Abstract
Introduction
Accumulated clinical trials had been focused on stem cell therapy in combination of core decompression (CD) in the treatment of avascular necrosis of the femoral head (ANFH). Nonetheless, the results were inconclusive. Here, we performed a systematic review and meta-analysis of previous randomized controlled trials (RCTs) and retrospective studies to assess whether combined stem cell augmentation with CD improved the outcomes of ANFH compared with CD alone.
Methods
The current study included 11 RCTs and 7 retrospective studies reporting the clinical outcomes of a total of 916 patients and 1257 hips. 557 and 700 hips received CD and CD plus stem cell therapy, respectively. To compare CD with CD plus stem cell therapy, we examined the clinical evaluating scores, the occurrence of the femoral head, radiologic progression and conversion to total hip arthroplasty (THA).
Results
Only 10 studies reported significantly greater improvement in hip functions while combining stem cell procedure with CD. The pooled results in subgroup analysis indicated that stem cell group had a lower collapse rate on a mid-term basis (P = 0.001), when combined with mechanical support (P < 0.00001), and with extracted stem cells (P = 0.0002). Likewise, stem cell group had a lower radiographic progression rate at 2- to 5-year follow-up [P = 0.003], when combined with structural grafting (P < 0.00001), and with extracted stem cells (P = 0.004). Stem cell therapy resulted in an overall lower THA conversion rate (P < 0.0001) except that at a follow-up longer than 5 years.
Conclusion
Stem cell therapy combined with core decompression was more effective in preventing collapse, radiographic progression and conversion to THA.
Trial Registration The current protocol has been registered in PROSPERO with the registration number: CRD42023417248.
Similar content being viewed by others
Introduction
Avascular necrosis of the femoral head (ANFH), a prevalent disease for orthopedics especially in Asian population, was caused by impaired circulation of the femoral head [1]. The interruption of the blood supply leaded to structural rebuilding of the femoral head, then causes collapse of articular cartilage, and eventually gives rise to dysfunction and disability of hip [2]. The etiology of ANFH was multifactorial, including long-term use of glucocorticoids, alcohol abuse, and hip trauma [3]. In spite of good results of total hip arthroplasty (THA), it had been proved that the revision rate was up to 13.8% based on the recent registry data [4, 5]. Therefore, hip-preserving surgery had drawn much attention for the early stage of ANFH, including physical therapy, administration of bisphosphonates and/or nonsteroidal anti-inflammatory drugs, weight-bearing restrictions, multiple epiphyseal drilling augmented with autologous bone marrow implantation, free vascularized fibular grafts and osteotomies [6, 7]. Core decompression (CD) was the most frequently used among hip-preserving procedures, and the purpose was to reduce the intraosseous pressure and improve the blood supply of the femoral head.
Although CD has been utilized for more than 50 years, its efficacy was still controversial, this procedure did not demonstrate superior outcomes compared to other treatment modalities, thereby necessitating further research to determine the optimal treatment approach for ANFH [8]. Steinberg et al. reported that average of 36% of patients after CD would ultimately receive THA [9]. CD alone may not be effective in improving the pain and function on a long-term basis, especially for the cases of mid-stages (Association Research Circulation Osseous, ARCO stage II/III) [10]. Recent research suggested that poor prognosis of the CD was associated with male gender, longer duration of symptoms prior to treatment, higher visual analogue scale (VAS) scores and lower Harris Hip Scores (HHS) [11]. In 1997, Hernigou el al. proposed the application of bone marrow cells for the treatment of ANFH [12]. In the past two decades, the literature had revealed that the pathogenesis of ANFH was strongly related to the decreased pool of osteoprogenitor cells in the bone marrow of the femoral head [12, 13]. It was well-known that stem cells had capacity of multipotent differentiation and could differentiate into osteoprogenitor cells, vascular progenitor cells, chondroblasts and osteoblasts, etc., improving revascularization and promoting the reconstruction of the bone tissue in femoral head. Based on this phenomenon, mesenchymal stem cells (MSCs) transplantation to the necrotic area was considered to be an effective treatment for early-stage cases.
Up till nowadays, several authors had published systematic reviews about CD plus stem cells therapy for ANFH [14,15,16,17,18]. Nonetheless, these previous studies included several limitations. First of all, the type and number of stem cells were not consistent in those studies. Secondly, although the incidence of collapse was proved to be a critical outcome, few studies have synthesized and assessed this parameter. Additionally, with the publishment of the long-term results of stem cells therapy, we believed that it is necessary to update the systematic review and meta-analysis in this field. The aim of the present systematic review and meta-analysis was to evaluate whether CD combined with stem cells therapy in the early stage of ANFH can reduce pain, improve hip function and prevent collapse of the head. The hypothesis was that: (1) the augmentation using cell therapy would postpone the progression of ANFH and reduce the conversion rate of THA. (2) The mechanical support of the subchondral bone in the femoral head would be advantageous. (3) The outcomes would not vary while using either MSCs or bone marrow aspirate concentrate (BMAC).
Methods
Protocol and registration
The current protocol has been registered in PROSPERO with the registration number: CRD42023417248, following PRISMA guidelines [19].
Eligibility criteria
The studies included in our present meta-analysis were in strict accordance with PICOS criteria as follows: patients (P): the patients were older than 18 years age and diagnosed with ANFH; intervention (I): the treatments were based on core decompression and mechanical supporting procedures, and various stem cells including peripheral blood mesenchymal stem cells, bone marrow stem cells, bone marrow aspirate concentrates, bone marrow mononuclear cells, etc., were added to the surgical site; Comparison (C): core decompression with mechanical supporting procedures without stem cells therapy was as direct comparison; Outcomes(O): the primary outcomes were the rate of conversion to THA and the rate of radiographic collapse after intervention; the secondary outcomes were diverse post-operative clinical evaluating scores including HHS, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and VAS, etc. All trials we included were controlled trials.
The exclusion criteria were (1) duplicated studies, animal or cadaver studies, biomechanical studies, reviews, correspondence or technical notes; (2) the hip of patients has received previous surgery; (3) uncontrolled trials; (4) biological augmentation interventions used by study group were without stem cells.
