimedium still focus on flavonoids compounds, especially in treating OA. Therefore, in the following sections, we will introduce in detail the effects and mechanisms of Epimedium and its ingredients, mainly flavonoids, in the OA treatment. Meanwhile, based on the anti-inflammatory and antioxidant activities of lignans and polysaccharides from Epimedium, and there having been studies on the treatment of OA with these components from other herbs, such as lignans of Schisandra chinensis (Turcz.) Baill. [43], total lignans of Vitex negundo var. cannabifolia (Sieb.et Zucc.) Hand.-Mazz. seeds [44], polysaccharides of Achyranthes bidentata Blume [45], and polysaccharides of Eucommia ulmoides Oliv. [46], we expect more Epimedium ingredients can participate in the research of OA and other diseases in the future.
Study on the mechanisms of Epimedium on OA
OA is the most common type of arthritis. All joint tissues can involve in the pathogenesis and progression of OA, including cartilage, synovium, subchondral bone, joint capsule and ligaments. The degeneration of cartilage and the loss of its biomechanical properties are the core pathological changes and outcomes of OA [47, 48]. Therefore, our study will summarize the recent in vivo and in vitro experimental studies in treating OA by Epimedium and its active ingredients from the perspective of cartilage, synovium and subchondral bone.
Maintaining structures and functions of articular cartilage
Normal articular cartilage consists of a small number of chondrocytes and a large amount of extracellular matrix [49]. The extracellular matrix is mainly composed of type II collagen (Col2) and proteoglycans [49]. Other collagens such as IX and XI, also play a vital role in cartilage development and function [50]. Proteoglycan is a complex sugar composed of protein and glycosaminoglycan (GAG), which plays a protective role on the skeleton formed by collagen [51].
Chondrocytes are dispersed in the matrix and maintain the synthesis and catabolism of extracellular matrix. The degradation of extracellular matrix mainly depends on the production of a series of proteases by chondrocytes [52]. Matrix metalloproteinases (MMPs) and aggrecanases are the key enzymes that perform the degradation and are the major mediators of OA matrix loss [49]. Among them, MMP13 is a collagenase with the strongest effect, which can directly degrade Col2 and cause matrix loss [53]. Aggrecan-degrading enzymes are represented by ADAMTS (ADAM with thrombospondin-1 domains)-4 and ADAMTS-5 [49].
Chondrocytes also control the matrix formation. Aggrecan is the most abundant proteoglycan in the matrix synthesized by chondrocytes [52]. It can interact with hyaluronic acid to provide osmotic properties for cartilage, and is considered as one of the markers of matrix formation [52]. However, the turnover rate of chondrocytes and matrix is extremely low, so articular cartilage has been considered as an inert tissue for a long time [54]. Chondrocytes are the only resident cell group in articular cartilage [54, 55]. They lack the nourishment of nerves, blood vessels and lymph, and are separated from each other by matrix, so they are vividly called solitary cells [54, 55]. Besides, they are highly differentiated cells, which means that once damaged, the self-healing ability of cartilage tissue is very poor [51]. Studies have shown that many pathological events occur in articular cartilage during the progression of OA, including inflammation, oxidative stress (OS), increased apoptosis, and decreased autophagy [56]. Eventually, the matrix is lost and the cartilage degenerates. Table 2 summarizes the literature about Epimedium and its active ingredients in treating OA from the aspect of articular cartilage. It can be seen that they can maintain structures and functions of cartilage from the following aspects: (1) anti-inflammation and anti-OS; (2) inhibiting chondrocyte apoptosis and pyroptosis, and improving autophagy; (3) promoting chondrogenic differentiation of mesenchymal stem cells (MSCs), as shown in Fig. 3.
Anti-inflammation and anti-OS
At present, more and more studies have shown that low-grade inflammation is closely related to OA [49]. Chondrocytes can not only respond to inflammatory factors in the joint tissue, but also produce proinflammatory factors themselves to further promote the release of degrading enzymes [49]. Tumor Necrosis Factor-α (TNF-α) and Interleukin- (IL-)1 are two crucial inflammatory cytokines in OA, which can promote the production of protease in chondrocytes [58, 77, 78]. Besides, they can also induce the synthesis of nitric oxide (NO) mediated by inducible nitric oxide synthase (iNOS), and prostaglandin mediated by COX-2 [58, 77, 78]. NO is another important player in OA and has several roles [77]. For example, NO can derive a series of highly oxidizing free radicals by interacting with compounds, such as reactive oxygen species (ROS), causing cell damage and apoptosis [79, 80].
Xianling Gubao capsules, made of five traditional Chinese herbs represented by Epimedium, have been widely used to treat osteoporosis and OA in China [81]. Ziadlou et al. selected 34 representative components from Xianling Gubao capsules [53]. Among the selected Epimedium components, OA chondrocytes 3D pellet culture with adding Epimedin C (25 µM) produced the most matrix (GAG/DNA) after 2 weeks. Further studies showed that 25 µM of Epimedin C can down-regulate COX-2 levels, reduce matrix degradation and promote matrix formation in human OA chondrocytes treated with IL-1β/TNF-α [53]. NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways are key pathways involved in OA inflammation, especially the NF-κB pathway [57, 58, 60]. Hypoxia-inducible factor-2alpha (HIF-2α) is the downstream molecule of NF-κB, which directly induces the expression of MMP9, MMP13, ADAMTS-4, iNOS and prostaglandin-endoperoxide synthase-2 [82, 83]. Based on in vitro and in vivo experiments, the study of Wang et al. showed that icariin can down-regulate NF-κB/HIF-2α signaling pathway to relieve inflammation and protect chondrocytes [57]. Hyperoside is another flavonoid glycoside isolated from Epimedium. Sun et al. found that hyperoside at the concentration of 10, 20 and 40 μM can reduce iNOS and COX-2 expressions, decrease the production of ADAMTS5, MMP3 and MMP13, and enhance the expression of cartilage matrix formation factors in IL-1β-stimulated C57BL/6 mouse chondrocytes for 24 h [58]. The nuclear factor erythroid 2-related factor 2 (Nrf2) is a vital antioxidant factor in the cytoplasm and regulates the activity of various antioxidant enzymes, including superoxide dismutase (SOD), heme oxygenase-1 (HO-1), and NAD(P)H quinine oxidoreductase-1 (NQO-1) by binding to the antioxidant reaction elements in the nucleus [59]. Furthermore, by adding phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT), extracellular signal-regulated kinase (ERK) and Nrf2 inhibitors, Sun et al. demonstrated that hyperoside can reduce inflammatory response and matrix loss in OA mainly by inhibiting PI3K/AKT/NF-κB and MAPK signaling pathways, and enhancing Nrf2/HO-1 signaling pathway [58]. In addition, they found that high ROS expression was associated with chondrocyte apoptosis, and hyperoside can reduce ROS production and chondrocyte apoptosis by up-regulating Nrf2 [58]. Zuo et al. pretreated human chondrocytes with icariin (10–3 μM) for 2 h and then stimulated with IL-1β [59]. Compared with direct IL-1β stimulation, they found that matrix degradation decreased in icariin pretreatment group. At the same time, ROS expression decreased, and Nrf2 and downstream antioxidant enzyme expression increased. Furthermore, they transfected human chondrocytes with Nrf2 siRNA, and they found the above phenomena and effects were eliminated. Therefore, they concluded that the regulation of Nrf2 is of great significance for the treatment of OA from the perspective of anti-inflammation and anti-OS [59].
Pain is still a thorny problem in OA, and there is no effective treatment yet [84]. Inflammation plays a vital role in causing pain [84]. Relevant studies have found that neuroregulation involved in regulating OA inflammation and pain [85]. Epimedium and its active ingredients can effectively inhibit OA inflammation, but whether they involve in the neuroregulation remains unclear. In the study of Li et al., they first confirmed the therapeutic effects of icariin on knee OA through in vivo and in vitro experiments, and exhibited the therapeutic effects are related to the inhibition of Toll-like receptor 4 (TLR4)/myeloid differentiation primary-response gene 88 (MyD88)/NF-κB inflammatory signaling pathway [61]. Second, based on functional magnetic resonance imaging and virus retrograde tracing techniques, they found that icariin can involve in the neural regulation by regulating the hypothalamic-mediated neuromodulation pathway and the endocannabinoid-related pathway. Further, by tandem mass tag-based quantitative proteomics and bioinformatics analyses, they verified levels of pain-related genes, such as Inα, Rtn4, changed after icariin treatment. Therefore, they concluded that icariin can participate in pain-related neural regulation of OA [61].
Inhibiting chondrocyte apoptosis, pyroptosis, and improving autophagy
The relationship between inflammation, OS, and apoptosis, pyroptosis and autophagy is very close. Apoptosis is programmed cell death to prevent excessive inflammation and damage to tissues. In OA, many factors such as inflammation, OS and autophagy state are related to chondrocyte apoptosis [86]. Epimedium and its active ingredients have been shown to inhibit chondrocyte apoptosis through various mechanisms.
