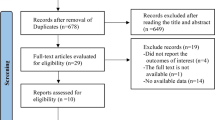
Abstract
The aim of this study was to evaluate the efficacy and safety of odanacatib (ODN) for the treatment of osteoporosis, using data in studies reported in the literature. We performed a literature search to compare the outcomes of applications of once-weekly ODN 50 mg and control. The outcomes of osteoporosis evaluated include primary outcome as bone mineral density (BMD) at different skeletal sites, and secondary outcomes, including adverse events (AEs), such as incidence of skin AEs, fracture, and serious adverse events (SAEs). Four trials were included. Mean difference (95 % CI) of lumbar spine BMD was 3.41 (1.57–5.24) at 12 months and 4.89 (2.72–7.05) at 24 months; mean difference (95 % CI) of femoral neck BMD was 1.90 (0.73–3.08) at 12 months and 3.85 (2.55–5.15) at 24 months; mean difference (95 % CI) of total hip BMD was 2.65 (1.20–4.09) at 12 months and 3.70 (1.76–5.64) at 24 months; risk ratio (95 % CI) of AEs was 0.98 (0.91–1.07); risk ratio (95 % CI) of SAEs was 1.11 (0.72–1.72); risk ratio (95 % CI) of skin AEs was 0.92 (0.63–1.35); and risk ratio (95 % CI) of fracture was 0.34 (0.16–0.70). In this study, application of 50 mg ODN produced significantly greater BMD increases and lower fracture incidence than that of the control. In addition, ODN was generally well tolerated.



Similar content being viewed by others
References
National Osteoporosis Foundation (2010) Clinician’s guide to prevention and treatment of osteoporosis. Available at: http://www.nof.org/sites/default/files/pdfs/NOF_ClinicianGuide2009_v7pdf
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Simonelli C, Burke MS (2006) Less frequent dosing of bisphosphonates in osteoporosis: focus on ibandronate. Curr Med Res Opin 22:1101–1108
Cusick T, Chen CM, Pennypacker BL, Pickarski M, Kimmel DB et al (2012) Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J Bone Miner Res 27:524–537
Masarachia PJ, Pennypacker BL, Pickarski M, Scott KR, Wesolowski GA et al (2012) Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res 27:509–523
Bone HG, McClung MR, Roux C, Recker RR, Eisman JA et al (2010) Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res 25:937–947
Brixen K, Chapurlat R, Cheung AM, Keaveny TM, Fuerst T et al (2013) Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab 98:571–580
Nakamura T, Shiraki M, Fukunaga M, Tomomitsu T, Santora AC et al (2014) Effect of the cathepsin K inhibitor odanacatib administered once weekly on bone mineral density in Japanese patients with osteoporosis—a double-blind, randomized, dose-finding study. Osteoporos Int 25:367–376
Bonnick S, De Villiers T, Odio A, Palacios S, Chapurlat R et al (2013) Effects of odanacatib on BMD and safety in the treatment of osteoporosis in postmenopausal women previously treated with alendronate: a randomized placebo-controlled trial. J Clin Endocrinol Metab 98:4727–4735
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Higgins JP, Green S (eds) (2006) Cochrane handbook for systematic reviews of interventions 4.2.6. In: The Cochrane Library, Issue 4. Wiley, Chichester (updated September 2006)
Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M et al (2006) Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91:2631–2637
Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD et al (2008) A comparison of the effect of alendronate and risedronate on bone mineral density in postmenopausal women with osteoporosis: 24-month results from FACTS-international. Int J Clin Pract 62:575–584
Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M et al (2000) Therapeutic equivalence of alendronate 70 mg once weekly and alendronate 10 mg daily in the treatment of osteoporosis. Aging Clin Exp Res 12:1–12
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M et al (2008) Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93:3785–3793
Boonen S, Marin F, Obermayer-Pietsch B, Simões ME, Barker C et al (2008) Effects of prior antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 93:852–860
Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L et al (2009) Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab 94:3772–3780
Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR et al (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Baron R, Ferrari S, Russell RG (2011) Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48:677–692
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture intervention trial research group. Lancet 348:1535–1541
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T et al (1999) Effects of risedronate treatment on vertebral and non vertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Miller PD, Recker RR, Reginster JY, Riis BJ, Czerwinski E et al (2012) Efficacy of monthly oral ibandronate is sustained over 5 years: the MOBILE long-term extension study. Osteoporos Int 23:1747–1756
Conflict of interest
There are no identified conflicts of interest with any of the authors.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Feng, S., Luo, Z. & Liu, D. Efficacy and safety of odanacatib treatment for patients with osteoporosis: a meta-analysis. J Bone Miner Metab 33, 448–454 (2015). https://doi.org/10.1007/s00774-014-0609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-014-0609-3