Abstract
Background
We analysed the clinical, biological, radiological profiles, and therapeutic patterns of the patients who underwent a surgical lower extremity amputation (LEA) in Togo from 2010 to 2020.
Methods
Retrospective analysis of clinical files of adult patients who underwent an LEA at a single centre (Sylvanus Olympio Teaching Hospital) from 1st January 2010 to 31st December 2020. Data were analysed by CDC Epi Info Version 7 and Microsoft Office Excel 2013 software.
Results
We included 245 cases. The mean age was 59.62 years (15.22 SD) (range: 15–90 years). The sex ratio was 1.99. The medical history of diabetes mellitus (DM) was found in 143/222 (64.41%) files. The amputation level found in 241/245 (98.37%) files was the leg in 133/241 (55.19%) patients, the knee in 14/241 (5.81%), the thigh in 83/241 (34.44%), and the foot in 11/241 (4.56%). The 143 patients with DM who underwent LEA had infectious and vascular diseases. Patients with previous LEAs were more likely to have the same limb affected than the contralateral one. The odds of trauma as an indication for LEA were twice as high in patients younger than 65 years compared to the older (OR = 2.095, 95% CI = 1.050–4.183). The mortality rate after LEA was 17/238 (7.14%). There was no significant difference between age, sex, presence or absence of DM, and early postoperative complications (P = 0.77; 0.96; 0.97). The mean duration of hospitalization marked in 241/245 (98.37%) files was 36.30 (1–278) days (36.20 SD). Patients with LEAs due to trauma had a significantly longer hospital admission than those with non-traumatic indications, F (3,237) = 5.505, P = 0.001.
Conclusions
Compared to previous decades, from 2010 to 2020, the average incidence of LEAs for all causes at Sylvanus Olympio Teaching Hospital (Lomé, Togo) decreased while the percentage of patients with DM who underwent LEAs increased. This setting imposes a multidisciplinary approach and information campaigns to prevent DM, cardiovascular diseases, and relative complications.
Similar content being viewed by others
Background
The term “amputation” derives from the Latin word “amputare” (“to excise” or “to cut out”), which means the removal of part or all of a body part delimited by the skin. Lower extremity amputation (LEA) consists of removing lower limbs by accident or surgery [1]. LEA is a severely disabling condition that imposes variable physical and psychological challenges as it entails lifestyle changes, self-concept alterations, impairments in physical functioning, and sensory effects [2,3,4,5]. LEA can occur as complications of trauma or underlying diseases, such as infections, tumours, cardiovascular or vascular diseases, especially diabetes mellitus (DM), and neglected congenital conditions [6, 7]. The incidence of LEA varied from 8.8 per 100.000 people in the Netherlands in 2004 to 92.5 per 100.000 in Ireland in 2009 [8, 9]. However, in Western countries, LEA has been recently reported as declining [10,11,12]. In Africa, the epidemiological studies about LEA for all the causes are limited. Although the predominant aetiologies of LEAs in the continent have been reported as trauma, infections of wounds, and leg ulcers [13, 14], recent data have referred most to DM. The incidence of DM-driven LEAs in Ghana increased from 0.6 in 2010 to 10.9 in 2015 [15], in Malawi, the rate was 10.6% in 2008–2016 [16] and, in South Africa, 53.7% in 2013–2018 [17]. In Nigeria, the LEA rate in patients with diabetic foot ulcer disease was 35.4% during 2016–2017 [18]. In the review of Abbas et al. (1960–2020), the amputation rate in patients with diabetic foot ranged from 3 to 61% [19]. In Togo, Abalo et al. reported a predominance of vascular causes of LEA from January 2000 to December 2004; among these vascular disorders, DM accounted for 48.2% [20]. In 2017, Amouzou et al. showed that all the LEAs due to leg ulcers were complications of diabetic foot [21].
The present study has described the clinical, biological, radiological profiles and therapeutic patterns of patients who underwent LEA in Togo from 2010 to 2020. In particular, we have analysed the characteristics of patients with DM who had LEA at Sylvanus Olympio Teaching Hospital (SOTH), which is the only one of the three tertiary hospitals in Togo equipped with a Wound Healing Unit.