The study of Mi et al. found that compared with the normal control group, the expressions of inflammatory cytokines (IL-1, IL-6, and IL-12), OS markers (NO, ROS), and apoptosis-related proteins (Bax, caspase-3, caspase-9) increased in TNF-α-treated SD rat chondrocytes. At the same time, the expression of autophagy markers (Atg5, Atg7, LC3-II) decreased [62]. However, the addition of icariin can partially block the above effects and is related to the inhibition of NF-κB pathway [62]. The non-coding RNAs (ncRNAs) is a kind of functional RNA that is transcribed from DNA but not translates into proteins [87]. More and more researches have shown that ncRNAs maintain the functions and homeostasis of cartilage, and also involve in the pathogenesis of OA [88]. The long non-coding RNAs (lncRNAs) refers to ncRNAs with a length of more than 200 nucleotides, which accounts for a considerable proportion of ncRNAs [87]. Wang et al. found that icariin can induce the upregulation of CYTOR, an IncRNA, to inhibit apoptosis of chondrocytes, and verified the findings by constructing CYTOR overexpression plasmids and CYTOR short hairpin RNA [63]. Zhou et al. studied the extraction method of Epimedium [64]. By using ethanol solution as extraction solvent and transfer rates of main flavonoids such as epimedins A–C, icariin and baohuoside I as evaluation index, they determined the optimal conditions for the ethanol extraction process of Epimedium, and demonstrated that the ethanol extract of Epimedium can reduce the apoptosis of chondrocytes induced by IL-1β [64]. Unlike apoptosis, the pyroptosis process is pro-inflammatory [89]. The typical pyroptosis pathway is mediated by caspase-1, in which nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome plays a key role [89]. Icariin has been shown to inhibit chondrocyte pyroptosis by inhibiting the expression of NLRP3 inflammasome and downstream factors, such as caspase-1 and GSDMD [56].
Autophagy, as a self-protection mechanism of cells, can use autophagosome and lysosome to remove damaged organelles and enhance the adaptabilities and survivals of cells [90]. Due to the unique structural characteristics of chondrocytes with absence of blood vessels, and lack of oxygen and nutrients, autophagy is particularly important in maintaining the structures and functions of chondrocytes [91, 92]. Targeting autophagy therapy is one of the hot topics in the treatment of OA [92]. Mammalian target of rapamycin (mTOR) is a negative regulator of autophagy, regulated by upstream PI3K/AKT and AMP-activated protein kinase (AMPK) signals [93]. In chondrocytes of OA rats established by the modified Hulth method, the activation of PI3K/AKT/mTOR pathway and increased chondrocytes apoptosis rate were observed [65]. After treatment with mTOR inhibitor, rapamycin, and different concentrations of icariin, the expression of PI3K/AKT/mTOR pathway-related factors decreased. However, the group of adding autophagy inhibitor, 3-methyladenine, showed significant chondrocytes apoptosis. The in vivo experiment results also exhibited that rapamycin and icariin can promote the expression of autophagy and improve the pathological status of OA articular cartilage [65].
Endoplasmic reticulum (ER) stress refers to the cellular state of protein misfolding and unfolded protein accumulation in ER caused by pathological conditions such as hypoxia, nutritional deficiency and continuous OS [94]. ER stress can activate autophagy to degrade misfolded proteins or damaged ER, and promote new ER generation, in order to maintain cell homeostasis [94, 95]. However, ER stress and long-term activation of autophagy tend to induce cell death [96]. Bone marrow-derived mesenchymal stem cells (BMSCs) have the ability to differentiate into chondrocytes after induction, which is one of the potential treatments for OA and cartilage defects [70]. However, in the OA environments with hypoxia and glucose deprivation, BMSCs will undergo ER stress, resulting in increased apoptosis, which greatly hinders the ability and efficiency of chondrogenic differentiation [67]. Icariin has been shown to reduce the expression of ER stress-related proteins, such as binding immunoglobulin protein (BIP), activating transcription factor 4 (ATF4) and XBP-1s (the active form of X-box-binding protein 1) in BMSCs through the inhibition of MAPK signaling pathway, thereby reducing the production of apoptosis proteins [67]. Besides, the expression of autophagy markers is also reduced, indicating that autophagy is also weakened [67]. In addition to enhancing the tolerance of cells to hypoxia and glucose deprivation by improving ER stress, icariin can also maintain the viability of chondrocytes in the hypoxia environment by increasing the expression of hypoxia-inducible factor-1α (HIF-1α), to promote glucose uptake and anaerobic glycolysis [68].
Promoting chondrogenic differentiation of MSCs
With the development of regenerative medicine, stem cell and cytokine therapy has gradually become a new choice for OA treatment [69]. Cartilage tissue engineering utilizes the potential of MSCs to differentiate into chondrocytes. These MSCs include BMSCs, adipose-derived MSCs (AMSCs), synovial membrane-derived MSCs (SM-MSCs), Umbilical cord blood-derived MSCs (UCB-MSCs), and induced pluripotent stem cells-derived MSCs (IPSC-MSCs) [97, 98]. Epimedium and its active ingredients play an important role in inducing chondrogenic differentiation of these MSCs, and reducing hypertrophy.
BMSCs are ideal, most widely studied source of autologous cell therapy for promoting cartilage repair [99]. Zhu et al. found in vitro that hydrogels loaded with different concentrations of icariin can promote BMSCs chondrogenic differentiation in varying degrees, and selected icariin at a concentration of 147.8 μM as the optimal concentration for in vivo experiments [69]. Then, the OA model was established by destabilizing medial meniscus surgery in SD rats, and BMSCs-loaded icariin hydrogel was injected into the articular cavity. Compared with the PBS group, the BMSCs group, and the icariin hydrogel group, the cartilage morphology of the BMSCs-loaded icariin hydrogel group was complete, and the expression of chondrogenic markers, such as Sox 9, Col2α1 and aggrecan increased. At the same time, the matrix metalloproteinase MMP13 decreased. Moreover, the factors related to wingless-related integration site (Wnt)/β-catenin pathway changed. These results demonstrated that icariin-loaded hydrogel can promote the chondrogenic differentiation of BMSCs and was related to the activation of Wnt/β-catenin pathway [69]. In addition, icariin can increase the tolerance of BMSCs to ischemia and hypoxia, increasing the long-term benefit of OA [70]. In addition to icariin, icaritin has also shown to promote BMSCs chondrogenic differentiation via the wnt/β-catenin pathway [71]. Hypertrophy is a common side effect of using growth factors such as transforming growth factor- (TGF-) β to induce MSCs differentiation [72]. X-type collagen (ColX), MMP13, osteocalcin, Indian hedgehog (IHh), and alkaline phosphatase are verified markers of hypertrophic chondrocytes [100]. Wang et al. found that after adding icariin, the ability of TGF-β to promote BMSCs chondrogenic differentiation enhanced, but did not increase hypertrophy reaction and fibrochondrogenesis [72]. There is a negative feedback regulation between parathyroid hormone-related protein (PHrP) and IHh [74]. In OA, chondrocytes acquire hypertrophy phenotype, and the expression of IHh increases [74, 100]. The increased IHh will up-regulate PHrP, and in turn inhibit IHh expression to maintain cartilage homeostasis [74, 100]. In the study of Luo et al., icariin was shown that can promote chondrocyte differentiation, and up-regulate PTHrP and down-regulate IHh to inhibit chondrocyte hypertrophy [74].
AMSCs are easier to obtain and may have better chondrogenic differentiation potential than BMSCs [75]. Bahrami et al. cultured TGF-β and/or icariin with human subcutaneous AMSC-fibrin constructors, and found that the icariin + TGF-β group can significantly increase the chondrogenic differentiation ability of the constructors, and the expression of hypertrophic markers (ColX) and fibro-chondrogenic markers (type I collagen) decreased compared with the TGF-β group [75]. Yu et al. injected 1 mL of autologous subcutaneous AMSC suspension, which was treated with 60 g/L icariin solution for 3 days, into the joint cavity of rabbit OA [76]. They found that compared with injecting saline, and injecting subcutaneous AMSCs without icariin pretreatment, the expression of NO, IL-1 and TNF-α in joint fluid decreased significantly after 14 days. Besides, the cartilage surface was intact, and the Mankin score of cartilage injury decreased. Moreover, they found that the expression of factors related to TLR4/NF-κB pathway decreased. Therefore, they concluded that icariin combined with AMSCs can treat OA [76].
SM and cartilage originate from a common cell pool during joint development, which means that SM-MSCs are more prone to chondrogenesis than BMSCs and AMSCs [101]. Zare et al. injected SM-MSCs and AMSCs into the articular cavity of OA rats, and found that they both improved radiological and pathological grading of articular cartilage [102]. By comparison, SM-MSCs showed better results [102]. UCB-MSCs are more accessible to obtain through painless extraction procedures [97]. Li et al. conducted a meta-analysis of clinical studies on UCB-MSCs in cartilage defects and OA treatment, and confirmed the efficacy and quality of UCB-MSCs treatment [103]. IPSCs refer to reprogramming terminally differentiated adult cells into PSCs by introducing specific transcription factors [98]. It is reported that IPSCs showed similar properties with embryonic stem cells in self-renewal and differentiation capacity [98]. Diederichs et al. reprogrammed human BMSCs to IPSCs and then derived MSCs [104]. They found that these stem cells had the morphology and surface markers of MSCs, but exhibited high heterogeneity compared with the originating parental BMSCs. In addition, they were less responsive to traditional BMSCs differentiation protocols. Therefore, the authors believe that the MSCs generated from IPSCs are a unique population of cells with mesenchymal characteristics [104]. Although the effects on IPSC-MSCs are still being determined, they are an attractive and promising source of cells considering their almost inexhaustible nature.
