Abstract
Background
Osteoporosis, a systemic skeletal disease, seriously affects the quality of life in postmenopausal women. As one type of cathepsin K (CatK) inhibitor, odanacatib (ODN) is a fresh medication for osteoporosis. Considering the potential of ODN, we further examined the effect and safety of ODN for postmenopausal osteoporosis (PMOP) with a meta-analysis.
Methods
PubMed, EMBASE, Cochrane Library, and Web of Science were searched for eligible studies from inception to December 29th, 2023. After that, we conducted a comprehensive meta-analysis following PRISMA guidelines. Risk of bias was meticulously investigated with the Cochrane Collaboration’s tool. Efficacy was assessed with bone mineral density (BMD) at different sites (lumbar spine, trochanter, radius, femoral neck) and biomarkers of bone turnover (P1NP, uNTx/Cr, s-CTx, BSAP). Safety was evaluated by analyzing total, serious, other, and skin adverse events (AEs).
Results
Four random clinical trials (RCTs) were involved in our research. All trials were rated as having high quality and met the eligibility criteria. In the current research, ODN was found to elevate BMD at lumbar spine, femoral neck, total hip, trochanter and forearm, while it decreased the levels of serum C-telopeptides of type I collagen (s-CTx) as well as urinary N-telopeptide/creatinine ratio (uNTx/Cr). No significant differences were observed in AEs between the ODN group and the control group.
Conclusions
ODN is a promising alternative for the treatment of PMOP on account of its excellent efficacy and credible safety. Unclear links between ODN and cardiovascular AEs require further research to clarify.
Similar content being viewed by others
Background
Osteoporosis, a systemic skeletal disease, has the characteristics of decreased bone mass as well as microarchitectural deterioration, resulting in heightened bone fragility and fracture susceptibility. Predominant sites for osteoporotic fractures include the spine, hip, and wrist [1]. Symptoms typically manifest only post-fracture occurrence, with even minor stress capable of inducing fractures in the presence of decreased bone mineral density (BMD). Osteoporosis-related chronic pain significantly hampers routine activities [2].
In developed countries, osteoporosis has a prevalence of 2–8% in males and 9–38% in females, depending on the diagnostic methodology [3]. China has witnessed an increasing prevalence of osteoporosis in recent years [4]. According to a recent multicenter survey, the age-standardized prevalence in individuals aged 50 years and older was 6.46% for men and 29.13% for women in China [5]. Particularly in older postmenopausal females, osteoporosis escalates the risk of fractures, with fractures at the hip and spine being linked to elevated morbidity and mortality in this population [6]. Postmenopausal bone loss, primarily attributable to estrogen deficiency, constitutes the main etiological factor for osteoporosis [7]. Postmenopausal osteoporosis (PMOP), the most common type of osteoporosis, is characterized by diminished BMD, microstructural disintegration, and elevated bone fragility in addition to fracture susceptibility [8].
Approximately 50% of postmenopausal women worldwide suffer from osteoporosis, with a reported 40% incidence of fractures among affected individuals [9]. In the National Health and Nutrition Examination Survey 2013–2014, 16.5% of American females at the age of 50 or older were diagnosed with osteoporosis, with a BMD T-score of -2.5 or lower at either the femoral neck (FN) or lumbar spine (LS) [10].
Current treatment regimens for osteoporosis encompass non-drug therapies, such as dietary balance and adequate protein, calcium, as well as the intake of vitamin D [1], and drug therapies including estrogen agonists/antagonists, sclerotin inhibitors, RANK ligand inhibitors, parathyroid hormone receptor agonists, and bisphosphonates [7]. While the treatment options have expanded over the past two decades [11, 12], the optimum intervention for postmenopausal females at a high risk of fracture warrants further investigation [13,14,15,16,17].
Odanacatib (ODN), a selective cathepsin K (CatK) inhibitor, represents a novel therapeutic approach based on CatK pivotal role in enzymatic bone degradation [18]. ODN, a potent, reversible nonpeptidic biaryl inhibitor, mitigates CatK proteolytic activity [19]. Clinical trials with ODN demonstrated progressive increases in BMD and reductions in bone resorption markers in postmenopausal women who have low BMD [20]. Assessing the efficacy and safety of ODN in treating PMOP involved utilizing BMD and biomarkers as primary efficacy indicators, with safety monitored through the observation of AEs, including cutaneous manifestations. A comprehensive literature analysis on ODN application in the treatment of PMOP was conducted to bolster conclusions and provide clinical treatment insights.
Methods
Registration
We conducted this present meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Also, the protocol of this study was registered with PROSPERO.
Search strategy
Electronic literature search was conducted for eligible studies from the inception of PubMed, Embase, Cochrane Library and Web of Science to December 29th, 2023, using core medical terms “odanacatib” and “postmenopause osteoporosis”. The search history is detailed in Supplementary materials.