Search strategy
The literature was searched using the following databases: PubMed, EMBASE, Web of Science databases, and the Cochrane Central Register of Controlled Trials for reports published from their commencement to March 2023 to identify the case-controlled studies, cohort studies, prospective studies and randomized controlled trials (RCT) that have compared the effects of CD with or without stem cells in the treatment of ANFH. The key term strings were used as follows: “osteonecrosis”, “avascular necrosis”, “femur head”, “stem cells”, “progenitor cells”, “cell therapy”, “core decompression”, “bone graft”. A search of the references on recent meta-analyses and reports of meetings was also undertaken. The language was restricted to English. Eligible studies were selected by screening the title or abstract. If this was deemed insufficient, the entire article was reviewed.
Selection and data collection
Two independent reviewers (MYL, DYC) followed a standardized form to extract data from articles without filters or constraints in the database search and independently assessed all the titles and abstracts for eligibility. The full text was obtained if at least one author judged a study to be eligible. Disagreements were resolved by consensus.
Data items
The extracted data elements included authors, publication date, evidence-based level, population, number of participants and hips, ratio of gender, mean follow-up, mean age, etiology, stage of necrosis (Ficat/ARCO), type of mechanical support after core decompression, type and number of stem cells. The number and rate of conversion to THA and radiographic collapse after intervention were recorded as primary outcome. The clinical functional scores of the hip, including HHS, WOMAC, VAS, were extracted as secondary outcomes. The post-operative data were based on the last time-point of follow-up because of the diverse follow-up time in the included studies.
Assessment of the risk of bias
Following the flowchart of the Cochrane Handbook for Systematic Reviews of Interventions [20], the reviewers (QJZ, YCM) independently assessed the random sequence generation, allocation concealment, blinding of participants and personnel, blinded evaluation of the outcome, the completeness of the outcome data, selective reporting, and other bias. Each of the domains was scored as “no risk of bias”, “high risk of bias”, or “unclear”. The Newcastle–Ottawa scale was introduced to assess the methodological quality of the included retrospective studies [21]. The scoring system included the representative of the study, exposure ascertainment, comparability of simultaneous group, assessment, follow-up, possible risk in selection bias and missing data. A score of 0–9 was allocated to each non-RCT, and the study scored higher than 6 was considered to be of high quality.
Assessment of the quality of recommendations
The quality of the evidence was evaluated based on the evidence profile using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system [22]. This approach enables a rating of the overall quality based on the evidence for risk of bias, publication bias, imprecision, inconsistency, and indirectness. The quality of evidence can be classified as very low-, low-, moderate-, or high-quality. The evidence quality was graded using the GRADE profile software (GRADEpro 3.6).
Data synthesis and analysis
Review Manager (RevMan 5.3, The Cochrane Collaboration, Copenhagen, Denmark) was used to extract data for statistical analysis. Chi-square test was used for heterogeneity testing if the research object, intervention measures and method of assessing outcome were identical. Mantel–Haenszel test (M–H) was used for enumeration data, and inverse variance (IV) was used for measurement data. The inspection was largely supported by the I2 index, which quantifies the proportion of variability in outcomes attributable to heterogeneity rather than chance across various trials. When I2 was less than 50%, it indicated that the heterogeneity among different studies was small, and a fixed-effects model can be used for statistical analysis. However, when I2 was greater than 50%, it indicated that the heterogeneity among different studies was large, and a random-effects model should be used for statistical analysis. The odds ratio (OR) was calculated for enumeration data; 95% confidence intervals (CIs) were also calculated for all meta-analyses (P < 0.05). The presence of publication bias of the primary outcome was tested by Egger test and illustrated as Funnel plot using STATA (STATA 17.0, The StataCorp, Texas, USA), P < 0.05 indicated significant publication bias.
Results
Search results
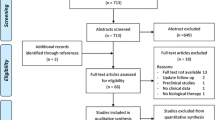
A total of 1325 articles were identified from the databases. A total of 141 studies were removed for duplication, and then, 729 studies were screened because they were correspondence or technical notes or irrelevant studies. A total of 317 studies were excluded because they were based on animal models or cadaver species, biomechanical studies and reviews. A further 85 non-controlled trials were also excluded. Twenty-seven trials were excluded from the remaining because their augmented interventions were not stem cells or not only stem cells. Of the remaining 26 studies, five were not in English, two did not have suitable clinical outcome, and one was not available in full-text articles, and therefore, they were also excluded. After the application of exclusion criteria, a total of 18 papers, all in English, were included in this meta-analysis (Fig. 1) [10, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. All selected studies used a conventional parallel group design, comparing CD versus CD plus stem cell therapy. Of the 18 identified studies, 11 studies were randomized controlled design [10, 28,29,30,31,32,33,34, 37,38,39], and the other 7 were retrospective studies [23,24,25,26,27, 35, 36].
Assessment of the risk of bias
Figure 2A, B illustrated the quality of each RCT. Table 1 indicated the quality of the 7 included retrospective studies.
Study characteristics
Seventeen of the included studies evaluated the conversion of hips to THA [10, 23,24,25,26,27, 29,30,31,32,33,34,35,36,37,38,39]. Eleven studies evaluated the radiographic progression [10, 23, 24, 26, 29,30,31,32,33,34, 39]. Nine studies recorded the radiographic progression [10, 23, 26, 29,30,31,32,33, 39]. With the definition of collapse as the femoral head as it progressing to ARCO stage III/Ficat stage IIB/Steinburg stage IV/Japanese Orthopaedic Association (JOA) stage II or subchondral fracture, eight studies evaluated the radiographic progression [10, 23, 24, 26, 32, 33, 35, 36].
Demographic, staging and etiologies matching
Table 2 provided the demographic, intervention and baseline data. A total of 905 patients and 1257 hips were included in the systematic review and meta-analysis. The age of the patients enrolled in these included studies ranging from 31 to 49.7. 556 and 701 hips received CD and CD plus stem cell therapy, respectively. The included studies had a minimum follow-up of 2 years. The study of the longest follow-up came from France, where Hernigou [35] reported a 25-year-result. Four kinds of staging systems were utilized in all of the studies. Among them, 11 studies [10, 24,25,26, 28, 30,31,32,33,34, 39] followed ARCO classification, 5 studies[27, 29, 36,37,38] reported data on the base of Ficat classification, and the left 2 studies used JOA [23] or Steinberg staging system [35]. Seventeen studies [10, 23,24,25,26,27,28,29,30,31,32,33, 35,36,37,38,39] documented and matched the etiologies of ANFH in their cases. Two well-known risk factors, Corticosteroid use and alcohol-abuse, took up 45% and 21% as the cause. Nonetheless, the etiology in a high proportion of patients (25%) was still ambiguous and thus, reported as idiopathic (Table 2).