Although the above cell therapies have their own advantages, it is challenging to maintain stable heredity and phenotype [105]. Therefore, many medical scientists have turned their attention to exosomes (Exos) [105]. Exos is a kind of extracellular vesicle secreted by cells, containing proteins, messenger RNA, microRNA and other substances, which is the carriers of cell communication [106]. MSCs can indirectly act on target cells by secreting Exos, which is important in disease treatment [105]. Zhu et al. injected IPSC-MSC-Exos and SM-MSC-Exos into collagenase-induced OA mice, and found that both Exos promoted chondrocyte proliferation and migration. In contrast, IPSC-MSC-Exos worked better [101].
Many studies have reported the advantages and disadvantages of MSCs derived from different sources in chondrogenic differentiation. However, there are few studies on the role of Epimedium and its active ingredients in promoting chondrogenic differentiation of synovial membrane-derived, umbilical cord blood-derived, and induced pluripotent stem cells-derived MSCs. Besides, there are few studies about OA treatment with Epimedium by regulating Exos. In the future, we expect more studies will focus on the above three stem cells in chondrogenic differentiation and the intervening role of Epimedium. Also, whether Exos mechanism participates in the Epimedium treating OA is worth studying.
Improving synovial inflammation
SM is a connective tissue membrane attached to the edge of articular cartilage and adhered to the joint capsule, which can be divided into the inner synovial layer and the sub-synovial layer [107, 108]. The inner synovial layer is dominated by cells, mainly composed of fibroblasts, macrophages and MSCs [107, 108]. The sub-synovial layer is dominated by blood vessels, lymph and nerves [107, 108]. SM can regulate the composition of joint fluid and provide nutrients to cartilage [108].
Synovitis is the main pathological process of SM, and is closely related to OA. In a prospective epidemiological study, synovitis was present in a majority of OA patients and was associated with pain and dysfunction in patients [109, 110]. It has been found that synovitis may be an early change of OA, and crosstalk between SM and cartilage plays an important role in OA progression [111]. Tissue debris from meniscus injury, anterior fork ligament injury, and cartilage degradation products all have the potential to trigger synovitis [112, 113]. Then, cytokines and proteases released by SM will cause cartilage to release more cytokines and proteases, resulting in progressive cartilage loss and persistent inflammation of SM, forming a vicious cycle [112, 113]. By summarizing the studies of Epimedium and its active ingredients in the treatment of OA, as detailed in Table 3, we found that they can treat synovial inflammation in OA through various mechanisms.
Fibroblasts are the dominant cell population in the SM, and their excessive proliferation is related to the release of inflammatory factors and the secretion of matrix degrading enzymes [118]. Pan et al. treated human fibroblast-like synovial cells in OA patients with different concentrations of icariin, and found that icariin can inhibit the proliferation and migration of fibroblast-like synovial cells in a dose-dependent manner [114]. In addition, the expression of IL-1, MMP14 and BIP, which are related to cartilage matrix degradation and ER stress also decreased [114]. Jin et al. established OA models by transecting the anterior cruciate ligament of the right knee joint in SD rats, and then treated with total flavonoids of Epimedium by gavage for 4 weeks [115]. They found that compared with the model control group, the total flavonoids of Epimedium group can relieve knee swelling, and increase pain threshold in OA rats. Besides, the synovial inflammatory cell infiltration and hyperplasia reduced, and the serum levels of IL-6, IL-1β and TNF-α decreased. Jin et al. also proved that the above effects were related to the inhibition of NF-κB signaling pathway [115]. Synovial macrophages, another important synovial cells, maintain the dynamic balance of anti-inflammation and pro-inflammation in synovial fluid through MI and M2 polarization states, with M1 promoting inflammation, and M2 inhibiting inflammation [119]. Quercetin is a flavonoid compound found in various herbs, including Epimedium. In vitro, quercetin at the concentrations of 2, 4, and 8 μM have been shown to inhibit the expression of inflammatory cytokines, the production of matrix-degrading enzyme, and chondrocyte apoptosis in a dose-dependent manner by inhibiting the AKT/NF-κB signaling pathway [116]. In addition, 8 μM quercetin can polarize RAW 264.7 cells into M2 macrophages, and release factors that promote cartilage growth, such as TGF-β1, TGF-β2, TGF-β3, insulin-like growth factor- (IGF-) 1, and IGF2, providing a microenvironment for chondrogenesis. In vivo experiments also confirmed the results obtained in vitro [116].
Ferroptosis is iron-dependent cell death associated with lipid peroxidation [120]. That is to say, iron accumulation and lipid peroxidation are crucial factors of ferroptosis [120]. Through the Fenton reaction, Fe2+ triggers lipid peroxidations to produce lipid hydroperoxides [121]. Malondialdehyde (MDA) is the main secondary metabolite of lipid hydroperoxides [121]. Glutathione (GSH) is a crucial antioxidant in the body, and cystine is the raw material of its synthesis [120, 121]. The cystine/glutamate antiporter system Xc− on the cell membrane can transport cystine into the cell, in which SLC7A11 as a system Xc− subunit, is highly specific for cystine/glutamate, thus facilitating the GSH formation [120, 121]. GSH peroxidase 4 (GPX4) is a GSH-dependent enzyme that ultimately mediates the reduction of lipid hydroperoxide or free hydrogen peroxide [120]. Yu et al. discovered that iron accumulation and decreased expression of GSH, GPX4, SLC7A11 and other factors related to antioxidant in human OA cartilage through the transcriptome RNA sequencing technology, indicating that ferroptosis is closely related to OA [122]. Synovitis is an important pathological process of OA. Luo et al. studied whether icariin can protect synovial cells by inhibiting ferroptosis to prevent OA development [117]. They found that icariin treatment of LPS-induced human synovial cells decreased the iron content, MDA and other factors related to ferroptosis, while increased the antioxidant-related factors such as GPX4, Nrf2 and SLC7A11. Besides, icariin counteracted the increased iron content and the imbalance between oxidate and antioxidant systems caused by GPX4 inhibitor-RSL3. Therefore, they concluded that icariin can protect synovial cells by inhibiting ferroptosis through Xc−/GPX4 axis [117].
Regulating subchondral bone remodeling
Cartilage and subchondral bone are not two separate joint tissues [123]. They are closely related in structure and function [123]. The calcified cartilaginous layer of cartilage is directly connected to the subchondral bone plate in a “comb tooth” structure [124]. The subchondral bone plate is a cortical lamellar bone structure that can withstand the stress directly from cartilage [125]. The subchondral cancellous bone (trabeculae) is distributed and arranged in the same direction as the stress and tension on the subchondral bone, helping to absorb joint stress and transfer them to the surroundings evenly [124, 125]. Guévremont et al. detected hepatocyte growth factor in OA cartilage [126]. However, it is not produced by chondrocytes, but by osteoblasts from subchondral bone plate, which indicates that there is a biochemical connection between cartilage and subchondral bone, and the calcified cartilage is effective on transporting such factors [126]. In conclusion, cartilage and subchondral bone, as a functional unit, are interconnected. Subchondral bone targeting therapy provides a new therapeutic idea for OA [127].
Osteoclasts dismantle and carry away old bone, and osteoblasts rebuild new bone. This process is called bone remodeling. In the early stage of OA, subchondral bone showed increased bone resorption and rapid bone remodeling, leading to structural changes such as decreased subchondral bone plate thickness, and increased porosity [128,129,130,131]. However, in the late stage of OA, subchondral bone showed decreased bone resorption and remodeling, and the bone formation relatively increased, with the image feature exhibiting subchondral osteosclerosis [128,129,130,131].
Epimedium is a common Chinese herb for the treatment of osteoporosis, and there are many studies on its effects on osteoporosis. It can promote osteogenic differentiation of BMSCs, promote bone formation, or inhibit bone resorption through various mechanisms to treat osteoporosis [132,133,134]. However, in the environment of OA, the effects of Epimedium and its ingredients on bone remodeling are limited. Gao et al. used the transection of anterior cruciate ligament method to establish OA mice models [135]. After 8 weeks, they found that the indicators of bone remodeling (CTX, osteocalcin) decreased. Histopathology showed the late OA manifestations, such as extensive exposure of subchondral bone, cartilage loss, thickening of subchondral cortical bone plate, and reduction of bone trabecula numbers. After treatment with icariin, bone remodeling of OA mice was regulated, and morphological structure of cartilage and subchondral bone was improved, especially early administrating icariin [135]. TGF-β plays a vital role in bone remodeling, which is secreted by osteoblasts and deposited in bone matrix [136]. Osteoclasts can activate it during bone resorption [136, 137]. Subsequently, TGF-β specifically induces MSCs recruitment to bone resorption sites and promotes their proliferation through signal transduction protein SMAD family, thus contributing to bone formation [136, 137]. Elevated TGF-β1 was observed in both animal and human OA, and has been shown to be associated with abnormal bone formation, OA severity, angiogenesis, and mineralization reduction [138, 139]. Xu et al. established OA rat models by modified Hulth method, and then administered icariin intragastrically for 12 weeks [140]. They found that the disorder of bone metabolism in OA rats was regulated, with 1,25-(OH)2D3, CTX, and osteocalcin increased, and the morphological structures of cartilage and subchondral bone improved. In addition, they found that factors related to the TGF-β signaling pathway decreased after treatment, suggesting that the therapeutic effects of icariin may be related to the inhibition of the TGF-β signaling pathway [140].