Study selection
The articles obtained were reserved for only the newest or most informative ones if they were detected duplicated in the established literature libraries. Then, two independent researchers were involved in screening titles and abstracts of the remaining research for further study selection on the basis of the criteria derived from PICOS rule [21]: (1) Population (P): The participants of the study were restricted in postmenopause women who had PMOP or less BMD during menopause; (2) Intervention (I) and comparison (C): The intervention measure was ODN, which was the only controlled difference between the experimental group and the control group in the study; (3) Outcome (O): Either efficacy indicators (BMD, bone turnover markers, etc.) or safety indicators (adverse events or AEs) should be investigated in the study; (4) Study design (S): Only randomized controlled trials (RCTs) in the clinical field can be included in the analysis, and other types of research such as animal experiment should be excluded.
Full-text review was carried out for confirmation of eligibility and comparability, independently by two researchers as well. The studies were excluded if they were not available, their data were incomplete, or their design was not reasonable. For different articles derived from the same series of clinical trials, the major one was included for convenience and was mentioned particularly.
Data extraction and quality assessment
All of the selected literature was reviewed by reading the whole texts. The related data were collected during the review process, covering the first author’s name, journal of publication, area covered by the study, sample size, year of publication, demographic characteristics of studied members, follow-up time, measure details of interventions and controls, and efficacy or safety outcomes. Further information was extracted from the websites where these studies were registered, such as clinical trial registry ( https://clinicaltrials.gov/ ). The data from the registration report were preferred when they were in conflict with the information in the articles.
Moreover, two investigators Jiaxuan Li and Qi Qiu assessed the quality of the RCTs using ‘Cochrane collaboration’s tool for assessing risk of bias in randomized trial’ as having ‘Low’ or ‘High’ risk of bias or ‘some concerns’ [22]. For the original data that were not provided or incomplete, the study authors were contacted to obtain or measure the data from relevant charts using GetData Graph Digitizer. Additionally, we referred to the Cochrane Handbook of Systematic Reviews to examine the quality of the included studies from several aspects like randomization, allocation concealment, blinding, measurement blinding, data integrity, selective reporting, and other bias assessments. If the two researchers disagreed with each other, a consensus or compromise was reached, or a third researcher was consulted if necessary. Review Manager 5.4 was employed to assess the quality of the included literature.
Data synthesis
We analyzed collected information with the support of StataSE 16.0. The data of AEs, serious AEs, and other AEs were investigated using the relative risk (RR) indicator. For quantitative indexes, heterogeneity was evaluated first using a fixed effects model based on I2 test [23]. I2 > 50% indicated great heterogeneity and a random effects model was utilized. The majority of analysis results were present in the forest plots, with 95% confidence intervals (CI) of each plot of data.
Results
Study collection
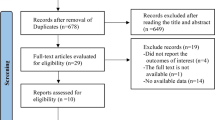
To sum up, totally 545 records were obtained from target databases. After deletion of duplicate literature (n = 208), retraction records (n = 5, all associated with the same article) and correction records (n = 2, all associated with the same article), 337 articles were screened by their titles and abstracts and 16 records were remained and further investigated by full-text reading. Finally, only 4 RCTs were included in the current analysis [24,25,26,27,28,29,30,31,32,33,34]. The study selection workflow is illustrated in Fig. 1.
Study characteristics
Table 1 illustrates the basic features of 4 included RCTs published between 2010 and 2019, involving 16,689 participants. The NCT numbers of these studies are NCT00529373, NCT00112437, NCT00729183 and NCT00885170, respectively. When scanning, we found that 11 out of the full-text-investigated 16 articles were involved in the above clinical trials [24,25,26,27,28,29,30,31,32,33,34]. Considering that the analysis of repeated populations or repeated subgroups might lead to greater risks of bias, we selected only one article containing the most complete data from each RCT in representation of the trial according to the eligibility and comparability of the article [24, 27, 28, 32].
It should be noted that the studies contain experimental groups with oral ODN dosage of 50 mg and control groups with the same dosage of placebo, and each study included the same intervention of vitamin D3 as well as calcium supplements in the experimental group and the control group. Additionally, Bonnick et al. conducted a survey on postmenopausal females with low BMD T-score at the total hip (TH) who have taken ALN for ≥ 3 years [27], and Bone et al. took the doses of ODN into consideration, including groups with oral ODN dosages of 3, 10, and 25 mg/week [24].