Surgical technique
CD was the fundamental procedure in all included studies. Nonetheless, the technique had discrepancies. Fourteen studies applied single drilling technique, whereas 3 studies chose multiple drilling. Kirschner wires [26, 34], burs [23], reamers [39], trephines [10, 27, 29,30,31,32,33, 36,37,38] and trocards [35] were utilized as the tools for decompression. The diameters of the tunnels were also diverse, ranging from 2 to 14 mm (Table 2).
Mechanical structural augmentations were described in 7 studies [23, 24, 29, 32, 33, 37, 38], 3 of which used autologous bone graft [29, 37], and Li [38] used auto bone-grafting plus angio-conductive bio-ceramic rod. Interconnected porous calcium hydroxyapatite [23], Granular porous medical nano-hydroxyapatite, polyamide 66 composite bone filling material [24], porous tantalum rod [33] and allograft bone [32] were also used for structural support. In the other 18 studies, no specific mechanical augmentation was employed (Table 2).
Type of stem cell therapy
All the included studied performed 4 kinds of cell therapy. Twelve studies [10, 25, 27,28,29,30, 32, 34,35,36,37,38] applied bone marrow aspirate concentrate (BMAC) in their cohorts. Three [23, 26, 31], two [24, 39] and one [33] used bone marrow mesenchymal stem cells (BMMSCs), bone marrow mononuclear cells (BMMCs) and peripheral blood stem cells (PBSCs) as biologic augmentation, respectively. There was a heterogeneity when concerning the number of cells. In the studies utilizing BMAC, the number ranged from 90,000 to 3.46 × 109. As for those applying stem cell direct injection, the number of cells ranged from 2 × 106 to 0.25 × 109 (Table 2).
Clinical outcome
The most frequently used clinical scoring system among all the included studies was Harris Hip Score (HHS) [24, 28, 33,34,35, 38, 39] and visual analogue scale (VAS) [10, 24, 26, 29, 30, 32, 34, 35, 37]. To add to that, 6 studies [10, 29, 30, 32, 35, 37] assessed the clinical function based on Western Ontario and McMaster Universities Arthritis Index osteoarthritis scoring (WOMAC). Three [29, 30, 37] and two [25, 36] studies reported Laseque index and Merle D’Aubigné and Postel score, respectively. However, since some data did not obey normal distribution and thus, was not reported in the form of “average ± standard difference” [10, 23, 25, 26, 36, 37, 39]. Some studies reported the scores in the figure or did not report the exact value [27, 30, 31, 34]. These reasons resulted in the difficulty to extract the data to synthesize a forest plot.
In 16 included studies, the pre-operative functional scores were matched between stem cell and control group despite of diverse scoring system. Two studies did not compare the post-operative clinical scores between groups [27, 31]. Ten studies reported significantly greater improvement in hip functions while combining stem cell procedure to CD despite of using different scoring systems and the diverse follow-up duration [24, 25, 28, 30,31,32,33, 35, 37, 38]. Nonetheless, the remaining 6 studies did not detect statistically significant differences in clinical scores between the two treatment groups [10, 26, 29, 34, 36, 39] (Table 3).
Synthesis of results
Collapse of the femoral head
Based on the current literature, whether the femoral head collapsing had been considered as a critical prognostic factor of hip-preserving procedure. Among the included studies, 5 [23, 24, 33, 35, 36] compared the occurrence of collapse directly, and 3 [10, 26, 32] evaluated collapses based on the classification system. We extracted the data from these 8 [10, 23, 24, 26, 32, 33, 35, 36] studies. Additionally, we divided the 8 studies into subgroups according the duration of follow-up, whether the structural support was employed and the type of stem cell therapy and conducted a meta-analysis of these subgroups.
The 8 studies were firstly categorized based on the duration of follow-up: (1) 2 year-follow-up: 12/35 (34.2%) hips in the stem cell group and 16/32 (50.0%) in the control group were observed with collapse of the femoral head. It was not statistically significant [OR = 4.50; 95% CI (0.07, 307.07); P = 0.49] (Fig. 3A). (2) 2- to 5 year-follow-up: 54/177 (30.5%) hips in the stem cell group and 56/126 (44.4%) in the control group were observed with collapse of the femoral head. It was statistically significant [OR = 2.46; 95% CI (1.43, 4.23); P = 0.001] (Fig. 3B). (3) longer than 5 year-follow-up: only 1 study was in this subgroup, collapses of the femoral heads were observed in 35/125 (28.0%) hips in the stem cell group and 90/125 (72.0%) in the control group (Fig. 3C).
Forest plots of the rate of collapse. Subgroup analysis according to A the duration of follow-up in 2 years, B the duration of follow-up in 2–5 years, C the duration of follow-up longer than 5 years, D with structural support, E without structural support, F stem cell therapy of the BMAC group, G stem cell therapy of the BMMSCs/BMMSs/PBSCs group. (BMAC bone marrow aspirate concentrate, BMMCs bone marrow mononuclear cells, BMMSCs bone marrow mesenchymal stem cells, CD core decompression, CI confidence interval, df degree of freedom, M-H Mantel–Haenszel test, PBSCs peripheral blood stem cells)
The included studies were then divided into CD with and without mechanical support subgroup: (1) CD with mechanical support: (18.6%) (22/118) of femoral heads in the stem cell group and (36/86) (41.9%) in the control group collapsed. It was statistically significant [OR = 6.22; 95% CI (2.79, 13.87); P < 0.00001] (Fig. 3D). (2) CD without mechanical support (79/219) hips (36.1%) in the stem cell group and (126/197) (64.0%) in the control group were observed with collapse of the femoral head. It was not statistically significant [OR = 1.82; 95% CI (0.58, 5.73); P = 0.31] (Fig. 3E).
