Abstract
Background
Congenital long QT syndrome (LQTS) can cause ventricular arrhythmic events with syncope and sudden death resulting from malignant torsades de pointes (TdP) followed by ventricular fibrillations (VFs). However, the syndrome is often overlooked prior to the development of arrhythmic events in patients with congenital heart diseases demonstrating right bundle branch block on electrocardiogram (ECG). We present a case of an adult patient with congenital heart disease who developed VFs postoperatively, potentially due to his mutation in a LQTS related gene, which was not identified on preoperative assessment due to incomplete evaluation of his family history.
Case presentation
A 64-year-old man was diagnosed as having multiple atrial septal defects. He presented with no symptoms of heart failure. His preoperative ECG showed complete right bundle branch block (CRBBB) with a corrected QT interval time of 478 ms. He underwent open-heart surgery to close the defects through median sternotomy access. Three hours after the operation, he developed multiple events of TdP and VFs in the intensive care unit. Cardiopulmonary resuscitation and multiple cardioversions were attempted for his repetitive TdP and VFs. He eventually reverted to sinus rhythm, and intravenous beta-blocker was administered to maintain the sinus rhythm. After this event, his family history was reviewed, and it was confirmed that his daughter and grandson had a medical history of arrhythmia. A genetic test confirmed that he had a missense mutation in CACNA1C, p.K1580 T, which is the cause for type 8.
Conclusions
This case highlights the importance of paying attention to other ECG findings in patients with CRBBB, which can mask prolonged QT intervals.
Similar content being viewed by others
Background
Congenital long QT syndrome (LQTS) causes arrhythmic events such as syncope and sudden death due to malignant torsades de pointes (TdP) followed by ventricular fibrillations (VFs). However, in the absence of arrhythmic events, the syndrome can be overlooked, especially in patients with congenital heart diseases, that demonstrate right bundle branch block (RBBB) on electrocardiograms (ECGs) [1, 2]. In addition, QT intervals in ECGs are known to increase for 2 days after open heart surgeries using cardiopulmonary bypasses as a result of electrolyte imbalance and QT prolonging medications. The prolongation of QT interval can trigger arrhythmic events after operations in patients with genetic disorders, such as long QT-related gene mutations or polymorphisms [1, 3]. If the diagnosis of the syndrome is made before open heart surgery, clinicians can avoid factors contributing to the prolongation of QT intervals and, as a result, may be able to prevent ventricular arrhythmias postoperatively [4]. We present an adult patient with congenital heart disease who developed VFs postoperatively, possibly due to his mutation in a LQTS-related gene, which were not identified preoperatively due to incomplete assessment of his family history.
Case presentation
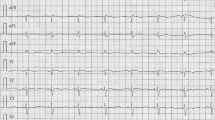
A 64-year-old man with a diagnosis of atrial septal defect (ASD) presented ECG features of incomplete or complete right bundle branch block (CRBBB) during a regular medical check-up. He was asymptomatic, and he had no other significant past medical history. He was referred to our hospital to assess the possibility of catheter device occlusion for ASD, and surgical correction was indicated due to multiple defects and the size of the defects. A chest radiograph showed enlargement of the pulmonary vasculature, indicating pulmonary high flow. An ECG showed CRBBB, and the corrected QT interval (Bazett formula) was 478 ms (Fig. 1). Echocardiography showed preserved left ventricular function, and enlarged right atrium and right ventricle with trivial tricuspid regurgitation and multiple secundum ASDs. The patient was not taking any medications known to prolong the QT interval, and his laboratory data indicated no significant electrolyte disorders. We were informed that he had a child receiving drug treatment for arrhythmia; however, we did not think that this was clinically relevant before the surgery as further information was not provided.
During the operation, anesthetic drugs were used as follows: sevoflurane, remifentanil, dexmedetomidine, propofol, fentanil and rocuronium were administered. Cefazolin was given for the prevention of surgical site infection. After the median sternotomy, an aortic cannula was placed in the ascending aorta, and venous cannulas were inserted into the superior vena cava and the inferior vena cava to establish the cardiopulmonary bypass (CPB). Once the aorta was cross-clamped, cold blood cardioplegic solution was infused through the cannula in the ascending aorta. The body temperature was cooled down to 33.8∘ Celsius. Under conditions of cardiac arrest, the right atrium was cut horizontally and opened. Then, three large ASDs were identified at the secundum with thin rims around the holes. The ASDs were closed directly with continuous 4–0 Prolene. After the de-airing, the aorta was de-clamped. The cross-clamp time was 38 min. The patient developed VF. The direct current (DC) cardioversion was successful to convert to sinus rhythm with one attempt of 10 J. The CPB was weaned uneventfully, and its running time was 76 min. The chest was closed using standard techniques, and the patient was sent to the intensive care unit (ICU) with tracheal intubation. In the ICU, dopamine at 4 mcg/kg/min and noradrenaline at 0.05 mcg/kg/min were administered for cardiac support. Propofol and dexmedetomidine were given at the adequate level for sedation. Bipolar temporary pacing wire was placed on the right atrium, and the setting of pacemaker was AAI pacing mode with HR 88 bpm, the sensitivity threshold of 3 mV and the output of 10 mA. The serum level of potassium was 3.8 mEq/l (normal serum potassium value: 3.8–4.8 mmol/l), and that of magnesium was not measured 2 h after the surgery as we do not routinely measure it after low risk operations and the patient was stable. Three hours after the operation, he suddenly presented R on T followed by TdP and Vfs (Fig. 2a). Chest compressions were initiated, and DC cardioversion was attempted. Sinus rhythm was achieved following cardioversion; however, the patient presented repetitive VF (14 times) (Fig. 2b).