Risk of bias within the studies
The risk of bias in selection, performance, detection, and reporting in these 4 RCTs [24, 27, 28, 32] was comparatively low, indicating a higher methodological quality. However, the studies by Bonnick 2013 [27], Brixen 2013 [28], and McClung O’Donoghue 2019 [32] exhibit a higher risk of bias related to result completeness, with attrition rates exceeding 10%. Additionally, 3 RCTs [27, 28, 32] manifest higher susceptibility to other biases, primarily attributed to concerns about potential sponsorship from relevant industry entities. Nonetheless, overall, these 4 RCTs [24, 27, 28, 32] demonstrated minimal bias, ensuring a robust foundation for further analysis and highlighting the efficacy of the treatment under investigation. Figure 2 shows the quality of the included studies.
Meta analysis
Based on the common outcome indicators in the 4 RCT studies [24, 27, 28, 32], we verified the efficacy of ODN, including BMD at LS, FN, trochanter and radius, and biomarkers of N-terminal propeptide of Type I collagen (P1NP), urinary N-telopeptide/creatinine ratio (uNTx/Cr), C-telopeptides of type 1 collagen (s-CTx), and bone-specific alkaline phosphatase (BSAP).
BMD increase
Overall, the results demonstrated that ODN dramatically increased BMD in individuals with PMOP, in comparison with placebo. Besides, with the extension of treatment time, the efficacy of ODN was better and more significant.
Lumbar spine BMD
Four RCTs [24, 27, 28, 32] reported BMD at LS, FN, total hip (TH), and trochanter, as well as distal BMD after 12 months and 24 months of treatment.
For the LS BMD after 12 months of treatment, we included 12,045 patients, with 6,026 in the experimental group and 6,019 in the control group. Due to heterogeneity among studies (P < 0.0001, I2 = 93.2%), the analysis was therefore completed with a random effects model. According to the results, the LS BMD of the experimental group, in comparison with the control group, was higher after treatment, with a statistical significance [WMD = 3.02, 95% CI (1.73, 4.31), P < 0.0001] (Fig. 3A).
For the LS BMD after 24 months of treatment, we included 12,045 patients, with 6,026 in the experimental group and 6,019 in the control group. Due to heterogeneity among studies (P < 0.0001, I2 = 88.8%), the analysis was therefore completed with a random effects model. According to the results, the LS BMD of the experimental group, in comparison with the control group, was higher after treatment, with a statistical significance [WMD = 5.01, 95% CI (3.68, 6.34), P < 0.001] (Fig. 3B).
Femoral Neck BMD
For the FN BMD after 12 months of treatment, we included 12,280 patients, with 6,139 in the experimental group and 6,141 in the control group. Heterogeneity was observed across the studies (P = 0.056, I2 = 60.4%), and a random effects model was therefore employed for analysis. According to the results, the FN BMD of the experimental group, in comparison with the control group, was higher after treatment, with a statistical significance [WMD = 1.95, 95% CI (1.36, 2.54), P = 0.056] (Fig. 4A).
For the FN BMD after 24 months of treatment, we included 12,280 patients, with 6,139 in the experimental group and 6,141 in the control group. Heterogeneity was observed across the studies (P = 0.117, I2 = 49.1%), and a fixed effects model was therefore used for analysis. According to the results, the FN BMD of the experimental group, in comparison with the control group, was higher after treatment, with a statistical significance [WMD = 4.34, 95% CI (4.16, 4.53), P = 0.117] (Fig. 4B).
Total hip BMD
For the TH BMD after 12 months of treatment, we included 12,280 patients, with 6,139 in the experimental group and 6,141 in the control group. Heterogeneity was observed across the studies (P = 0.002, I2 = 80.5%), and the analysis was therefore completed with a random effects model. According to the results, the TH BMD of the experimental group, in comparison with the control group, was higher after treatment, with statistical significance [WMD = 1.96, 95% CI (1.28, 2.65), P = 0.002] (Fig. 5A).
For the TH BMD after 24 months of treatment, we included 12,280 patients, with 6,139 in the experimental group and 6,141 in the control group. Heterogeneity was observed across the studies (P = 0.006, I2 = 75.9%), and a random effects model was therefore used for analysis. Based on the results, the TH BMD of the experimental group, in comparison with the control group, was higher after treatment, with a statistical significance. [WMD = 3.76, 95% CI (2.89, 4.63), P = 0.006] (Fig. 5B).
Trochanter BMD
For trochanter BMD after 12 months of treatment, we included 12,280 patients, with 6,139 in the experimental group and 6,141 in the control group. Heterogeneity was observed across the studies (P < 0.0001, I2 = 83.4%), and the analysis was therefore completed with a random effects model. According to the results, the trochanter BMD of the experimental group, compared with the control group, was higher after treatment, with a statistical significance [WMD = 2.51, 95% CI (1.38, 3.64), P < 0.001] (Fig. 6A).