Toxicity
Unlike Tripterygium wilfordii Hook.f., its toxicity is widely known in TCM [141]. In recent years, with the increased reports of adverse reactions to Epimedium and its proprietary Chinese medicines, Epimedium is considered as a potential toxic drug. By searching and summarizing the toxicity of Epimedium and its main components, we found that the toxicity of Epimedium is mainly related to the flavonoids, and hepatotoxicity was more reported [142,143,144]. Cao combined with network toxicology and cellular experiments to target and verify that icaritin is the material basis of hepatotoxicity [145]. Further, through metabolomics technology, she considered that the mechanism might be related to GSH metabolism. In addition, the study of Cao also found that the combination with Ligustrum lucidum W.T.Aiton, another common kidney tonic herb, can inhibit the enrichment of the main flavonoids of Epimedium in the body, suggesting that the combination of the two is of great importance [145]. In addition, Morinda officinalis F.C. How has also been reported to have a detoxification effect on Epimedium [146]. Yang et al. conducted a study on the content of heavy metals in Epimedium [147]. They found that the content of heavy metals varied greatly among different species of Epimedium, with copper and lead being the main heavy metals, and the levels of copper and lead were higher in wild Epimedium than in cultivated Epimedium. In addition, they found that some samples sold in the Chinese market had excessive heavy metal content. Therefore, they suggested that toxic heavy metal contamination is also an issue that needs attention in the application of Epimedium [147]. Xianling Gubao Capsule is a proprietary Chinese medicine that has been widely used in the treatment of bone diseases such as fractures and osteoporosis in recent years, and is also the proprietary Chinese medicine with the most reported adverse reactions to Epimedium preparations in recent years [148]. Peng analyzed 2796 reports of adverse drug reactions/adverse events (ADRs/AEs) after using Xianling gubao capsule/tablet from the National Adverse Drug Reaction Monitoring Center from 2004 to mid-2006, and found that ADRs/AEs mainly manifested as nausea, rash, itching, gastrointestinal disorders, and dizziness, and severe ADRs/AEs mainly manifested as abnormal liver function, accounting for 44.12% of all severe ADRs/AEs manifestations [149]. Based on the above, it is suggested that liver functions of patients should be paid close attention when the Xianling gubao capsule is used in clinical practice.
However, several toxicity tests have shown no acute, subchronic toxicity or genotoxicity of Epimedium extraction or its major ingredients [150,151,152]. The clinical manifestations, blood physiological and biochemical parameters, histopathology, and cell proliferation capacity of the test animals were not significantly different from those of the control animals. The differences in results may be related to the species, extraction method, processing method, dosage, duration, and compatibility of Epimedium [148]. Gao et al. reviewed the literature on Epimedium toxicity and proposed a drug-specific response theory of Epimedium [153]. In other words, there is no obvious direct toxicity of this drug, and the occurrence of toxicity is mainly related to the patient’s metabolism or immune idiosyncrasy. If the body is under immune stress, it is more likely to trigger hepatotoxicity.
In summary, Epimedium is relatively safe in clinical use, but cautions should be exercised to avoid taking large dosages or long periods. Besides, attentions should be paid to patient’s own immune and metabolic states when using Epimedium.
Conclusions
OA is a complex disease, which has not been effectively conquered clinically. At present, there are many studies on OA therapeutic targets. TCM has the characteristics of multiple targets, and has great prospects in treating OA. Epimedium is one of the commonly used kidney tonic herbs, which can nourish the liver and kidney, strengthen the muscles and bones, dispel wind and dampness, and can effectively treat both the symptoms and the root cause of OA. Through searching and summarizing literature on the treatment of OA by Epimedium and its ingredients, we found that their treatments of OA are relatively comprehensive, involving various pathological processes and signaling pathways. We reviewed the therapeutic effects of Epimedium and its active ingredients on OA from three aspects: maintaining cartilage structures and functions, improving synovial inflammation, and regulating subchondral bone remodeling. However, we also found that there are some deficiencies in the treatment of OA with Epimedium. For example, the bioavailability of main flavonoids in Epimedium are not high, and the studies on OA treatment mainly focus on flavonoids, especially icariin, and there are few studies on other ingredients, such as Epimedium polysaccharides, and Epimedium lignans. Therefore, improving the bioavailability of flavonoids in Epimedium by chemical modification and other methods, and paying more attentions to other components that have the potential to treat OA, are the key to enhancing the application value of Epimedium in OA treatment. In addition, Exos have increasingly become the hot topic in the field of regenerative medicine, and play an important role in the progress and treatment of OA [154, 155]. Whether Epimedium and its ingredients can treat OA through Exos mechanism is also one of the future research directions.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- OA:
-
Osteoarthritis
- TCM:
-
Traditional Chinese Medicine
- ROS:
-
Reactive oxygen species
- NLRP3:
-
Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing protein 3
- Col2:
-
Type II collagen
- GAG:
-
Glycosaminoglycan
- MMPs:
-
Matrix metalloproteinases
- ADAMTS:
-
ADAM with thrombospondin-1 domains
- OS:
-
Oxidative stress
- MSCs:
-
Mesenchymal stem cells
- TNF-α:
-
Tumor Necrosis Factor-α
- IL-:
-
Interleukin-
- NO:
-
Nitric oxide
- Inos:
-
Inducible nitric oxide synthase
- COX-2:
-
Cyclooxygenase-2
- NF-:
-
Nuclear factor-
- MAPK:
-
Mitogen-activated protein kinase
- HIF-2α:
-
Factor-2alpha
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- SOD:
-
Superoxide dismutase
- HO-1:
-
Heme oxygenase-1
- NQO-1:
-
NAD(P)H quinine oxidoreductase-1
- PI3K:
-
Phosphatidylinositol 3 kinase
- AKT:
-
Protein kinase B
- ERK:
-
Extracellular signal-regulated kinase
- TLR4:
-
Toll-like receptor 4
- MyD88:
-
Myeloid differentiation primary-response gene 88
- ncRNAs:
-
Non-coding RNAs
- lncRNAs:
-
Long non-coding RNAs
- mTOR:
-
Mammalian target of rapamycin
- AMPK:
-
AMP-activated protein kinase
- ER:
-
Endoplasmic reticulum
- BMSCs:
-
Bone marrow-derived mesenchymal stem cells
- BIP:
-
Binding immunoglobulin protein
- ATF4:
-
Activating transcription factor 4
- HIF-1α:
-
Hypoxia-inducible factor-1α
- AMSCs:
-
Adipose-derived MSCs
- SM-MSCs:
-
Synovial membrane-derived MSCs
- UCB-MSCs:
-
Umbilical cord blood-derived MSCs
- IPSC-MSCs:
-
Induced pluripotent stem cells-derived MSCs
- Wnt:
-
Wingless-related integration site
- TGF-:
-
Transforming growth factor-
- ColX:
-
X-type collagen
- IHh:
-
Indian hedgehog
- PHrP:
-
Parathyroid hormone-related protein
- Exos:
-
Exosomes
- IGF-:
-
Insulin-like growth factor-
- MDA:
-
Malondialdehyde
- GSH:
-
Glutathione
- GPX4:
-
GSH peroxidase 4
- ADRs/AEs:
-
Adverse drug reactions/adverse events
References
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30. https://doi.org/10.1136/annrheumdis-2013-204763.
Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66(9):246–53. https://doi.org/10.15585/mmwr.mm6609e1.
Ong KL, Niazi F, Lau E, Mont MA, Concoff A, Shaw P, et al. Knee OA cost comparison for hyaluronic acid and knee arthroplasty. J Orthop Surg Res. 2020;15(1):305. https://doi.org/10.1186/s13018-020-01848-7.
Liu Q, Wang S, Lin J, Zhang Y. The burden for knee osteoarthritis among Chinese elderly: estimates from a nationally representative study. Osteoarthr Cartil. 2018;26(12):1636–42. https://doi.org/10.1016/j.joca.2018.07.019.
Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–78. https://doi.org/10.1001/jama.2020.22171.
Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr Cartil. 2020;28(3):242–8. https://doi.org/10.1016/j.joca.2020.01.002.
Zhu Y, Luo L, Zhang M, Song X, Wang P, Zhang H, et al. Xuanfei Baidu formula attenuates LPS-induced acute lung injury by inhibiting the NF-κB signaling pathway. J Ethnopharmacol. 2023;301: 115833. https://doi.org/10.1016/j.jep.2022.115833.
Chen B, Yu X, Zhang L, Huang W, Lyu H, Xu Y, et al. Clinical efficacy of Jingyin granules, a Chinese patent medicine, in treating patients infected with coronavirus disease 2019. Phytomedicine. 2023;108: 154496. https://doi.org/10.1016/j.phymed.2022.154496.