Refractory ventricular fibrillations after the atrial septal defect repair. a Three hours after the surgical correction of multiple atrial septal defects, the patient suddenly presented R on T followed by Torsades de Pointes in the intensive care unit, and eventually, the electrocardiogram shifted to a ventricular fibrillation pattern. b The patient presented with repetitive ventricular fibrillations even after he reverted to sinus rhythm with direct current cardioversions
Intravenous amiodarone and lidocaine were not effective in managing the arrhythmic storm. After the administration of magnesium and nifecalant, the patient finally recovered to sinus rhythm. Echocardiography revealed normal left ventricular wall motion, which indicated no heart ischemia. After the event, he underwent 24 h of hypothermic treatment to prevent brain damage, and, fortunately, there remained no evidence of brain damage on computed tomography. Landiolol had been administered intravenously to maintain sinus rhythm until the patient was able to take bisoprolol tablets orally. Given that his VFs were refractory and the patient had no coronary artery disease and relevant valve disease, we supposed that he might have been a carrier of familial arrhythmic disorders causing repetitive VFs. Therefore, we reviewed his family history and found that his daughter and grandson had congenital LQTS diagnosed at other hospitals, and his grandson was being treated with a beta blocker for this disease.
After obtaining informed consent from the patient, genetic screening for LQTS related genes was conducted to confirm the diagnosis. In the genetic analysis, a CACNA1c missense mutation, c. 4739a > c, p.K1580 T, was identified. CACNA1C is a causative gene for LQTS type 8 (Fig. 3(a)-(c)) [5,6,7]. The mutation was novel and not reported in the cohort databases; ExAC (http://exac.broadinstitute.org/), gnomAD (http://gnomad.broadinstitute.org/) and HGVD (http://www.hgvd.genome.med.kyoto-u.ac.jp/index.html?). We could not perform genetic analysis for the family members who were diagnosed with LQTS.
He was discharged 2 weeks after the operation with the prescription of bisoprolol.
Discussion
Congenital LQTS is a disease resulting from impaired expression or dysfunctions of cardiac ion channels as a result of their encoding gene variants. It can cause the prolongation of QT intervals and, as a consequence, result in life threatening events such as VFs. Itoh et al. reported that 28% of acquired LQTS patients were actually “congenital” [8]. Moreover, only 60% of patients show symptoms prior to the diagnosis of congenital LQTS [9]. Given that our patient was asymptomatic until the operation, it is presumable that stimulating factors related to perioperative and postoperative procedures or the use of medicines strongly contributed to the event of postoperative TdP followed by VFs. The factors associated with fatal arrhythmias for acquired LQTS are medicines including anesthetic agents, inotropes, hypokalemia, bradycardia, emotional stress, hypothermia and the combinations of these factors [8, 10]. If we had known that the patient had a genetic background of CACNA1C mutations, some of which were reported as cardiac-only Timothy syndrome, we could have avoided potential factors that triggered malignant TdP and VFs [5,6,7, 10]. In order to prevent malignant arrhythmias postoperatively, some authors have recommended the use of appropriate premedications, avoidance of hypothermia, proper anesthetic agents, adequate pain relief, continuous use of beta blockers during and after operations, avoidance of the excess use of diuretics, and maintenance of proper level of electrolytes such as calcium and magnesium [3, 4, 10, 11]. In our case, sevoflurane, propofol, dopamine and dexmedetomidine were used for anesthesia and postoperative care, and these were categorized as possible cause of arrhythmic events or should be avoided in CredibleMeds (https://www.crediblemeds.org/). If the diagnosis was made preoperatively, these possible unsafe drugs could not have been used and, as a result, the patient could not have experienced the refractory VFs in the ICU.
The congenital LQTS is often overlooked and difficult to recognize before the occurrence of arrhythmic events, especially in patients with congenital heart diseases, as in our case. However, preoperative diagnosis of this syndrome is essential as it may help physicians to avoid arrhythmic factors [1, 2, 12]. Diagnostic difficulty is due to the fact that patients with LQTS and ASDs occasionally show a RBBB preoperatively or after surgical correction of tetralogy of Fallot, and this ECG abnormality can mask the prolonged QT time [1]. It has also been reported that QT interval is prolonged after surgical correction of ASD in 42% of patients due to increased coronary flow after the correction [13]. Thus, physicians should carefully evaluate patients’ family medical history, especially when they present RBBB and a strong family history of arrhythmias. It is important to note that Schwartz and Moss score can help medical practitioners to diagnose the LQTS, even in the absence of genetic screening for LQTS [14, 15].