For the trochanter BMD after 24 months of treatment, we included 12,280 patients, with 6,139 in the experimental group and 6,141 in the control group. Heterogeneity was observed across the studies (P < 0.05, I2 = 76.5%), and the analysis was therefore completed using a random effects model. Based on the results, the trochanter BMD of the experimental group, in comparison with the control group, was higher after treatment, with a statistical significance [WMD = 5.28, 95% CI (3.97, 6.60), P = 0.005]. (Fig. 6B)
Distal BMD
For the distal BMD after 12 months of treatment, we included 1,706 patients, with 834 in the experimental group and 872 in the control group. No significant heterogeneity was found across the studies (P = 0.157, I2 = 42.5%), and a fixed effects model was therefore used for analysis. According to the results, the distal BMD of the experimental group, compared with the control group, was higher after treatment, with a statistical significance [WMD = 0.89, 95% CI (0.55, 1.24), P < 0.001] (Fig. 7A).
For the distal BMD after 24 months of treatment, we included 1,706 patients, with 834 in the experimental group and 872 in the control group. Heterogeneity was found across the studies (P = 0.099, I2 = 52.3%), and the analysis was therefore completed with a random effects model. According to the results, the distal BMD of the experimental group, compared with the control group, was higher after treatment, with a statistical significance [WMD = 1.37, 95% CI (0.64, 2.11), P = 0.099] (Fig. 7B).
Biomarkers
Four RCTs [24, 27, 28, 32] reported bone turnover markers of P1NP, uNTx/Cr, s-CTx, and BSAP after 12 months and 24 months of treatment. Overall, the results revealed that ODN dramatically lowered biomarkers in individuals with PMOP, in comparison with the placebo.
For P1NP after 12 months of treatment, we included 1,777 patients, with 876 in the experimental group and 901 in the control group. Heterogeneity was found across the studies (P = 0.003, I2 = 79.0%), and the analysis was therefore completed with a random effects model. Based on the results, in comparison with the control group, P1NP, which was proportional to the increase of fracture risk, decreased in the experimental group, and the fracture risk decreased, with a statistical significance [WMD=-20.56, 95% CI (-34.65, -6.47), P = 0.003] (Fig. 8A).
For the P1NP after 24 months of treatment, we included 1,777 patients, with 876 in the experimental group and 901 in the control group. Heterogeneity was observed across the studies (P = 0.007, I2 = 75.2%), and the analysis was therefore completed using a random effects model. Based on the results, in comparison with the control group, the bone turnover marker P1NP, which was proportional to the increase of fracture risk, decreased in the experimental group, and the fracture risk decreased, with a statistical significance [WMD=-8.22, 95% CI (-22.65, 6.21), P = 0.007] (Fig. 8B).
uNTx/Cr
For the uNTx/Cr after 12 months of treatment, we included 1,283 patients, with 634 in the experimental group and 649 in the control group. Due to the lack of data from Brixen (2013) [28] and heterogeneity between studies (P = 0.583, I2 = 0.00%), a fixed effects model was adopted for analysis. Based on the results, the bone turnover marker uNTx/Cr, which was proportional to the increase of fracture risk, reduced in the experimental group in comparison with the control group, and the fracture risk decreased, with a statistical significance [WMD=-53.49, 95% CI (-57.89, -49.10), P = 0.583] (Fig. 9A).
For the uNTx/Cr after 24 months of treatment, we included 1,283 patients, with 634 in the experimental group and 649 in the control group. Due to the lack of data from Brixen (2013) [28]and heterogeneity between studies (P = 0.369, I2 = 0.00%), a fixed effects model was adopted for analysis. Based on the results, the bone turnover marker uNTx/Cr, which was proportional to the increase of fracture risk, decreased in the experimental group in comparison with the control group, and the fracture risk decreased, with a statistical significance [WMD=-54.81, 95% CI (-60.06, -49.57), P = 0.369] (Fig. 9B).
s-CTx
For the s-CTx after 12 months of treatment, we included 1,468 patients, with 721 in the experimental group and 747 in the control group. Heterogeneity was found across the studies (P = 0.021, I2 = 69.0%), and the analysis was therefore conducted using a random effects model. According to the results, s-CTx, which was proportional to the increase of fracture risk, decreased in the experimental group in comparison with the control group, and the fracture risk decreased, with a statistical significance [WMD=-52.01, 95% CI (-64.33, -39.70), P = 0.021] (S1 File. A).
For the s-CTx after 24 months of treatment, we included 1,468 patients, with 721 in the experimental group and 747 in the control group. Heterogeneity was found across the studies (P = 0.009, I2 = 73.9%), and the analysis was therefore completed using a random effects model. According to the results, s-CTx, which was proportional to the increase of fracture risk, decreased in the experimental group in comparison with the control group, and the fracture risk decreased, with a statistical significance [WMD=-41.63, 95% CI (-59.25, -24.01), P = 0.009] (S1 File. B).