Lastly, we performed subgroup analysis based on the type of biologic augmentation: (1) BMAC group: 32.8% (66/201) of femoral heads in the stem cell group collapsed, whereas the proportion in the control group was 65.3% (115/176). The pooled data indicated that it was not statistically significant [OR = 2.78; 95% CI (0.64, 12.13); P = 0.17] (Fig. 3F). (2) BMMSCs/BMMSs/PBSCs group: 35/136 (25.7%) hips in the stem cell group and 47/107 (43.9%) in the control group were observed with collapse of the femoral head. It was statistically significant [OR = 3.30; 95% CI (1.74, 6.25); P = 0.0002] (Fig. 3G).
Radiological progression
Failure of intervention was defined as the radiological progression of necrotic zone, so this outcome was extracted from 11 [10, 23, 24, 26, 29,30,31,32,33,34, 39] studies.
First of all, subgroup analysis was based on the follow-up duration. (1) 2 year-follow-up: 15.5% (16/103) of femoral heads in the stem cell group and 29.5% (31/105) in the control group progressed radiologically. It was not statistically significant [OR = 2.31; 95% CI (1.14, 4.66); P = 0.02] (Fig. 4A). (2) 2 to 5 year-follow-up: 48/225 (21.3%) hips in the stem cell group and 75/185 (40.5%) in the control group progressed. The pooled data were statistically significant [OR = 4.50; 95% CI (1.69, 12.03); P = 0.003] (Fig. 4B).
Forest plots of the rate of radiographic progression. Subgroup analysis according to A the duration of follow-up in 2 years, B the duration of follow-up in 2–5 years, C with structural support, D without structural support, E stem cell therapy of the BMAC group, F stem cell therapy of the BMMSCs/BMMSs/PBSCs group. (BMAC bone marrow aspirate concentrate, BMMCs bone marrow mononuclear cells, BMMSCs bone marrow mesenchymal stem cells, CD core decompression, CI confidence interval, df degree of freedom, M-H Mantel–Haenszel test, PBSCs peripheral blood stem cells)
In the subgroup analysis was based on whether subchondral mechanical support was performed, we discovered that (1) in CD with mechanical support group, 25/145 (17.2%) and 50/115 (43.5%) hips experienced radiological progressions in stem cell group and control group [OR = 6.61; 95% CI (3.28, 13.34); P < 0.00001] (Fig. 4C), and (2) in CD without mechanical support group, (39/183) (21.3%) and (56/175) (32.0%) hips experienced radiological progressions in stem cell group and control group [OR = 2.20; 95% CI (0.88, 5.53); P = 0.09] (Fig. 4D).
We thirdly performed subgroup analysis according to the type of cell therapy: (1) BMAC group: 22.1% (19/86) of femoral heads in the stem cell group progressed, while in the control group the figure was 35/86 (40.7%) [OR = 2.44; 95% CI (1.24, 4.81); P = 0.01] (Fig. 4E). (2) BMMSCs/BMMSs/PBSCs group: 45/242 hips (18.6%) in the stem cell group and 71/204 (34.8%) in the control group were observed with radiological progression. The pooled data indicated a statistically significance [OR = 4.37; 95% CI (1.58, 12.06); P = 0.004] (Fig. 4F).
Conversion to THA
THA is the ultimate surgery for those failed hip-preserving cases. And therefore, conversion to THA is a crucial outcome of these studies and this was documented in 17 [10, 23,24,25,26,27, 29,30,31,32,33,34,35,36,37,38,39] of the included studies.
In the subgroup analysis based on the follow-up duration, (1) 22/125 (17.6%) hips in stem cell group and 40/134 (30.0%) hips in control group received THA during 2 years’ follow-up. The pooled results revealed a significant difference between the groups [OR = 1.69; 95% CI (1.13, 2.51); P = 0.01] (Fig. 5A). (2) When the follow-up duration lasted to 5 years, 42/276 (15.2%) hips in stem cell group and 63/214 (29.4%) hips in control group received THA. It was statistically significant [OR = 1.94; 95% CI (1.38, 2.71); P = 0.0001] (Fig. 5B). However, (3) for the data of follow-up longer than 5 years, 82/274 (29.9%) and 116/176 (65.9%) hips were conversed to THA, respectively, and the pooled data did not indicate statistically significance [OR = 3.17; 95% CI (0.62, 16.14); P = 0.16] (Fig. 5C).
Forest plots of the rate of THA conversion. Subgroup analysis according to A the duration of follow-up in 2 years, B the duration of follow-up in 2–5 years, C the duration of follow-up longer than 5 years, D with structural support, E without structural support, F stem cell therapy of the BMAC group, G stem cell therapy of the BMMSCs/BMMSs/PBSCs group. (BMAC bone marrow aspirate concentrate, BMMCs bone marrow mononuclear cells, BMMSCs bone marrow mesenchymal stem cells, CD core decompression, CI confidence interval, df degree of freedom, M–H Mantel–Haenszel test, PBSCs peripheral blood stem cells)
Secondly, subgroup analysis was based on the utilization of subchondral mechanical support. (1) CD with mechanical support: 8.5% (16/188) of femoral heads in the stem cell group and 26.2% (43/164) in the control group conversed to THA ultimately. It was statistically significant [OR = 3.80; 95% CI (2.02, 7.12); P < 0.0001] (Fig. 5D). (2) CD without mechanical support: 130/487 hips (26.7%) in the stem cell group and 176/360 (48.9%) in the control group had THA terminally. The pooled data were statistically significant [OR = 2.37; 95% CI (1.12, 5.04); P = 0.02] (Fig. 5E).
When examining the kind of cell therapy, we discovered the pooled resulted favored stem cell group in both BMAC (SG: 123/433, 28.4% vs CG: 168/320, 52.5%; OR = 2.53; 95% CI (1.23, 5.20); P = 0.01) (Fig. 5F) and BMMSCs/BMMSs/PBSCs subgroup (SG: 23/242, 9.5% vs CG: 51/204, 25.0%; OR = 3.25; 95% CI (1.85, 5.70); P < 0.0001) (Fig. 5G).