Methods
This retrospective study was based on data retrieved from clinical files of adult (> 15-year-old) patients admitted and treated in the Wound Healing Unit and Traumatology-Orthopaedics department of Sylvanus Olympio Teaching Hospital (SOTH), in Lomé, the capital of Togo, from 1st January 2010 to 31st December 2020. Togo is a country in West Africa of 56,600 km2, with a population of 8,849,000 in 2022, among which 5% were above 60 years. Togo was the first country to eradicate four tropical diseases (dracuncunculiasis, lymphatic filariasis, human African trypanosomiasis, and trachoma) [22]. Togo’s health system is relatively well-equipped in terms of infrastructures, and 70.9% of the population has access to facilities. However, geographical, economic, and social disparities regarding the supply and accessibility of essential health care persist. Analysis of the distribution of human resources for health indicates that most of the health workforce is in the capital; rural areas are disadvantaged in this respect [23]. The health system in Togo is pyramidal with three levels: central, intermediate, and peripheral. The base represents the peripheral level with district hospitals, maternal and infant protection centres (PMI), and peripheral care units (USP). The middle of the pyramid represents the intermediate or regional level and corresponds to six health regions with six regional hospitals. Finally, the top of the pyramid, called the central or national level, encompasses the office of the Minister of Health, its national directions, and the three teaching hospitals. Two of the three teaching hospitals are in the capital, among which is SOTH, and one in the North of the country in Kara.
The patients admitted at SOTH enrolled in the current study came from Lomé and its surrounding areas.
This study was conducted according to the World Medical Association’s Declaration of Helsinki (1964, version 2013) [24], Good Clinical Practice, and approved by the medical. Institutional Board of SOTH. The drafting of the manuscript complied with the STROBE guidelines [25].
From the clinical files, we retrieved the following data:
-
Socio-demographic parameters Age (“aged” patients were those 65-year-old and older), sex, job;
-
Past medical conditions and chronic diseases DM, hypertension, sickle cell disease;
-
Charlson Comorbidity Index (CCI) was calculated from the files, and distributed in 4 groups: 0 (no comorbidity), [1–4], ≥ 5 [26, 27];
-
Clinical characteristics Ischaemic or wet gangrene, tumour, crush syndrome;
-
Biological parameters Haemoglobin rate, white cells count;
-
Radiological findings Vascular lower limb ultrasonography;
-
Histological nature of the amputated part;
-
Therapeutic aspects and operative records Type of anaesthesia, side of amputation, amputation level (minor amputation: toe or part of the foot; major amputation: above and below knee), duration between indication and surgery, duration of hospital admission;
-
Operative course Immediate and early postoperative complications (present or absent), which included death, psychological troubles, wound infections (surgical site infections), the persistence of ischaemia, and disorders on the contralateral limb. The immediate complications were defined as those that happened in the first 24 h after surgery and the early those that happened from the first 24 h after surgery to the supposed date wound healing;
-
Clavien–Dindo classification modified for foot and ankle surgery Ia (likely negligible), Ib (likely minimal), IIa (likely minimal), IIb (likely significant), IIIa (likely minimal), IIb (likely significant), IV, V (serious) [28, 29].
We included clinical files of all adult patients who underwent an LEA at SOTH, of both sexes. We excluded missing or incomplete clinical files. We defined the incomplete files as those with more than half of the above parameters missing.
We performed a descriptive statistical analysis of the data with CDC Epi Info Version 7.2.0.1 and Microsoft Office Excel 2013 software and provided the Chi-square value, degree of freedom, Fisher test, Odds Ratio (OR), confidence interval, and cell value P, if necessary. The cell values under 5% were considered significant.
We used Microsoft Office Excel 2013 software for drafting tables and figures. We used the mean value to measure the age of the patients, rate of haemoglobin, hospital stay, and duration from indication to LEA.
Results
General data
In the study period of 11 years (2010–2020), a total of 10,731 patients were admitted to the Traumatology-Orthopaedics department and Wound Healing Unit of Sylvanus Olympio Teaching Hospital. LEA was conducted in 363/10731 (3.38%) patients, and the average number of LEAs per year was 33. Figure 1 displays the distribution of all the patients admitted to both the Traumatology-Orthopaedics department and Wound Healing Unit (Fig. 1A), to the only Traumatology-Orthopaedics department (Fig. 1B), and the only Wound Healing Unit (Fig. 1C).
The clinical files of 98/363 (27%) patients operated on for LEA were missing. Of the 363 clinical files, 20 (5.51%) had incomplete information, and 245 (67.49%) contained enough information to be included in this study. Figure 2 shows the flow diagram of the participants throughout the study phases.
Demographic data
The mean age of the 245 patients who underwent LEA was 59.62 years (15.22 SD) (range: 15–90 years). Figure 3A displays the age distribution; patients more than 65-year-old accounted for 94/245 (38.37%).
Among the various patients’ professions, tailoring was the most frequent (22.86%, P = 0.023) (Table 1).
The sex ratio of our patient population was 1.99. There was a statistically significant difference between male (163, 66.53%) and female patients (82, 33.47%) (P < 0.001).
Medical and social history data
In the medical history of the 245 patients included in this study, 26 (10.61%) had a previous LEA. The previous LEAs of the 26 patients were minor (50%) and major (50%) amputations.