BASP
For the BASP after 12 months of treatment, we included 1,350 patients, with 667 in the experimental group and 683 in the control group. Due to the lack of data from Brixen (2013) [28] and heterogeneity between studies (P < 0.0001, I2 = 90.0%), a random effects model was adopted for analysis. Based on the results, the bone turnover marker BASP, which was proportional to the increase of fracture risk, decreased in the experimental group in comparison with the control group, and the fracture risk decreased, with a statistically significant difference [WMD=-5.38, 95% CI (-19.92, 9.16), P < 0.001] (S2 File. A).
For the BASP after 24 months of treatment, we included 1,350 patients, with 667 in the experimental group and 683 in the control group. Due to the lack of data from Brixen (2013) [28] and heterogeneity across the studies (P = 0.029, I2 = 71.8%), the analysis was completed with a random effects model. According to the results, the bone turnover marker BASP, which was proportional to the increase of fracture risk, decreased in the experimental group in comparison with the control group, and the fracture risk decreased, with a statistical significance [WMD=-7.00, 95% CI (-18.45, 4.45), P = 0.029] (S2 File. B).
Safety/Adverse events
Four RCTs [24, 27, 28, 32] reported the incidence of total AEs after treatment among 4 groups of patients. We included 16,689 patients, with 8,352 in the experimental group and 8,337 in the control group. No heterogeneity was found across the studies (P = 0.791, I2 = 0.0%), and the analysis was therefore completed using a fixed effects model. was According to the results, no statistically significant difference was observed in the incidence of AEs between the observation group and the control group [RR = 1.00, 95% CI (0.99, 1.01), P = 0.791] (S3 File).
Four RCTs [24, 27, 28, 32] reported the occurrence of serious AEs after treatment in 4 groups of patients. We included 16,689 patients, with 8,352 in the experimental group and 8,337 in the control group. No heterogeneity was found across the studies (P = 0.413, I2 = 0.0%), and the analysis was completed using a fixed effects model. According to the results, no statistically significant difference was observed in the incidence of AEs between the observation group and the control group [RR = 0.97, 95% CI (0.92, 1.03), P = 0.413] (S4 File).
Four RCTs [24, 27, 28, 32] reported the incidence of other AEs after treatment in 4 groups of patients. We included 16,693 patients, with 8,356 in the experimental group and 8,337 in the control group. No heterogeneity was found across the studies (P = 0.648, I2 = 0.0%), and the analysis was therefore completed using a fixed effects model. According to the results, no statistical significance was observed in the incidence of other AEs between the observation group and the control group [RR = 1.00, 95% CI (0.98, 1.02), P = 0.648] (S5 File).
Three RCTs [24, 27, 28] reported the occurrence of skin AEs after treatment in 3 groups of patients. We included 618 patients, with 309 in the experimental group and 309 in the control group. No heterogeneity was found across the studies (P = 0.293, I2 = 16.6%), and a fixed effects model was adopted for analysis. According to the results, no statistical significance was observed in the incidence of skin AEs between the observation group and the control group [RR = 0.93, 95% CI (0.63, 1.35), P = 0.293]. (S6 File)
Discussion
We carried out this systematic review to evaluate whether ODN could be effective and well-tolerated in postmenopausal females with osteoporosis, with 4 RCTs included (4 RCTs were published as 11 articles). The results of the present research illustrated that the overall effective rate of ODN medication was dramatically higher than that of placebo. BMD at LS, FN, TH, trochanter and forearm in experimental groups was significantly increased compared with control groups. The changes from the baseline of two biomarkers, uNTx/Cr and s-CTx, saw a dramatic dive in two years’ treatment, demonstrating the efficacy of ODN. In addition, all kinds of AEs in ODN groups were similar to that of the control groups.
Our analysis of the BMD of all tested bone locations at 12 and 24 months revealed a significant increase in BMD with ODN. Similar findings were observed in previous meta-analyses investigating the effects of ODN in osteoporosis across the age spectrum [35, 36]. The gain might be mostly attributed to ODN’s inhibition of CatK, a key protease that is involved in bone resorption and secreted by osteoclasts [37]. Studies have clarified that since CatK regulates the resorption ability of osteoclasts but not its life cycle, ODN decreases the degree of bone resorption reaction by suppressing the normal function of CatK and keeps osteoclasts alive, making it possible to transmit bone formation signals for bone rebuilding [9, 38]. This mechanism can also illuminate the decrease of uNTx/Cr and s-CTx, as these two biomarkers reflect the extent of bone resorption. However, no significant difference was seen in P1NP (at 24 months) and BASP (at 12 and 24 months), though they are bone formation indicators. Animal studies showed that ODN suppressed bone markers of bone formation in adult ovariectomized Rhesus monkeys [39]. Chen R et al. also reported the same phenomenon of P1NP in middle-aged and elderly people in their meta-analysis [40]. Unlike other included research in our study, only Bonnick S et al. discovered the increase of P1NP and BASP [27]. The main reason might lie in the administration of alendronate for at least 3 years before the clinical trial and the interaction of these two drugs. When ODN is taken without other treatments, bone formation biomarkers are reduced, implying that ODN might also curb the bone formation pathway in some way in postmenopausal women with osteoporosis. This could not be clarified by the above mechanism and needs further investigation. Interestingly, long-term clinical research shows that P1NP and BASP recover around the baseline in 4 or 5 years [32, 41]. Hence, the possible curb on bone formation might be a temporary condition and could be tolerated by the human body.