Publication bias
Egger test was carried out for all the forest plots which included more than 2 studies, and the results are shown in Table 4. For the rate of radiographic progression of 2-to-5-year follow-up, using subchondral bone graft and specific stem cells transplantation, P of Egger test was 0.001, 0.016 and 0.025, respectively, and therefore, possible publication bias was suggested. Funnel plots of standard error by effect size was illustrated in (Additional file 1: Fig. S1, S2, S3).
Assessment of the quality of recommendations
The GRADE system was used to evaluate all subgroups’ results of the three main outcomes in the present study. For the outcome of femoral head collapse, very low-quality evidence was found in the subgroups of 2-year follow-up, non-mechanical support and BMAC, while the quality was rated as low in the subgroups of 2–5-year follow-up, longer than 5-year follow-up, with mechanical support, and BMMSCs/BMMSs/PBSCs. For the outcome of radiological progression, very low-quality evidence was found in the subgroups of 2–5-year follow-up, non-mechanical support and BMMSCs/BMMSs/PBSCs, while moderate quality evidence was found in the subgroups of 2-year follow-up and with mechanical support, and high-quality evidence was found in the BMAC subgroup. For the outcome of conversion to THA, very low-quality evidence was found in the subgroups of longer than 5-year follow-up, non-mechanical support and BMAC, while low-quality evidence was found in the subgroups of 2–5-year follow-up, mechanical support, and BMMSCs/BMMSs/PBSCs (Table 5).
Discussion
Stem cell therapy combined with core decompression, as utilized in more than 20 clinical studies [40], was a prevalent hip-preserving strategy to treat ANFH. Nonetheless, the efficacy of stem cell therapy was inconclusive based on the current literature [41]. We included the level III to level I evidence in the present systematic review and meta-analysis with the purpose to analyze the outcome after stem cell therapy in a comprehensive way by further subgroup analysis. Overall, our results supported our initial hypothesis that the hypothesis was that: (1) the augmentation using cell therapy would postpone the progression of ANFH and reduce the conversion rate of THA. (2) The mechanical support of the subchondral bone in the femoral head would be advantageous. (3) The outcomes would not vary while using either MSCs or bone marrow aspirate concentrate (BMAC). Additionally, in the subgroup analysis, we found that stem cell therapy could lowering the rate of collapse, radiographic progression and THA conversion on a mid-term basis. We also confirmed the necessity of subchondral mechanical support after CD for its advantage in avoiding collapse and disease progression. Thirdly, the utilization of a specific type of stem cell was indicated to be more efficient than BMAC.
Core decompression is a hip-preserving surgical technique that aims to mitigate edema and improve circulation of the femoral head by decreasing intraosseous pressure, and thus, it has the potential to prevent or postpone THA [42]. Conversely, the clinical results of CD alone were still controversial in the current literature because of the inconclusive success rate especially for cases of collapse stage [43]. Mont [44] reported a success of only 47% in ARCO stage III cases. Similarly, Song [45] reported the survival rate of Ficat stage I, II and III was 79%, 77% and 35%, respectively, in a study with a minimum 5-year follow-up. These unsatisfying results could be attributed to large diameter core decompression, deprivation of regional MSCs, inaccurate or incomplete bone graft compaction or early postoperative weight-bearing [24].
In recent decades, enthusiasm has been aroused for applying osteogenic precursors to necrotic lesions in ANFH for their capacity to differentiate to diverse cell lineages. The scientific foundation underlying stem cell therapy is to provide osteoprogenitor and vascular progenitor cells to facilitate bone remodeling and repair in the necrotic area [46]. To add to that, strategies that stimulate and enhance the mobilization and homing capacity of MSCs also attracted growing interests [47]. Individual studies of stem cell therapy combined with CD revealed promising results. Gangji [30] claimed that the strategy of stem cell application afforded a significant improved hip function, reduced volume of necrotic lesions, and delayed radiographic progression. 25-year study conducted by Hernigou [35] indicated that stem cell therapy reduced collapse and THA conversion rate while comparing with CD alone. Our previous 10-year result also favored the employment of stem cell since it provided better subjective assessment scores and longer average survival time[37]. These were in accordance with the results of our meta-analysis that stem cell augmentation plus CD reduced the collapse rate by 2.97 times, the radiographic progression rate by 3.52 times and THA conversion rate by 2.85 times compared with CD alone. Nan et al. had demonstrated that resveratrol (Res) can potentially reverse abnormal osteogenesis during ANFH by suppressing osteoclastogenesis via modulating levels of sirtuin1 (Sirt1), nuclear transcription factor-κB (NF-κB), and receptor activator of NF-κB ligand (RANKL) [48]. Zhang et al. found that during treatment of ANFH with BMSCs, the transplanted cells underwent significant stress-induced apoptosis and senescence in the oxidative stress microenvironment of the necrotic area, significantly limiting their efficacy. Subsequent studies by the authors revealed that upregulation of Parkin and downregulation of P53 in BMSCs effectively counteracted stress-induced apoptosis and senescence and improved the therapeutic effect of BMSC transplantation in early steroid-induced ANFH [49]. All of these findings provided new avenues for the subsequent treatment of ANFH.
Nonetheless, owing to the lack of a standardization in the regard of the qualitative and quantitative guidelines of the harvest methods, processing and transplantation of cells, there was a dramatic heterogeneity in the current published studies. Various mesenchymal cells were applied in the hip-preserving procedures, including BMMSCs, BMMCs, PBSCs, human umbilical cord mesenchymal stem cells, etc. [50, 51]. These cells would promote the secretion of osteogenic and angiogenic factors in the necrotic area [52]. Aside from those, BMAC, proposed by Hernigou [53] firstly, was preferred in numerous studies because of its convenience in harvesting and processing. BMAC is indicated to provide higher concentration of chondrogenic, affirmative stromal cells, lymphocytes, neutrophils, monocytes, and platelets in various stages of differentiation [54, 55]. However, it should be noticed that the number of cells in BMAC range from 90,000 to 3.46 × 109 and the transplanted volume varied from 1 to 60 ml. Moreover, most of the cells in BMAC are not mesenchymal or vascular progenitor cells [3]. We suspected that it might attributed to the ambiguous results of CD plus BMAC therapy with respect of collapse rate and progression rate while comparing with CD alone in our pooled data.