Of the 245 clinical files, the past medical conditions were missing in 23 (9.39%) clinical files and present in 222 (90.61%). Of the 222 clinical files with data about past medical conditions, 143 patients had DM. Of them, 94 (42.34%) had DM alone, and 49 (22.07%) had DM associated with other comorbidities (Fig. 3B). Two hundred and four out of 222 (91.90%) patients had comorbidity for more than one year, 6 (2.70%) between 6 months and one year, and 12 (5.40%) for less than 6 months. The Charlson Comorbidly Index (CCI) was 0 in 18.92%, and below 2 in 89.19% (Fig. 3C).
Twenty-six out of 222 (11.71) patients had previous LEAs; of them, 20 (76.92%) were diabetic, and 6 (23.08%) were non-diabetic.
Clinical, biological, radiological data and LEA causes
Gangrene was the clinical presentation of the limb at hospitalization in 212/245 (86.53%) patients. Other clinical presentations were crush syndrome in 23/245 (9.39%), bone tumour in 5/245 (2.04%), osteomyelitis in 2/245 (0.82%), chronic ulcer in 2/245 (0.82%), and necrotizing fasciitis in 1/245 (0.40%) cases. Figure 4 shows the clinical aspects of some patients who underwent LEA. We found the data about the type of gangrene in 194/212 (91.51%) clinical files; of them, 71 (36.60%) were ischaemic gangrene, and 123 (63.40%) were wet. Of the 123 patients with wet gangrene, 16 (13.01%) were with gas gangrene.
The 26 patients with previous amputations were more likely to have the same limb affected than the contralateral limb. The ipsilateral limb was amputated in 57.7% of patients compared to 42.5% of those with a contralateral limb (P < 0.001). The contralateral limb was amputated in 23.08% of patients after a minor amputation and in 53.85% after a major one.
Previous amputation was less common in patients with traumatic indications (5.9%) than in those with non-traumatic (11.9%), yet this difference did not reach statistical significance ((OR = 0.465, 95% CI = 0.134–1.614), P = 0.308).
A complete blood count was registered in 235/245 (95.92%) clinical files. Patients with hyperleukocytosis accounted for 153/235 (65.11%). The count of the white blood cells was in the normal range for 80/235 (34.04%) patients; 2/235 (0.85%) patients had leukopenia. The average count of white blood cells was 15,646 elements/mm3 (9.25DS) (range: 3200–44,700). The rate of white cell anomalies (leukopenia and hyperleukocytosis) was significantly higher in patients with DM compared to those without DM (98% vs. 43%, P = 0.01). Patients with infection had the highest white blood cell counts, while those with tumours had the lowest haemoglobin (Table 2). The mean rate of haemoglobin was 9.33 g/dl (2.38DS) (range: 2.5–17); 36 out of 235 patients had a haemoglobin rate under 7 g/dl.
Doppler-ultrasound was performed in 91/245 (37.14%) patients; 18/91 (19.78%) patients were in the normal range, 42/91 (46.15%) displayed arterial abnormality (peripheral artery disease), 6/91 (6.59%) had venous disorders (superficial insufficiency or deep venous thrombosis), and 25/91 (27.48%) a combined artery and venous disorders.
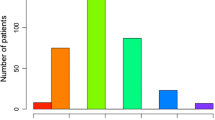
The causes of LEA were infections in 143/245 (58.37%) cases and bone or skin tumours in 5/245 (2.04%) (Fig. 5A). No data about histology types of the tumour was documented. The 143 patients with DM who underwent LEA were involved in infectious and vascular aetiologies.
Numerical distribution of patients by causes of LEA (N = 245) (A), number of traumatic or nontraumatic LEA according to the range of patient’s age (< 65 years or > 65 years) (N = 245) (B), numerical distribution of the early complications (N = 238) (C), and the distribution of complications according to the modified Clavien–Dindo classification for foot and ankle surgery (D)
When we categorized age into less or more than 65 years, there was a significant association between age and the cause of LEA. Patients younger than 65-year-old had significantly more traumatic indications for LEA than those older (P = 0.023). The odds of trauma as an indication for LEA were twice as high in those younger than 65 years compared to the older (OR = 2.095, 95% CI = 1.050–4.183). Figure 5B shows the relationship between traumatic indications for LEA and the patient’s age.
Therapeutic data
Spinal anaesthesia was administered in 242/245 (98.78%) patients and general anaesthesia to 3/245 (1.22%). The side of the LEA was reported in 239/245 (97.55%) clinical files and missing in 6/245 (2.45%). LEA was left in 116/239 (48.54%) patients and right in 123/239 (51.46%). We found data about the amputation level in 241/245 (98.37%) clinical files: leg in 133/241 (55.19%) patients, knee in 14/241 (5.81%), thigh in 83/241 (34.44%), and foot in 11/241 (4.56%). There was no significant association between the aetiology of the LEA and the amputation level (P = 0.052) (Table 3). The amputation level below the knee was significantly higher in patients with DM than in those without (96% vs. 39%, P = 0.01).