Pan L, Jing L, Wang GB, Wang XJ, Yan Q, Liu XX. Research progress on knee osteoarthritis in traditional Chinese and western medicine. World Chin Med. 2022;17(16):2373–7.
Gong HQ, Gao M, Chai YH, Miao DQ, Wu YZ, Li Q, et al. Research progress on chemical constituents and pharmacological effects of Epimedium. J Hubei Minzu University (Medical Edition). 2021;38(4):75–8. https://doi.org/10.13501/j.cnki.42-1590/r.2021.04.018.
WFO. Epimedium L. 2023. http://www.worldfloraonline.org/taxon/wfo-4000013613. Accessed 26 Mar 2023.
Ma H, He X, Yang Y, Li M, Hao D, Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharmacol. 2011;134(3):519–41. https://doi.org/10.1016/j.jep.2011.01.001.
Chinese FE, Commission P. Pharmacopeia of the People’s Republic of China, vol. I. Beijing: China Medical Science Press; 2015.
Yuan H, Cao SP, Chen SY, Guo LN, Zheng J, Lin RC. Research progress on chemical constituents and quality control of Epimedii Folium. Chin Tradit Herb Drugs. 2014;45(24):3630–40. https://doi.org/10.7501/j.issn.0253-2670.2014.24.024.
Wang G, Li X, Li N, Wang X, He S, Li W, et al. Icariin alleviates uveitis by targeting peroxiredoxin 3 to modulate retinal microglia M1/M2 phenotypic polarization. Redox Biol. 2022;52: 102297. https://doi.org/10.1016/j.redox.2022.102297.
Zeng Y, Xiong Y, Yang T, Wang Y, Zeng J, Zhou S, et al. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: from effects to molecular mechanisms. Biomed Pharmacother. 2022;147: 112642. https://doi.org/10.1016/j.biopha.2022.112642.
Chen G, Cao Z, Shi Z, Lei H, Chen C, Yuan P, et al. Microbiome analysis combined with targeted metabolomics reveal immunological anti-tumor activity of icariside I in a melanoma mouse model. Biomed Pharmacother. 2021;140: 111542. https://doi.org/10.1016/j.biopha.2021.111542.
Shi Y, Wu Y, Li F, Zhang Y, Hua C, Yang J, et al. Identifying the anti-metastasis effect of anhydroicaritin on breast cancer: coupling network pharmacology with experimental validation. J Ethnopharmacol. 2022;293: 115326. https://doi.org/10.1016/j.jep.2022.115326.
Cho WK, Ma JY. Antiviral activity of Epimedium koreanum Nakai water extract against influenza viruses. Biomed Pharmacother. 2022;146: 112581. https://doi.org/10.1016/j.biopha.2021.112581.
Huang J, Ding J, Wang Z, Li Y, He Y, Wang X, et al. Icariside II attenuates methamphetamine-induced neurotoxicity and behavioral impairments via activating the Keap1-Nrf2 pathway. Oxid Med Cell Longev. 2022;2022:8400876. https://doi.org/10.1155/2022/8400876.
Li LR, Sethi G, Zhang X, Liu CL, Huang Y, Liu Q, et al. The neuroprotective effects of icariin on ageing, various neurological, neuropsychiatric disorders, and brain injury induced by radiation exposure. Aging (Albany NY). 2022;14(3):1562–88. https://doi.org/10.18632/aging.203893.
Zheng Y, Deng Y, Gao JM, Lv C, Lang LH, Shi JS, et al. Icariside II inhibits lipopolysaccharide-induced inflammation and amyloid production in rat astrocytes by regulating IKK/IκB/NF-κB/BACE1 signaling pathway. Acta Pharmacol Sin. 2020;41(2):154–62. https://doi.org/10.1038/s41401-019-0300-2.
Hu L, Wang Z, Li H, Wei J, Tang F, Wang Q, et al. Icariin inhibits isoproterenol-induced cardiomyocyte hypertropic injury through activating autophagy via the AMPK/mTOR signaling pathway. Biochem Biophys Res Commun. 2022;593:65–72. https://doi.org/10.1016/j.bbrc.2022.01.029.
Shen R, Wang JH. The effect of icariin on immunity and its potential application. Am J Clin Exp Immunol. 2018;7(3):50–6.
Jiang J, Song J, Jia XB. Phytochemistry and ethnopharmacology of Epimedium L. species. Chin Herb Med. 2015;7(3):204–22. https://doi.org/10.1016/S1674-6384(15)60043-0.
Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. https://doi.org/10.1017/jns.2016.41.
Ramesh P, Jagadeesan R, Sekaran S, Dhanasekaran A, Vimalraj S. Flavonoids: classification, function, and molecular mechanisms involved in bone remodelling. Front Endocrinol. 2021;12: 779638. https://doi.org/10.3389/fendo.2021.779638.
Chen Y, Wang J, Jia X, Tan X, Hu M. Role of intestinal hydrolase in the absorption of prenylated flavonoids present in Yinyanghuo. Molecules. 2011;16(2):1336–48. https://doi.org/10.3390/molecules16021336.
Chen Y, Zhao YH, Jia XB, Hu M. Intestinal absorption mechanisms of prenylated flavonoids present in the heat-processed Epimedium koreanum Nakai (Yin Yanghuo). Pharm Res. 2008;25(9):2190–9. https://doi.org/10.1007/s11095-008-9602-7.
Sun E, Wei YJ, Zhang ZH, Cui L, Xu FJ, Jia XB. Study on the processing mechanism of stir-fried Epimedium based on the flavonoids absorption and metabolism. Zhongguo Zhong Yao Za Zhi. 2014;39(3):383–90.
Jiang J, Cui L, Sun E, Li J, Cheng XD, Ding SM, et al. Material basis for anti-osteoporosis efficacy of Epimedium flavonoids based on their in vivo metabolism. Chin Tradit Herb Drugs. 2014;45(5):721–9. https://doi.org/10.7501/j.issn.0253-2670.2014.05.024.
Wang M, Gao H, Li W, Wu B. Icariin and its metabolites regulate lipid metabolism: from effects to molecular mechanisms. Biomed Pharmacother. 2020;131: 110675. https://doi.org/10.1016/j.biopha.2020.110675.
Szabó R, Rácz CP, Dulf FV. Bioavailability improvement strategies for icariin and its derivates: a review. Int J Mol Sci. 2022;23(14):7519. https://doi.org/10.3390/ijms23147519.
Sun E, Huang R, Ding K, Wang L, Hou J, Tan X, et al. Integrating strategies of metabolomics, network pharmacology, and experiment validation to investigate the processing mechanism of Epimedium fried with suet oil to warm kidney and enhance yang. Front Pharmacol. 2023;14:1113213. https://doi.org/10.3389/fphar.2023.1113213.
Li MY, Sun E, Xu FJ, Xu JD, Jia XB. Analysis changes of Epimedii Folium’s flavonoids before and after processing based on UPLC-Q/TOF-MS. Chin Tradit Herb Drugs. 2020;51(11):2900–7. https://doi.org/10.7501/j.issn.0253-2670.2020.11.007.
Ti H, Wu P, Xu L, Wei X. Anti-inflammatory neolignans from Epimedium pseudowushanese. Nat Prod Res. 2017;31(22):2621–8. https://doi.org/10.1080/14786419.2017.1289200.
Sze SC, Tong Y, Ng TB, Cheng CL, Cheung HP. Herba Epimedii: anti-oxidative properties and its medical implications. Molecules. 2010;15(11):7861–70. https://doi.org/10.3390/molecules15117861.
Ding Y, Xu XY, Wang YF, Gao YL, Shen MH. Protective of Epimedium alkaloid on reproductive system injury induced by cyclophosphamide in male mice. Sci Tech Food Ind. 2021;42(21):353–60. https://doi.org/10.13386/j.issn1002-0306.2020110125.
He J, Zang S, Liu N, Ji M, Ma D, Ji C. Epimedium polysaccharides attenuates hematotoxicity by reducing oxidative stress and enhancing immune function in mice model of benzene-induced bone marrow failure. Biomed Pharmacother. 2020;125: 109908. https://doi.org/10.1016/j.biopha.2020.109908.
Li C, Chen FF, Jia XB, Feng L, Tan XB. Effects and mechanism of Epimedium polysaccharide on solubility of icariin and baohuoside I. Zhongguo Zhong Yao Za Zhi. 2021;46(22):5825–31. https://doi.org/10.19540/j.cnki.cjcmm.20210317.302.
Zhang H, Wang H, Wei J, Chen X, Sun M, Ouyang H, et al. Comparison of the active compositions between raw and processed Epimedium from different species. Molecules. 2018;23(7):1656. https://doi.org/10.3390/molecules23071656.
Gao M, Fu C, Liu J, Guo B, Yang X, Chen D, et al. Distribution and influencing factors of magnoflorine in Epimedium. Zhongguo Zhong Yao Za Zhi. 2011;36(18):2475–8.