With the concern of subchondral structural weakness after CD, mechanical augmentation is another fundamental approach in the treatment of ANFH [56]. There are various choices to enhance the mechanical support, including vascularized fibula grafts, autologous cancellous bone grafts, allografts and porous tantalum, etc. [57, 58]. Chen [59] reported the optimizing mid- and long-term results of bone graft impaction through the CD track, especially for those early pre-collapse cases. In addition to structural enhancement, bone grafts also provide a microenvironment for bone remodeling and angiogenesis [60]. Dou [61] founded that porous tantalum could promote the proliferation and adhesion of BMSCs via activation of the MAPK/ERK pathway, so that it could up-regulate the expression of osteogenic genes and promote the osteogenic differentiation of BMSCs in vitro. Our pooled data also supported mechanical enhancing procedures for lowering the risk of disease progression. It is worthy noticing the utilization of synthesized and bio-inductive material. Liu [24] proposed a 10-mm single drilling technique in combination with granular porous medical nano-hydroxyapatite / polyamide 66 composite bone filling material transplantation and reported promising clinical results. Previously, our center introduced angioconductive bioceramic rod grafting combined with BMAC to treat early-stage cases and reported satisfying results of improved hip function and a higher survivorship as well [38].
Our pooled data in subgroup analysis based on the duration of follow-up favored the use of stem cell therapy, which is in accordance with the current literature [62]. Conversely, the efficacy of stem cell augmentation was inconclusive on a short-term basis, which was consistent with the systematic review of Andronic [41]. For the studies with a follow-up of 2 years, Hauzeur [10] only included the cases of ARCO stage III and more than a half with the etiology of corticosteroid-use. Similarly, corticosteroid-use and idiopathic factor took high proportion for the risk factor of patients included in Rastogi’s study [39]. As was revealed in the current literature, corticosteroid would influence the treatment outcome because MSCs in these patients not only had impaired activity but also tend to differentiate into adipose cells instead of osteoblasts, by imposing adverse effects on bone matrix, cell apoptosis, lipid metabolism and angiogenesis [63,64,65]. Therefore, this etiology was considered a negative prognostic factor for hip preservation. In regard of the long-term results, although Hernigou [35] reported a lower collapse rate and THA conversion rate, it was not consistent with Li [37] and Lim [27]. Future studies with follow-up longer than 5 years and larger sample sizes may provide more persuasive evidence.
This systematic review and meta-analysis had some limitations. First, due to the limited RCTs, we included 7 retrospective studies with good quality. Despite of this, the enrolled numbers of patients of hips was still small. Therefore, it needs large sample size, multi-center, prospective, randomized controlled studies to test and verify this inference. Second, although we performed subgroup analysis to balance the heterogeneity of follow-up duration, surgical technique and type of cell therapy, the approach of bone grafting and the numbers of cells in the treatment were still diverse. Thirdly, all the included studies reported the positive outcomes of stem cell therapy, which might introduce publication bias. Fourth, the included studies involved various types of scoring system and the data were reported in different forms, and thus, we did not extract and synthesize the quantitative data. Additionally, although two investigators reviewed the results and data based on the standardized form and came to an agreement, search bias and extractor bias may still have occurred. Last but not least, we only included studies published in English which would lead to language bias.
Conclusion
Stem cell therapy combined with core decompression was more effective in preventing collapse, radiographic progression and conversion to THA.
Availability of data and materials
The data and materials used and/or analyzed during the current study are not publicly available but available from the corresponding author on reasonable request.
Abbreviations
- ANFH:
-
Avascular necrosis of the femoral head
- ARCO:
-
Association Research Circulation Osseous
- BMAC:
-
Bone marrow aspirate concentrate
- BMMCs:
-
Bone marrow mononuclear cells
- BMMSCs:
-
Bone marrow mesenchymal stem cells
- CD:
-
Core decompression
- CG:
-
Control group
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- HHS:
-
Harris hip score
- JOA:
-
Japanese Orthopaedic Association
- LoE:
-
Level of evidence
- MAP:
-
Merle D’Aubigné and Postel score
- ANFH:
-
Avascular necrosis of the femoral head
- OR:
-
Odd Ratio
- PBSCs:
-
Peripheral blood stem cells
- SG:
-
Stem cell group
- VAS:
-
Visual analogue scale
- WOMAC:
-
Western Ontario and McMaster Universities Arthritis Index osteoarthritis scoring
References
Zhao D, Zhang F, Wang B, et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J Orthop Translat. 2020;21:100–10.
Hua K-C, Yang X-G, Feng J-T, et al. The efficacy and safety of core decompression for the treatment of femoral head necrosis: a systematic review and meta-analysis. J Orthop Surg Res. 2019;14(1):306.
Mont MA, Salem HS, Piuzzi NS, et al. Nontraumatic osteonecrosis of the femoral head: where do we stand today?: A 5-year update. J Bone Joint Surg Am. 2020;102(12):1084–99.
Magill P, Blaney J, Hill JC, et al. Impact of a learning curve on the survivorship of 4802 cementless total hip arthroplasties. Bone Jt J. 2016;98B(12):1589–96.
Xu Y, Jiang Y, Xia C, et al. Stem cell therapy for osteonecrosis of femoral head: opportunities and challenges. Regenerative therapy. 2020;15:295–304.
Migliorini F, La Padula G, Oliva F, et al. Operative management of avascular necrosis of the femoral head in skeletally immature patients: a systematic review. Life (Basel). 2022;12(2).
Quaranta M, Miranda L, Oliva F, et al. Osteotomies for avascular necrosis of the femoral head. Br Med Bull. 2021;137(1).
Sadile F, Bernasconi A, Russo S, et al. Core decompression versus other joint preserving treatments for osteonecrosis of the femoral head: a meta-analysis. Br Med Bull. 2016;118(1):33–49.
Steinberg ME, Larcom PG, Strafford B, et al. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–8.
Hauzeur J-P, De Maertelaer V, Baudoux E, et al. Inefficacy of autologous bone marrow concentrate in stage three osteonecrosis: a randomized controlled double-blind trial. Int Orthop. 2018;42(7):1429–35.
Migliorini F, Maffulli N, Baroncini A, et al. Prognostic factors in the management of osteonecrosis of the femoral head: A systematic review. Surgeon. 2023;21(2):85–98.