Conclusions
This case highlights the importance of taking a thorough family history in patients due to undergo open heart surgeries. Furthermore, we should pay attention to other ECG findings in patients with CRBBB, which can mask prolonged QT intervals. If preoperative diagnosis of the LQTS is made, we may be able to avoid factors triggering ventricular arrhythmias and prevent arrhythmic events after open heart surgeries.
Abbreviations
- ASD:
-
Atrial septal defect
- CPB:
-
Cardiopulmonary bypass
- CRBBB:
-
Complete right bundle branch block
- DC:
-
Direct current
- ECG:
-
Electrocardiogram
- RBBB:
-
Right bundle branch block
- TdP:
-
Torsades de Pointes
- Vf:
-
Ventricular fibrillation
References
Chiu SN, Wu MH, Su MJ, Wang JK, Lin MT, Chang CC, et al. Coexisting mutations/polymorphisms of the long qt syndrome genes in patients with repaired tetralogy of fallot are associated with the risks of life-threatening events. Hum Genet. 2012;131:1295–304.
Walls J, Sanatani S, Hamilton R. Post-hoc diagnosis of congenital long qt syndrome in patients with tetralogy of fallot. Pediatr Cardiol. 2005;26:107–10.
Krasner BS, Girdwood R, Smith H. The effect of slow releasing oral magnesium chloride on the qtc interval of the electrocardiogram during open heart surgery. Can Anaesth Soc J. 1981;28:329–33.
Kim SJ, Pundi KN, Bos JM, Ackerman MJ. Ventricular fibrillation after elective surgery in an adolescent with long qt syndrome. BMJ Case Reports. 2015. doi:10.1136/bcr-2015-212365.
Fukuyama M, Wang Q, Kato K, Ohno S, Ding WG, Toyoda F, et al. Long qt syndrome type 8: novel cacna1c mutations causing qt prolongation and variant phenotypes. Europace. 2014;16:1828–37.
Boczek NJ, Best JM, Tester DJ, Giudicessi JR, Middha S, Evans JM, et al. Exome sequencing and systems biology converge to identify novel mutations in the l-type calcium channel, cacna1c, linked to autosomal dominant long qt syndrome. Circ Cardiovasc Genet. 2013;6:279–89.
Boczek NJ, Ye D, Jin F, Tester DJ, Huseby A, Bos JM, et al. Identification and functional characterization of a novel cacna1c-mediated cardiac disorder characterized by prolonged qt intervals with hypertrophic cardiomyopathy, congenital heart defects, and sudden cardiac death. Circ Arrhythm Electrophysiol. 2015;8:1122–32.
Itoh H, Crotti L, Aiba T, Spazzolini C, Denjoy I, Fressart V, et al. The genetics underlying acquired long qt syndrome: impact for genetic screening. Eur Heart J. 2016;37:1456–64.
Ackerman MJ. The long qt syndrome: ion channel diseases of the heart. Mayo Clin Proc. 1998;73:250–69.
Kies SJ, Pabelick CM, Hurley HA, White RD, Ackerman MJ. Anesthesia for patients with congenital long qt syndrome. Anesthesiology. 2005;102:204–10.
Nathan AT, Berkowitz DH, Montenegro LM, Nicolson SC, Vetter VL, Jobes DR. Implications of anesthesia in children with long qt syndrome. Anesth Analg. 2011;112:1163–8.
Park YM, Kim SJ, Park CH, Kang WC, Shin MS, Koh KK, et al. Repeated aborted sudden cardiac death with long qt syndrome in a patient with anomalous origin of the right coronary artery from the left coronary cusp. Korean Circ J. 2013;43:830–3.
Aburawi EH, Souid AK, Liuba P, Zoubeidi T, Pesonen E. Early changes in myocardial repolarization and coronary perfusion after cardiopulmonary bypass surgery for asd repair in children. BMC Cardiovasc Disord. 2013;13:67.
Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long qt syndrome. Circulation. 1993;88:782–4.
Tester DJ, Will ML, Haglund CM, Ackerman MJ. Effect of clinical phenotype on yield of long qt syndrome genetic testing. J Am Coll Cardiol. 2006;47:764–8.
Acknowledgements
none.
Funding
none.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Author information
Authors and Affiliations
Contributions
AI, FS, TO, TH and SU carried out the operation and postoperative care of the patient. NK and TS gathered figures and data to prepare for this manuscript. SO and MH conducted the gene analysis of this case and revised the manuscript. YK, TY and HI supervised and corrected the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent for this publication were waived by Ehime University Committee.
Consent for publication
Written informed consent was gained from the patient for the publication of this article. A copy of the consent form is available for review by the Editors-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kojima, A., Shikata, F., Okamura, T. et al. Refractory ventricular fibrillations after surgical repair of atrial septal defects in a patient with CACNA1C gene mutation - case report. J Cardiothorac Surg 12, 118 (2017). https://doi.org/10.1186/s13019-017-0683-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-017-0683-4