Min L, Wu Y, Cao G, Mi D, Chen C. A network pharmacology strategy to investigate the anti-osteoarthritis mechanism of main lignans components of Schisandrae Fructus. Int Immunopharmacol. 2021;98: 107873. https://doi.org/10.1016/j.intimp.2021.107873.
Ban Y, Wang Y, Qiao L, Zhang C, Wang H, He X, et al. Total lignans from Vitex negundo seeds attenuate osteoarthritis and their main component vitedoin A alleviates osteoclast differentiation by suppressing ERK/NFATc1 signaling. Phytother Res. 2023;37(4):1422–34. https://doi.org/10.1002/ptr.7750.
Fu C, Qiu Z, Huang Y, Lin Q, Jin L, Tu H, et al. Achyranthes bidentata polysaccharides alleviate endoplasmic reticulum stress in osteoarthritis via lncRNA NEAT1/miR-377-3p pathway. Biomed Pharmacother. 2022;154: 113551. https://doi.org/10.1016/j.biopha.2022.113551.
Sun Y, Huang K, Mo L, Ahmad A, Wang D, Rong Z, et al. Eucommia ulmoides polysaccharides attenuate rabbit osteoarthritis by regulating the function of macrophages. Front Pharmacol. 2021;12: 730557. https://doi.org/10.3389/fphar.2021.730557.
Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–75. https://doi.org/10.1038/s41584-022-00749-9.
Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386(9991):376–87. https://doi.org/10.1016/s0140-6736(14)60802-3.
Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3):224. https://doi.org/10.1186/ar2592.
Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30–5. https://doi.org/10.1186/ar380.
Yu SY, Liu JH, Zhang XY, Xu ZW, Xie GX, Wu HB, et al. Molecular mechanism of icariin effects on articular chondrocytes, subchondral bone and synovium in the treatment of osteoarthritis. J Clin Rehabil Tis Eng Res. 2020;24(14):2243–9. https://doi.org/10.3969/j.issn.2095-4344.2474.
Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–8. https://doi.org/10.1177/1941738109350438.
Ziadlou R, Barbero A, Stoddart MJ, Wirth M, Li Z, Martin I, et al. Regulation of inflammatory response in human osteoarthritic chondrocytes by novel herbal small molecules. Int J Mol Sci. 2019;20(22):5745. https://doi.org/10.3390/ijms20225745.
Xia B, Di C, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95(6):495–505. https://doi.org/10.1007/s00223-014-9917-9.
Ziadlou R, Barbero A, Martin I, Wang X, Qin L, Alini M, et al. Anti-inflammatory and chondroprotective effects of vanillic acid and epimedin C in human osteoarthritic chondrocytes. Biomolecules. 2020;10(6):932. https://doi.org/10.3390/biom10060932.
Zu Y, Mu Y, Li Q, Zhang ST, Yan HJ. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J Orthop Surg Res. 2019;14(1):307. https://doi.org/10.1186/s13018-019-1307-6.
Wang P, Meng Q, Wang W, Zhang S, Xiong X, Qin S, et al. Icariin inhibits the inflammation through down-regulating NF-κB/HIF-2α signal pathways in chondrocytes. 2020. Biosci Rep. https://doi.org/10.1042/bsr20203107.
Sun K, Luo J, Jing X, Xiang W, Guo J, Yao X, et al. Hyperoside ameliorates the progression of osteoarthritis: an in vitro and in vivo study. Phytomedicine. 2021;80: 153387. https://doi.org/10.1016/j.phymed.2020.153387.
Zuo S, Zou W, Wu RM, Yang J, Fan JN, Zhao XK, et al. Icariin alleviates IL-1β-induced matrix degradation by activating the Nrf2/ARE pathway in human chondrocytes. Drug Des Dev Ther. 2019;13:3949–61. https://doi.org/10.2147/dddt.S203094.
Zeng L, Rong XF, Li RH, Wu XY. Icariin inhibits MMP-1, MMP-3 and MMP-13 expression through MAPK pathways in IL-1β-stimulated SW1353 chondrosarcoma cells. Mol Med Rep. 2017;15(5):2853–8. https://doi.org/10.3892/mmr.2017.6312.
Li X, Xu Y, Li H, Jia L, Wang J, Liang S, et al. Verification of pain-related neuromodulation mechanisms of icariin in knee osteoarthritis. Biomed Pharmacother. 2021;144: 112259. https://doi.org/10.1016/j.biopha.2021.112259.
Mi B, Wang J, Liu Y, Liu J, Hu L, Panayi AC, et al. Icariin activates autophagy via down-regulation of the NF-κB signaling-mediated apoptosis in chondrocytes. Front Pharmacol. 2018;9:605. https://doi.org/10.3389/fphar.2018.00605.
Wang G, Zhang L, Shen H, Hao Q, Fu S, Liu X. Up-regulation of long non-coding RNA CYTOR induced by icariin promotes the viability and inhibits the apoptosis of chondrocytes. BMC Complement Med Ther. 2021;21(1):152. https://doi.org/10.1186/s12906-021-03322-1.
Zhou AZ, Wang L, Cheng B. Ethanol extraction technology of Epimedii Folium and protective effect of ethanol extract on chondrocyte. Zhongguo Zhong Yao Za Zhi. 2020;45(5):1097–104. https://doi.org/10.19540/j.cnki.cjcmm.20191221.309.
Tang Y, Li Y, Xin D, Chen L, Xiong Z, Yu X. Icariin alleviates osteoarthritis by regulating autophagy of chondrocytes by mediating PI3K/AKT/mTOR signaling. Bioengineered. 2021;12(1):2984–99. https://doi.org/10.1080/21655979.2021.1943602.
Chen Y, Pan X, Zhao J, Li C, Lin Y, Wang Y, et al. Icariin alleviates osteoarthritis through PI3K/Akt/mTOR/ULK1 signaling pathway. Eur J Med Res. 2022;27(1):204. https://doi.org/10.1186/s40001-022-00820-x.
Liu D, Tang W, Zhang H, Huang H, Zhang Z, Tang D, et al. Icariin protects rabbit BMSCs against OGD-induced apoptosis by inhibiting ERs-mediated autophagy via MAPK signaling pathway. Life Sci. 2020;253: 117730. https://doi.org/10.1016/j.lfs.2020.117730.
Wang P, Xiong X, Zhang J, Qin S, Wang W, Liu Z. Icariin increases chondrocyte vitality by promoting hypoxia-inducible factor-1α expression and anaerobic glycolysis. Knee. 2020;27(1):18–25. https://doi.org/10.1016/j.knee.2019.09.012.
Zhu Y, Ye L, Cai X, Li Z, Fan Y, Yang F. Icariin-loaded hydrogel regulates bone marrow mesenchymal stem cell chondrogenic differentiation and promotes cartilage repair in osteoarthritis. Front Bioeng Biotechnol. 2022;10: 755260. https://doi.org/10.3389/fbioe.2022.755260.
Tang W, Zhang H, Liu D, Jiao F. Icariin accelerates cartilage defect repair by promoting chondrogenic differentiation of BMSCs under conditions of oxygen-glucose deprivation. J Cell Mol Med. 2022;26(1):202–15. https://doi.org/10.1111/jcmm.17073.
Wang JY, Yin CC, Wu CC, Geng SG, Yin M. Icaritin promotes chondrogenic differentiation of BMSCs by Wnt/β-catenin signaling pathway. Zhongguo Zhong Yao Za Zhi. 2016;41(4):694–9. https://doi.org/10.4268/cjcmm20160425.
Wang ZC, Sun HJ, Li KH, Fu C, Liu MZ. Icariin promotes directed chondrogenic differentiation of bone marrow mesenchymal stem cells but not hypertrophy in vitro. Exp Ther Med. 2014;8(5):1528–34. https://doi.org/10.3892/etm.2014.1950.
Wang Z, Li K, Sun H, Wang J, Fu Z, Liu M. Icariin promotes stable chondrogenic differentiation of bone marrow mesenchymal stem cells in self-assembling peptide nanofiber hydrogel scaffolds. Mol Med Rep. 2018;17(6):8237–43. https://doi.org/10.3892/mmr.2018.8913.
Luo Y, Zhang Y, Huang Y. Icariin reduces cartilage degeneration in a mouse model of osteoarthritis and is associated with the changes in expression of Indian Hedgehog and parathyroid hormone-related protein. Med Sci Monit. 2018;24:6695–706. https://doi.org/10.12659/msm.910983.
Bahrami M, Valiani A, Amirpour N, Ra Rani MZ, Hashemibeni B. Cartilage tissue engineering via icariin and adipose-derived stem cells in fibrin scaffold. Adv Biomed Res. 2018;7:36. https://doi.org/10.4103/2277-9175.225925.
Yu YL, Wu JS, Emeti R, Zhou Q, Liu HH, Guan XF. Effects of Icariin on repair of knee osteoarthritis by adipose derived mesenchymal stem cells. Chin J Immunol. 2021;37(3):301–6.
Kim HA, Yeo Y, Jung HA, Jung YO, Park SJ, Kim SJ. Phase 2 enzyme inducer sulphoraphane blocks prostaglandin and nitric oxide synthesis in human articular chondrocytes and inhibits cartilage matrix degradation. Rheumatology (Oxford). 2012;51(6):1006–16. https://doi.org/10.1093/rheumatology/ker525.