Hernigou P, Bernaudin F, Reinert P, et al. Bone-marrow transplantation in sickle-cell disease. Effect on osteonecrosis: a case report with a four-year follow-up. J Bone Joint Surg Am. 1997;79(11):1726–30.
Emadedin M, Karimi S, Karimi A, et al. Autologous bone marrow-derived CD133 cells with core decompression as a novel treatment method for femoral head osteonecrosis: a pilot study. Cytotherapy. 2019;21(1):107–12.
Migliorini F, Maffulli N, Eschweiler J, et al. Core decompression isolated or combined with bone marrow-derived cell therapies for femoral head osteonecrosis. Expert Opin Biol Ther. 2021;21(3):423–30.
Saini U, Jindal K, Rana A, et al. Core decompression combined with intralesional autologous bone marrow derived cell therapies for osteonecrosis of the femoral head in adults: a systematic review and meta-analysis. Surgeon. 2023;21(3):e104–17.
Li J, Su P, Li J, et al. Efficacy and safety of stem cell combination therapy for osteonecrosis of the femoral head: a systematic review and meta-analysis. J Healthcare Eng. 2021;2021:9313201.
Migliorini F, Maffulli N, Baroncini A, et al. Failure and progression to total hip arthroplasty among the treatments for femoral head osteonecrosis: a Bayesian network meta-analysis. Br Med Bull. 2021;138(1):112–25.
Mao L, Jiang P, Lei X, et al. Efficacy and safety of stem cell therapy for the early-stage osteonecrosis of femoral head: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res Ther. 2020;11(1):445.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
JPT H, J T, J C, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) 2021. www.training.cochrane.org/handbook.
Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Yamasaki T, Yasunaga Y, Ishikawa M, et al. Bone-marrow-derived mononuclear cells with a porous hydroxyapatite scaffold for the treatment of osteonecrosis of the femoral head: a preliminary study. J Bone Joint Surg Br. 2010;92(3):337–41.
Liu Y, Liu S, Su X. Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2013;133(1):125–33.
Pilge H, Bittersohl B, Schneppendahl J, et al. Bone marrow aspirate concentrate in combination with intravenous iloprost increases bone healing in patients with avascular necrosis of the femoral head: a matched pair analysis. Orthop Rev (Pavia). 2016;8(4):6902.
Kang JS, Suh YJ, Moon KH, et al. Clinical efficiency of bone marrow mesenchymal stem cell implantation for osteonecrosis of the femoral head: a matched pair control study with simple core decompression. Stem Cell Res Ther. 2018;9(1):274.
Lim YW, Kim YS, Lee JW, et al. Stem cell implantation for osteonecrosis of the femoral head. Exp Mol Med. 2013;45: e61.
Sen RK, Tripathy SK, Aggarwal S, et al. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012;27(5):679–86.
Ma Y, Wang T, Liao J, et al. Efficacy of autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of femoral head: a prospective, double-blinded, randomized, controlled study. Stem Cell Res Ther. 2014;5(5):115.
Gangji V, De Maertelaer V, Hauzeur J-P. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–9.
Zhao D, Cui D, Wang B, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30.
Tabatabaee RM, Saberi S, Parvizi J, et al. Combining concentrated autologous bone marrow stem cells injection with core decompression improves outcome for patients with early-stage osteonecrosis of the femoral head: a comparative study. J Arthroplasty. 2015;30(9 Suppl):11–5.
Mao Q, Wang W, Xu T, et al. Combination treatment of biomechanical support and targeted intra-arterial infusion of peripheral blood stem cells mobilized by granulocyte-colony stimulating factor for the osteonecrosis of the femoral head: a randomized controlled clinical trial. J Bone Miner Res. 2015;30(4):647–56.
Pepke W, Kasten P, Beckmann NA, et al. Core decompression and autologous bone marrow concentrate for treatment of femoral head osteonecrosis: a randomized prospective study. Orthop Rev (Pavia). 2016;8(1):6162.
Hernigou P, Dubory A, Homma Y, et al. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop. 2018;42(7):1639–49.
Cruz-Pardos A, Garcia-Rey E, Ortega-Chamarro JA, et al. Mid-term comparative outcomes of autologous bone-marrow concentration to treat osteonecrosis of the femoral head in standard practice. Hip Int. 2016;26(5):432–7.
Li M, Ma Y, Fu G, et al. 10-year follow-up results of the prospective, double-blinded, randomized, controlled study on autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of the femoral head. Stem Cell Res Ther. 2020;11(1):287.
Li Q, Liao W, Fu G, et al. Combining autologous bone marrow buffy coat and angioconductive bioceramic rod grafting with advanced core decompression improves short-term outcomes in early avascular necrosis of the femoral head: a prospective, randomized, comparative study. Stem Cell Res Ther. 2021;12(1):354.
Rastogi S, Sankineani SR, Nag HL, et al. Intralesional autologous mesenchymal stem cells in management of osteonecrosis of femur: a preliminary study. Musculoskelet Surg. 2013;97(3):223–8.
Piuzzi NS, Chahla J, Jiandong H, et al. Analysis of cell therapies used in clinical trials for the treatment of osteonecrosis of the femoral head: a systematic review of the literature. J Arthroplasty. 2017;32(8):2612–8.
Andronic O, Hincapié CA, Burkhard MD, et al. Lack of conclusive evidence of the benefit of biologic augmentation in core decompression for nontraumatic osteonecrosis of the femoral head: a systematic review. Arthroscopy. 2021;37(12).
Cohen-Rosenblum A, Cui Q. Osteonecrosis of the femoral head. Orthop Clin North Am. 2019;50(2):139–49.
Atilla B, Bakırcıoğlu S, Shope AJ, et al. Joint-preserving procedures for osteonecrosis of the femoral head. EFORT Open Rev. 2019;4(12):647–58.
Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–78.
Song WS, Yoo JJ, Kim Y-M, et al. Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res. 2007;454:139–46.
Goodman SB. The biological basis for concentrated iliac crest aspirate to enhance core decompression in the treatment of osteonecrosis. Int Orthop. 2018;42(7):1705–9.
Lin W, Xu L, Zwingenberger S, et al. Mesenchymal stem cells homing to improve bone healing. J Orthop Translat. 2017;9:19–27.