In terms of safety, we observed no statistically significant difference in total AEs, serious AEs and other AEs between ODN groups and placebo groups. Several meta-analyses [35, 36, 40] had confirmed safety outcomes in broader population, suggesting that ODN could be a reliable choice for the treatment of PMOP. While some studies reported that patients treated with CatK inhibitors like balicatib might have a higher risk of morphea-like skin lesions [42, 43], and similarly was ODN [44], our research showed no strong evidence for the risk of skin AEs. M. R. McClung et al. revealed a hazard ratio (HR) 1.12 (95% CI 0·95–1·34; p = 0·18) for cardiovascular death, myocardial infarction, or stroke (ODN vs. placebo), leading to the clue that ODN might increase the risk of cardiovascular AEs [32]. However, it should be noted that cardiovascular AEs in former clinical trials were unknown, resulting in lower repeatability and credibility of the conclusion. More research is required to figure out whether ODN is strongly linked to cardiovascular AEs and how ODN affects the cardiovascular system.
Our review restricted participants to postmenopausal women, reducing bias caused by age and sex for a more precise analysis of ODN, compared with other previous meta-analyses. Nevertheless, some limitations in the current research still need to be addressed. To start with, significant differences in study design, intervention and characteristics of included patients could not be eliminated even though the inclusion and exclusion criteria had been determined, contributing to existing bias. In addition, no unpublished data were included in our study. Last, the research did not investigate the effect of different doses of ODN on postmenopausal women. More high-quality investigations are required to validate the results of our meta-analysis.
Conclusion
According to our meta-analysis, ODN is a promising alternative for the treatment of PMOP on account of its excellent efficacy and credible safety. Unclear link between ODN and cardiovascular AEs requires further research to clarify.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CatK:
-
Cathepsin K
- ODN:
-
Odanacatib
- PMOP:
-
Postmenopausal osteoporosis
- BMD:
-
Bone mineral density
- AEs:
-
Adverse events
- RCTs:
-
Random clinical trials
- s-CTx:
-
Serum C-telopeptides of type I collagen
- uNTx/Cr:
-
Urinary N-telopeptide/creatinine ratio
- P1NP:
-
N-terminal propeptide of Type I collagen
- FN:
-
Femoral neck
- LS:
-
Lumbar spine
- RR:
-
Relative risk
- TH:
-
Total hip
- BSAP:
-
Bone-specific alkaline phosphatase
References
Management of Postmenopausal Osteoporosis: ACOG Clinical Practice Guideline No. Obstet Gynecol. 2022;139(4):698–717. https://doi.org/10.1097/aog.0000000000004730. 2.
Cauley JA. Public health impact of osteoporosis. J Gerontol Biol Sci Med Sci. 2013;68(10):1243–51. https://doi.org/10.1093/gerona/glt093.
Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9:182. https://doi.org/10.1007/s11657-014-0182-3.
Chen P, Li Z, Hu Y. Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health. 2016;16(1):1039. https://doi.org/10.1186/s12889-016-3712-7.
Zeng Q, Li N, Wang Q, Feng J, Sun D, Zhang Q, et al. The prevalence of osteoporosis in China, a Nationwide, Multicenter DXA Survey. J Bone Min Res. 2019;34(10):1789–97. https://doi.org/10.1002/jbmr.3757.
Management of osteoporosis in postmenopausal women. 2010 position statement of the North American Menopause Society. Menopause. 2010;17(1):25–54. https://doi.org/10.1097/gme.0b013e3181c617e6. quiz 5–6.
Management of osteoporosis in. Postmenopausal women: the 2021 position statement of the North American Menopause Society. Menopause. 2021;28(9):973–97. https://doi.org/10.1097/gme.0000000000001831.
Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374(3):254–62. https://doi.org/10.1056/NEJMcp1513724.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet (London England). 2011;377(9773):1276–87. https://doi.org/10.1016/s0140-6736(10)62349-5.
Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos Int. 2017;28(6):1979–88. https://doi.org/10.1007/s00198-017-3996-1.
Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;1Cd001155. https://doi.org/10.1002/14651858.CD001155.pub2.
Wells GA, Hsieh SC, Zheng C, Peterson J, Tugwell P, Liu W. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2022;5(5):Cd004523. https://doi.org/10.1002/14651858.CD004523.pub4.
Reginster J, Bianic F, Campbell R, Martin M, Williams SA, Fitzpatrick LA. Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporos Int. 2019;30(7):1465–73. https://doi.org/10.1007/s00198-019-04947-2.
Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, et al. Efficacy of pharmacological therapies for the Prevention of fractures in Postmenopausal women: A Network Meta-Analysis. J Clin Endocrinol Metab. 2019;104(5):1623–30. https://doi.org/10.1210/jc.2019-00192.
Freemantle N, Cooper C, Diez-Perez A, Gitlin M, Radcliffe H, Shepherd S, et al. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int. 2013;24(1):209–17. https://doi.org/10.1007/s00198-012-2068-9.
Liu GF, Wang ZQ, Liu L, Zhang BT, Miao YY, Yu SN. A network meta-analysis on the short-term efficacy and adverse events of different anti-osteoporosis drugs for the treatment of postmenopausal osteoporosis. J Cell Biochem. 2018;119(6):4469–81. https://doi.org/10.1002/jcb.26550.
Messori A, Fadda V, Maratea D, Trippoli S, Marinai C. Anti-reabsorptive agents in women with osteoporosis: determining statistical equivalence according to evidence-based methods. J Endocrinol Invest. 2014;37(8):769–73. https://doi.org/10.1007/s40618-014-0124-3.
Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong LT, Falgueyret JP, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008;18(3):923–8. https://doi.org/10.1016/j.bmcl.2007.12.047.
Pérez-Castrillón P JL, Pinacho F, De Luis D, Lopez-Menendez M, Dueñas Laita A. Odanacatib, a new drug for the treatment of osteoporosis: review of the results in postmenopausal women. J Osteoporos. 2010;2010. https://doi.org/10.4061/2010/401581.
Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR, Rizzoli R, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Min Res. 2011;26(2):242–51. https://doi.org/10.1002/jbmr.212.
Suvan J, Leira Y, Moreno Sancho FM, Graziani F, Derks J, Tomasi C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J Clin Periodontol. 2020;47(Suppl 22):155–75. https://doi.org/10.1111/jcpe.13245.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Bone HG, McClung MR, Roux C, Recker RR, Eisman JA, Verbruggen N, et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Min Res. 2010;25(5):937–47. https://doi.org/10.1359/jbmr.091035.
Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR, Rizzoli R, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res. 2011;26(2):242–51. https://doi.org/10.1002/jbmr.212.
Langdahl B, Binkley N, Bone H, Gilchrist N, Resch H, Rodriguez Portales J, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Miner Res. 2012;27(11):2251–8. https://doi.org/10.1002/jbmr.1695.
Bonnick S, De Villiers T, Odio A, Palacios S, Chapurlat R, DaSilva C, et al. Effects of odanacatib on BMD and safety in the treatment of osteoporosis in postmenopausal women previously treated with alendronate: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2013;98(12):4727–35. https://doi.org/10.1210/jc.2013-2020.
Brixen K, Chapurlat R, Cheung AM, Keaveny TM, Fuerst T, Engelke K, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98(2):571–80. https://doi.org/10.1210/jc.2012-2972.
Cheung AM, Majumdar S, Brixen K, Chapurlat R, Fuerst T, Engelke K, et al. Effects of odanacatib on the radius and tibia of postmenopausal women: improvements in bone geometry, microarchitecture, and estimated bone strength. J Bone Miner Res. 2014;29(8):1786–94. https://doi.org/10.1002/jbmr.2194.
Engelke K, Fuerst T, Dardzinski B, Kornak J, Ather S, Genant HK, et al. Odanacatib Treatment affects trabecular and cortical bone in the femur of Postmenopausal women: results of a two-year placebo-controlled trial. J Bone Miner Res. 2015;30(1):30–8. https://doi.org/10.1002/jbmr.2292.
Rizzoli R, Benhamou CL, Halse J, Miller PD, Reid IR, Rodríguez Portales JA, et al. Continuous treatment with odanacatib for up to 8 years in postmenopausal women with low bone mineral density: a phase 2 study. Osteoporos Int. 2016;27(6):2099–107. https://doi.org/10.1007/s00198-016-3503-0.
McClung MR, O’Donoghue ML, Papapoulos SE, Bone H, Langdahl B, Saag KG, et al. Odanacatib for the treatment of postmenopausal osteoporosis: results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT extension study. Lancet Diabetes Endocrinol. 2019;7(12):899–911. https://doi.org/10.1016/s2213-8587(19)30346-8.