Sun W, Xie W, Huang D, Cui Y, Yue J, He Q, et al. Caffeic acid phenethyl ester attenuates osteoarthritis progression by activating NRF2/HO-1 and inhibiting the NF-κB signaling pathway. Int J Mol Med. 2022;50(5):1–14. https://doi.org/10.3892/ijmm.2022.5190.
Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129: 110452. https://doi.org/10.1016/j.biopha.2020.110452.
Jiang H, Ji P, Shang X, Zhou Y. Connection between osteoarthritis and nitric oxide: from pathophysiology to therapeutic target. Molecules. 2023;28(4):1683. https://doi.org/10.3390/molecules28041683.
Liu W, Xu D, Qi Q, Li J, Ou L. Chinese herbal medicine Xianling Gubao capsule for knee osteoarthritis: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2022;101(3): e28634. https://doi.org/10.1097/md.0000000000028634.
Zhou X, Zheng Y, Sun W, Zhang Z, Liu J, Yang W, et al. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2α-dependent manner. Cell Prolif. 2021;54(11): e13134. https://doi.org/10.1111/cpr.13134.
Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16(6):687–93. https://doi.org/10.1038/nm.2153.
Wood MJ, Miller RE, Malfait AM. The genesis of pain in osteoarthritis: inflammation as a mediator of osteoarthritis pain. Clin Geriatr Med. 2022;38(2):221–38. https://doi.org/10.1016/j.cger.2021.11.013.
Berenbaum F, Meng QJ. The brain-joint axis in osteoarthritis: nerves, circadian clocks and beyond. Nat Rev Rheumatol. 2016;12(9):508–16. https://doi.org/10.1038/nrrheum.2016.93.
Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–54. https://doi.org/10.3390/ijms161125943.
Gu J, Rao W, Huo S, Fan T, Qiu M, Zhu H, et al. MicroRNAs and long non-coding RNAs in cartilage homeostasis and osteoarthritis. Front Cell Dev Biol. 2022;10:1092776. https://doi.org/10.3389/fcell.2022.1092776.
Razmara E, Bitaraf A, Yousefi H, Nguyen TH, Garshasbi M, Cho WC, et al. Non-coding RNAs in cartilage development: an updated review. Int J Mol Sci. 2019;20(18):4475. https://doi.org/10.3390/ijms20184475.
Wang S, Wang H, Feng C, Li C, Li Z, He J, et al. The regulatory role and therapeutic application of pyroptosis in musculoskeletal diseases. Cell Death Discov. 2022;8(1):492. https://doi.org/10.1038/s41420-022-01282-0.
Li Y, Wu Y, Jiang K, Han W, Zhang J, Xie L, et al. Mangiferin prevents TBHP-induced apoptosis and ECM degradation in mouse osteoarthritic chondrocytes via restoring autophagy and ameliorates murine osteoarthritis. Oxid Med Cell Longev. 2019;2019:8783197. https://doi.org/10.1155/2019/8783197.
Wang P, Zhang F, He Q, Wang J, Shiu HT, Shu Y, et al. Flavonoid compound icariin activates hypoxia inducible factor-1α in chondrocytes and promotes articular cartilage repair. PLoS ONE. 2016;11(2): e0148372. https://doi.org/10.1371/journal.pone.0148372.
Duan R, Xie H, Liu ZZ. The role of autophagy in osteoarthritis. Front Cell Dev Biol. 2020;8: 608388. https://doi.org/10.3389/fcell.2020.608388.
Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthr Cartil. 2020;28(4):400–9. https://doi.org/10.1016/j.joca.2020.02.027.
Qi Z, Chen L. Endoplasmic reticulum stress and autophagy. Adv Exp Med Biol. 2019;1206:167–77. https://doi.org/10.1007/978-981-15-0602-4_8.
Fernández A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res. 2015;59(3):292–307. https://doi.org/10.1111/jpi.12264.
Kwon J, Kim J, Kim KI. Crosstalk between endoplasmic reticulum stress response and autophagy in human diseases. Anim Cells Syst (Seoul). 2023;27(1):29–37. https://doi.org/10.1080/19768354.2023.2181217.
Hwang JJ, Rim YA, Nam Y, Ju JH. Recent developments in clinical applications of mesenchymal stem cells in the treatment of rheumatoid arthritis and osteoarthritis. Front Immunol. 2021;12: 631291. https://doi.org/10.3389/fimmu.2021.631291.
Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6(1):10. https://doi.org/10.1186/scrt546.
Ding N, Li E, Ouyang X, Guo J, Wei B. The therapeutic potential of bone marrow mesenchymal stem cells for articular cartilage regeneration in osteoarthritis. Curr Stem Cell Res Ther. 2021;16(7):840–7. https://doi.org/10.2174/1574888x16666210127130044.
Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, et al. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol. 2019;165:49–65. https://doi.org/10.1016/j.bcp.2019.02.036.
Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1):64. https://doi.org/10.1186/s13287-017-0510-9.
Zare R, Tanideh N, Nikahval B, Mirtalebi MS, Ahmadi N, Zarea S, et al. Are stem cells derived from synovium and fat pad able to treat induced knee osteoarthritis in rats? Int J Rheumatol. 2020;2020:9610261. https://doi.org/10.1155/2020/9610261.
Lee DH, Kim SA, Song JS, Shetty AA, Kim BH, Kim SJ. Cartilage regeneration using human umbilical cord blood derived mesenchymal stem cells: a systematic review and meta-analysis. Medicina (Kaunas). 2022. https://doi.org/10.3390/medicina58121801.
Diederichs S, Tuan RS. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev. 2014;23(14):1594–610. https://doi.org/10.1089/scd.2013.0477.
Watanabe Y, Tsuchiya A, Terai S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin Mol Hepatol. 2021;27(1):70–80. https://doi.org/10.3350/cmh.2020.0194.
Wu C, He Y, Yao Y, Yang H, Lu F. Exosomes treating osteoarthritis: hope with challenge. Heliyon. 2023;9(1): e13152. https://doi.org/10.1016/j.heliyon.2023.e13152.
Li F, Tang Y, Song B, Yu M, Li Q, Zhang C, et al. Nomenclature clarification: synovial fibroblasts and synovial mesenchymal stem cells. Stem Cell Res Ther. 2019;10(1):260. https://doi.org/10.1186/s13287-019-1359-x.
Li BJ, Zheng J, Yang F, Yuan PW, Dong B. Research progress on the pathological mechanism of osteoarthritis synovium. Chin J Bone Joint. 2022. https://doi.org/10.3969/j.issn.2095-252X.2022.10.010.
Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804–9. https://doi.org/10.1136/ard.2011.150243.
Griffin TM, Scanzello CR. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin Exp Rheumatol. 2019;37(Suppl 120):57–63.
Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep. 2013;15(5):323. https://doi.org/10.1007/s11926-013-0323-5.
Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. https://doi.org/10.1186/s13075-017-1229-9.
Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4(4):269–85. https://doi.org/10.1177/1759720x12448454.
Pan L, Zhang Y, Chen N, Yang L. Icariin regulates cellular functions and gene expression of osteoarthritis patient-derived human fibroblast-like synoviocytes. Int J Mol Sci. 2017;18(12):2656. https://doi.org/10.3390/ijms18122656.
Jin JF, He WY, Zhou YL. Effect of total flavonoids of Epimedium on the development of knee osteoarthritis in rats by inhibiting NF-kappaB signaling pathway. Chin J Tradit Med Traumatol Orthop. 2020;28(3):5–14.
Hu Y, Gui Z, Zhou Y, Xia L, Lin K, Xu Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic Biol Med. 2019;145:146–60. https://doi.org/10.1016/j.freeradbiomed.2019.09.024.
Luo H, Zhang R. Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc−/GPX4 axis. Exp Ther Med. 2021;21(1):72. https://doi.org/10.3892/etm.2020.9504.
Xiong A, Xiong RP, Peng Y, Li Y, Jiang X, Xu JZ. Establishment and characteristic analysis of a model of knee fibroblast-like synoviocytes inlipopolysaccharide-induced Sprague-Dawley rats. Acta Lab Anim Sci Sin. 2020;28(4):436–46. https://doi.org/10.3969/j.issn.1005-4847.2020.04.002.
Gao L, Zhou XC, Zhang CY, Ni GX. Role of macrophage polarization in osteoarthritis. Chem Life. 2022;42(2):208–16. https://doi.org/10.13488/j.smhx.20210816.
Zhang S, Xu J, Si H, Wu Y, Zhou S, Shen B. The role played by ferroptosis in osteoarthritis: evidence based on iron dyshomeostasis and lipid peroxidation. Antioxidants (Basel). 2022;11(9):1668. https://doi.org/10.3390/antiox11091668.
Gao L, Hua W, Tian L, Zhou X, Wang D, Yang Y, et al. Molecular mechanism of ferroptosis in orthopedic diseases. Cells. 2022;11(19):2979. https://doi.org/10.3390/cells11192979.
Miao Y, Chen Y, Xue F, Liu K, Zhu B, Gao J, et al. Contribution of ferroptosis and GPX4’s dual functions to osteoarthritis progression. EBioMedicine. 2022;76: 103847. https://doi.org/10.1016/j.ebiom.2022.103847.