Nan K, Pei J-P, Fan L-H, et al. Resveratrol prevents steroid-induced osteonecrosis of the femoral head via miR-146a modulation. Ann N Y Acad Sci. 2021;1503(1):23–37.
Zhang F, Peng W, Zhang J, et al. P53 and Parkin co-regulate mitophagy in bone marrow mesenchymal stem cells to promote the repair of early steroid-induced osteonecrosis of the femoral head. Cell Death Dis. 2020;11(1):42.
Zhao J, Meng H, Liao S, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells in early traumatic osteonecrosis of the femoral head. J Orthop Translat. 2022;37:126–42.
Cao H, Guan H, Lai Y, et al. Review of various treatment options and potential therapies for osteonecrosis of the femoral head. J Orthop Translat. 2016;4:57–70.
Xu H, Wang C, Liu C, et al. Cotransplantation of mesenchymal stem cells and endothelial progenitor cells for treating steroid-induced osteonecrosis of the femoral head. Stem Cells Transl Med. 2021;10(5):781–96.
Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23.
Chiang H, Hsieh C-H, Lin Y-H, et al. Differences between chondrocytes and bone marrow-derived chondrogenic cells. Tissue Eng Part A. 2011;17(23–24):2919–29.
Filardo G, Madry H, Jelic M, et al. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717–29.
Phemister DB. Treatment of the necrotic head of the femur in adults. J Bone Joint Surg Am. 1949;31A(1):55–66.
Pierce TP, Elmallah RK, Jauregui JJ, et al. A current review of non-vascularized bone grafting in osteonecrosis of the femoral head. Curr Rev Musculoskelet Med. 2015;8(3):240–5.
Wei BF, Ge XH. Treatment of osteonecrosis of the femoral head with core decompression and bone grafting. Hip Int J Clin Exp Res Hip Pathol Therapy. 2011;21(2):206–10.
Chen L, Hong G, Hong Z, et al. Optimizing indications of impacting bone allograft transplantation in osteonecrosis of the femoral head. Bone Joint J. 2020;102B(7):838–44.
Sultan AA, Khlopas A, Surace P, et al. The use of non-vascularized bone grafts to treat osteonecrosis of the femoral head: indications, techniques, and outcomes. Int Orthop. 2019;43(6):1315–20.
Dou X, Wei X, Liu G, et al. Effect of porous tantalum on promoting the osteogenic differentiation of bone marrow mesenchymal stem cells in vitro through the MAPK/ERK signal pathway. J Orthop Translat. 2019;19:81–93.
Zhang C, Fang X, Huang Z, et al. Addition of bone marrow stem cells therapy achieves better clinical outcomes and lower rates of disease progression compared with core decompression alone for early stage osteonecrosis of the femoral head: a systematic review and meta-analysis. J Am Acad Orthop Surg. 2020;28(23):973–9.
Houdek MT, Wyles CC, Packard BD, et al. Decreased Osteogenic Activity of Mesenchymal Stem Cells in Patients With Corticosteroid-Induced Osteonecrosis of the Femoral Head. J Arthroplasty. 2016;31(4):893–8.
Larson E, Jones LC, Goodman SB, et al. Early-stage osteonecrosis of the femoral head: where are we and where are we going in year 2018? Int Orthop. 2018;42(7):1723–8.
Xie X-H, Wang X-L, Yang H-L, et al. Steroid-associated osteonecrosis: Epidemiology, pathophysiology, animal model, prevention, and potential treatments (an overview). J Orthop Translat. 2015;3(2):58–70.
Acknowledgements
Not applicable. All authors were agreed on the search and data extraction.
Funding
This study was supported by Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515110511), the Program of Administration of Traditional Chinese Medicine of Guangdong Province (No. 20212033) and the Start-up Funding of National Natural Science Foundation of China (No. 8210090461 and 8200090080).
Author information
Authors and Affiliations
Contributions
Study design was contributed by MZ and QZ. Administrative support was contributed by QZ and YM. Study conduct was contributed by ML and DC. Data collection was contributed by ML and DC. Data analysis and data interpretation were contributed by ML and DC. Drafting manuscript was contributed by ML and DC. Revising manuscript content was contributed by ML, MZ, and QZ. Approving final version of manuscript was contributed by MZ and QZ. MZ and QZ took responsibility for the integrity of the data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1:
Funnel plots of the rate of collapse. Subgroup analysis according to (A) the duration of follow-up in 2 years, (B) the duration of follow-up in 2–5 years, (C) with structural support, (D) without structural support, (E) stem cell therapy of the BMAC group, (F) stem cell therapy of the BMMSCs/BMMSs/PBSCs group. (BMAC bone marrow aspirate concentrate, BMMCs bone marrow mononuclear cells, BMMSCs bone marrow mesenchymal stem cells, PBSCs peripheral blood stem cells).
Additional file 2: Figure S2:
Funnel plots of the rate of radiographic progression. Subgroup analysis according to (A) the duration of follow-up in 2 years, (B) the duration of follow-up in 2–5 years, (C) with structural support, (D) without structural support, (E) stem cell therapy of the BMAC group, (F) stem cell therapy of the BMMSCs/BMMSs/PBSCs group. (BMAC bone marrow aspirate concentrate, BMMCs bone marrow mononuclear cells, BMMSCs bone marrow mesenchymal stem cells, PBSCs peripheral blood stem cells).
Additional file 3: Figure S3:
Funnel plots of the rate of radiographic progression. Subgroup analysis according to (A) the duration of follow-up in 2 years, (B) the duration of follow-up in 2–5 years, (C) the duration of follow-up longer than 5 years, (D) with structural support, (E) without structural support, (F) stem cell therapy of the BMAC group, (G) stem cell therapy of the BMMSCs/BMMSs/PBSCs group. (BMAC bone marrow aspirate concentrate, BMMCs bone marrow mononuclear cells, BMMSCs bone marrow mesenchymal stem cells, PBSCs peripheral blood stem cells). (PDF 1870 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, M., Chen, D., Ma, Y. et al. Stem cell therapy combined with core decompression versus core decompression alone in the treatment of avascular necrosis of the femoral head: a systematic review and meta-analysis. J Orthop Surg Res 18, 560 (2023). https://doi.org/10.1186/s13018-023-04025-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04025-8