Recker R, Dempster D, Langdahl B, Giezek H, Clark S, Ellis G, et al. Effects of Odanacatib on Bone structure and quality in Postmenopausal Women with osteoporosis: 5-Year Data from the phase 3 Long-Term Odanacatib Fracture Trial (LOFT) and its extension. J Bone Miner Res. 2020;35(7):1289–99. https://doi.org/10.1002/jbmr.3994.
Papapoulos S, Bone H, Cosman F, Dempster DW, McClung MR, Nakamura T, et al. Incidence of hip and Subtrochanteric/Femoral Shaft Fractures in Postmenopausal Women with osteoporosis in the phase 3 Long-Term Odanacatib Fracture Trial. J Bone Miner Res. 2021;36(7):1225–34. https://doi.org/10.1002/jbmr.4284.
Feng S, Luo Z, Liu D. Efficacy and safety of odanacatib treatment for patients with osteoporosis: a meta-analysis. J Bone Min Metab. 2015;33(4):448–54. https://doi.org/10.1007/s00774-014-0609-3.
Gajic-Veljanoski O, Tomlinson G, Srighanthan J, Adachi JD, Josse R, Brown JP, et al. Effect of odanacatib on BMD and fractures: estimates from bayesian univariate and bivariate meta-analyses. J Clin Endocrinol Metab. 2014;99(9):3070–9. https://doi.org/10.1210/jc.2014-1162.
Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP, Cathepsin K. Its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol. 2011;7(8):447–56. https://doi.org/10.1038/nrrheum.2011.77.
Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907. https://doi.org/10.1016/s2213-8587(17)30188-2.
Masarachia PJ, Pennypacker BL, Pickarski M, Scott KR, Wesolowski GA, Smith SY, et al. Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Min Res. 2012;27(3):509–23. https://doi.org/10.1002/jbmr.1475.
Chen R, Chen C, Geng B, Yang C, Xiao H, Yang F, et al. Efficacy and safety of odanacatib for osteoporosis treatment: a systematic review and meta-analysis. Arch Osteoporos. 2023;18(1):67. https://doi.org/10.1007/s11657-023-01261-7.
Langdahl B, Binkley N, Bone H, Gilchrist N, Resch H, Rodriguez Portales J, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: five years of continued therapy in a phase 2 study. J Bone Min Res. 2012;27(11):2251–8. https://doi.org/10.1002/jbmr.1695.
Peroni A, Zini A, Braga V, Colato C, Adami S, Girolomoni G. Drug-induced morphea: report of a case induced by balicatib and review of the literature. J Am Acad Dermatol. 2008;59(1):125–9. https://doi.org/10.1016/j.jaad.2008.03.009.
Rünger TM, Adami S, Benhamou CL, Czerwiński E, Farrerons J, Kendler DL, et al. Morphea-like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol. 2012;66(3):e89–96. https://doi.org/10.1016/j.jaad.2010.11.033.
Saag KG, de Villiers TJ, Alexandersen P, Jacobe H, Kovarik C, Werth VP, et al. Morphea-like skin lesions reported in the phase 3 Long-Term Odanacatib Fracture Trial (LOFT) in postmenopausal women with osteoporosis. J Am Acad Dermatol. 2021;84(4):1113–9. https://doi.org/10.1016/j.jaad.2020.06.036.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key R&D Program of China (2023YFC3603404), National Natural Science Foundation of China (82072506, 92268115), Hunan Provincial Science Fund for Distinguished Young Scholars (2024JJ2089), Hunan Young Talents of Science and Technology (2021RC3025), National Clinical Research Center for Geriatric Disorders, Xiangya Hospital (2021LNJJ05).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.L. and Q.Q.; methodology, J.L. and Q.Q.; software, J.L. and Q.Q.; validation, Y.L.; formal analysis, J.S.; investigation, J.S.; resources, S.J.; data curation, V.P.; writing—original draft preparation, J.L. and Q.Q.; writing—review and editing, S.J., J.S., V.P. and Y.L.; visualization, Y.L.; supervision, Y.L.; project administration, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13018_2024_5008_MOESM1_ESM.tif
Supplementary Material 1: S1 File - Forest plot showing the effect of odanacatib on s-CTx. A. After 12 months of treatment. B. After 24 months of treatment.
13018_2024_5008_MOESM2_ESM.jpg
Supplementary Material 2: S2 File -Forest plot showing the effect of odanacatib on BASP. A. After 12 months of treatment. B. After 24 months of treatment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Qiu, Q., Jiang, S. et al. Efficacy and safety of odanacatib in the treatment of postmenopausal women with osteoporosis: a meta-analysis. J Orthop Surg Res 19, 521 (2024). https://doi.org/10.1186/s13018-024-05008-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-05008-z