Li JL. Research progress of subchondral bone in osteoarthritis. Chin J Bone Joint Injury. 2020;35(6):670–2. https://doi.org/10.7531/j.issn.1672-9935.2020.06.045.
Mahjoub M, Berenbaum F, Houard X. Why subchondral bone in osteoarthritis? The importance of the cartilage bone interface in osteoarthritis. Osteoporos Int. 2012;23(Suppl 8):S841–6. https://doi.org/10.1007/s00198-012-2161-0.
Hu YZ, Ruan ZH, Han BQ, Xu T, Zhao TF, Zhang YP, et al. Subchondral bone changes in knee osteoarthritis. Rheum Arthritis. 2022;11(8):42–6. https://doi.org/10.3969/j.issn.2095-4174.2022.08.011.
Guévremont M, Martel-Pelletier J, Massicotte F, Tardif G, Pelletier JP, Ranger P, et al. Human adult chondrocytes express hepatocyte growth factor (HGF) isoforms but not HgF: potential implication of osteoblasts on the presence of HGF in cartilage. J Bone Miner Res. 2003;18(6):1073–81. https://doi.org/10.1359/jbmr.2003.18.6.1073.
Yu D, Xu J, Liu F, Wang X, Mao Y, Zhu Z. Subchondral bone changes and the impacts on joint pain and articular cartilage degeneration in osteoarthritis. Clin Exp Rheumatol. 2016;34(5):929–34.
Chen WJ, L YJ, Zheng XF, Wang HJ. Pathological changes of subchondral bone and their roles in pathogenesis of osteoarthritis. Chin Orthop J Clin Basic Res. 2020;12(4):234–41. https://doi.org/10.3969/j.issn.1674-666X.2020.04.007.
Yu DG, Tang TT, Zhu ZA. Research progress of subchondral bone changes and their effects in osteoarthritis. Int J Orthop. 2015;36(3):172–8.
Goldring SR. The role of bone in osteoarthritis pathogenesis. Rheum Dis Clin N Am. 2008;34(3):561–71. https://doi.org/10.1016/j.rdc.2008.07.001.
Batiste DL, Kirkley A, Laverty S, Thain LM, Spouge AR, Holdsworth DW. Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthr Cartil. 2004;12(12):986–96. https://doi.org/10.1016/j.joca.2004.08.010.
Luo G, Xu B, Wang W, Wu Y, Li M. Study of the osteogenesis effect of icariside II and icaritin on canine bone marrow mesenchymal stem cells. J Bone Miner Metab. 2018;36(6):668–78. https://doi.org/10.1007/s00774-017-0889-5.
Gao L, Zhang SQ. Antiosteoporosis effects, pharmacokinetics, and drug delivery systems of icaritin: advances and prospects. Pharmaceuticals (Basel). 2022;15(4):397. https://doi.org/10.3390/ph15040397. (Epub 2022/04/24).
Liu Y, Chai LJ, Huang JY, Wang Q, Song L, Zhou K, et al. Effects of epimedin A on osteoclasts and osteoporotic male mice. Chin J Vet Sci. 2021;41(7):1359–64.
Gao K, Wang S, Wang Q. Effect of icariin on serum bone turnover markers expressions and histology changes in mouse osteoarthritis model. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(8):963–9. https://doi.org/10.7507/1002-1892.201703044.
Greene B, Russo RJ, Dwyer S, Malley K, Roberts E, Serrielo J, et al. Inhibition of TGF-β increases bone volume and strength in a mouse model of osteogenesis imperfecta. JBMR Plus. 2021;5(9): e10530. https://doi.org/10.1002/jbm4.10530.
Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J Clin Invest. 2014;124(2):466–72. https://doi.org/10.1172/jci70050.
Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–12. https://doi.org/10.1038/nm.3143.
Zhang LZ, Wei Z, Zhang SM. Roles of TGF-beta signaling in the development of osteoarthritis. Chin J Bone Joint Surg. 2019;12(9):727–32. https://doi.org/10.3969/j.issn.2095-9958.2019.09.17.
Xu YT, Jia LL, Xu LM, He XJ, Li H, Wang SJ, et al. Study on the mechanism of kidney-tonifying Chinese herbs regulating subchondral bone metabolism disorder in knee osteoarthritis. In: 2019 Chutian orthopedics summit forum and the 26th China integrated traditional and western orthopedics and traumatology annual conference. Hubei, Wuhan; 10 Sep 2019.
Tong X, Qiao Y, Yang Y, Liu H, Cao Z, Yang B, et al. Applications and mechanisms of Tripterygium wilfordii Hook. F. and its preparations in kidney diseases. Front Pharmacol. 2022;13: 846746. https://doi.org/10.3389/fphar.2022.846746.
Song Z, Li Z, Wen X, Liu R, Tian X. UPLC-MS/MS method for simultaneously determining nucleosides and methyl-nucleosides in liver mRNA of epimedin C-induced liver injury mouse model. Chin Med. 2021;16(1):91. https://doi.org/10.1186/s13020-021-00501-7.
Song L, Zhou Y, Zhai Y, Huo X, Chen M, Shi H, et al. Sub-chronic toxicity of an aqueous extract of Epimedium sagittatum (Sieb. Et Zucc.) Maxim. in rats. Drug Chem Toxicol. 2023;46(3):451–61. https://doi.org/10.1080/01480545.2022.2050749.
Zhang L, Wang T, Zhao BS, Zhang JX, Yang S, Fan CL, et al. Effect of 2″-O-rhamnosyl icariside II, baohuoside I and baohuoside II in herba epimedii on cytotoxicity indices in HL-7702 and HepG2 cells. Molecules. 2019;24(7):1263. https://doi.org/10.3390/molecules24071263.
Cao YJ. Preliminary study on the molecular mechanism of Epimedium induced liver injury based on metabolomics and network toxicology. Beijing: Beijing University of Chinese Medicine; 2021.
Ling J, Wang M, Chen Y, Song J, Sun E, Shi ZQ, et al. Analysis of folium Epimedium toxicity in combination with Radix Morindae Officinalis based on zebrafish toxicity/metabolism synchronization. Acta Pharm Sin. 2018;53(1):74–83. https://doi.org/10.16438/j.0513-4870.2017-0756.
Yang XH, Zhang HF, Niu LL, Wang Y, Lai JH. Contents of heavy metals in Chinese edible herbs: evidence from a case study of Epimedii Folium. Biol Trace Elem Res. 2018;182(1):159–68. https://doi.org/10.1007/s12011-017-1075-2.
Wang D, Jia DX, Li ZZ, Niu YM, Xie WC, Wang XK, et al. Discussion on safety evaluation and risk control measures of Epimedii Folium. Zhongguo Zhong Yao Za Zhi. 2019;44(8):1715–23.
Peng SY, Xie YM, Wang ZF, Zhang YL, Yi DH. Analysis of adverse reactions of Xianling Gubao preparation based on real world SRS data. Zhongguo Zhong Yao Za Zhi. 2020;45(10):2316–21.
Tong YM, Zhang Y, Shi J, Hu TJ, Wei YY, Yang HZ. Safety evaluation of Epimedium extracts. Feed Res. 2021;8:61–7. https://doi.org/10.13557/j.cnki.issn1002-2813.2021.08.015.
Niu HM. Experimental study on acute toxicity of icariin. Acta Neuropharm. 2018;8(2):40.
Hwang YH, Yang HJ, Yim NH, Ma JY. Genetic toxicity of Epimedium koreanum Nakai. J Ethnopharmacol. 2017;198:87–90. https://doi.org/10.1016/j.jep.2016.11.050.
Gao Y, Wang JB, Xiao XH, Bai ZF. Exploring the safe and rational use of Epimedii Folium based on the evolution of efficacy/toxicity records in ancient and modern literature. Acta Pharm Sin. 2023;58(2):246–57. https://doi.org/10.16438/j.0513-4870.2022-0801.
Dai Y, Chen Y, Hu Y, Zhang L. Current knowledge and future perspectives on exosomes in the field of regenerative medicine: a bibliometric analysis. Regen Med. 2023;18(2):123–36. https://doi.org/10.2217/rme-2022-0141.
Chen A, Chen Y, Rong X, You X, Wu D, Zhou X, et al. The application of exosomes in the early diagnosis and treatment of osteoarthritis. Front Pharmacol. 2023;14:1154135. https://doi.org/10.3389/fphar.2023.1154135.
Acknowledgements
We are grateful for the support from Orthopaedics Institute of Tianjin, Tianjin Hospital.
Funding
This research was funded by the National Key R&D Program of China (Grant number: 2022YFC3601904), and the Tianjin Natural Science Foundation Key Program (Grant number: 22JCZDJC00340).
Author information
Authors and Affiliations
Contributions
XT and YW wrote the manuscript. B-CD supplied materials and assembled the tables. YL and SL prepared the figures. J-XM and X-LM conceived and revised the article. All authors have read and approved the final submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tong, X., Wang, Y., Dong, B. et al. Effects of genus Epimedium in the treatment of osteoarthritis and relevant signaling pathways. Chin Med 18, 92 (2023). https://doi.org/10.1186/s13020-023-00788-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-023-00788-8