The mean duration from the indication of amputation to the surgery found in 239/245 (97.55%) clinical files was 6.75 (1–78) days (11.59 SD).
In the 237 out of 245 (96.73%) clinical files with recorded postoperative course, there was no immediate postoperative complications; there was a postoperative complication in 3/237 (1.27%); specifically, two patients had a stroke after the surgery, and one had a perioperative shock that raised favourably on medical treatment. Data regarding early postoperative complications were available in 238/245 (97.14%) clinical files. Complications were present in 56/238 (23.53%); the type of complications was reported in 47/56 (83.92%) clinical files and missing in 9/56 (16.08%) (Fig. 5C). According to Clavien–Dindo classification modified for foot and ankle surgery, the complications were likely minimal in 60%, and serious (life-threatening or involved central nervous system) in 40% (Fig. 5D).
Early postoperative complications did not significantly differ according to age (P = 0.77), sex (P = 0.96), level of amputation (P = 0.05), and presence or absence of DM (P = 0.97) (Table 4).
The mortality rate after LEA was 17/238 (7.14%). The mean hospital stay was marked in 241/245 (98.37%) clinical files and consisted of 36.30 (1–278) days (36.20 SD). The average hospital duration was 34.27 (27.86 SD) days for infections, 19.60 (20.62SD) for tumour, 32.44 (25.05 SD) for vascular aetiologies, and 63.25 (78.02 SD) for traumatic aetiologies. Patients with LEAs due to trauma had significantly longer hospital admission than those with non-traumatic indications (F (3,237) = 5.505, P = 0.001).
Discussion
Our study has provided the updated pattern of LEA in Togo based on the data collected between 2010 and 2020 at the country’s main hospital (SOTH). The results have shown an overall improvement, but the causes of LEA and the socio-demographic features of the patients changed compared with the previous decades. However, the present study had an incomplete series of clinical files with data available for analyses.
Considering the increase of the population of Togo from 5.01 million in 2000, 6.57 million in 2010 to 8.44 million in 2020 [20, 30], the average number of LEA per year of 33 LEA found in our study has decreased compared to that previously reported of 32 by Abalo et al. in 2004. Therefore, in the last decades, the prevention of LEA in our country improved. However, the most remarkable increase in patients who underwent LEA occurred in the Wound Healing Unit of SOTH (Fig. 1C), especially from 2013 to 2020. This increase can be due to the more conservatory management of open injuries of lower limbs conducted in the Traumatology-orthopaedic department by the Plastic surgery team, which reduced the rate of traumatic LEAs. Although the Togolese ortho-plastic team was young, the protocol for the reconstruction of severe open fractures as in international guidelines was the radical “ortho-plastic” treatment with radical debridement, skeletal stabilization and soft tissue coverage [31]. Moreover, the first microvascular free gracilis microvascular reconstruction in April 2018 introduced microsurgery in Togo [32]. This intervention provided a relevant improvement and started a new era in the management of open injuries of lower limbs in our country.
Of the 363 patients who underwent LEA at SOTH during the study period (2010–2020), we found 245 folders available for analysis. Therefore, the missed data represent a bias of the present study given the considerable number of absent or incomplete clinical files. Moreover, the incomplete series of sociodemographic or clinical characteristics of inpatient cases, such as marital status, address, religion, ethnic group, residual limb length, or phantom limb sensation, prevented us from assessing a possible association between these characteristics and LEA and reduced the range of feasible statistical tests. However, the information obtained from the complete clinical files accounted for relevant features of the LEA in our setting.
The mean age of our patients who underwent LEA was 59.6 years. Ouchemi et al. in Tchad found a mean age of 47.0 years in 2016; Abalo et al. in Togo reported a mean age of 43.1 years in 2011, Kouassi et al. in Ivory Coast reported a mean age above 40 years in 2016; while Tobome et al. in Benin found a mean age of 37.4 years in 2015 [13, 14, 20, 33]. The mean age of our patients higher than those reported in the literature could be related to the high incidence of underlying cardiovascular disorders.
The sex ratio of our patient population was 1.99, with a statistically significant difference between female and male. Various authors reported the same male predominance in worldwide literature [1, 13, 20, 33, 34]. The greater tendency to inobservance and involvement in the trauma of men, which led to complications, could explain the higher proportion of LEA due to traumatic causes in men than women [35].
Concerning the occupation of the patients, although the percentage of tailors is nearly 23% of the professions, we have not identified any clear association between work activities and LEA.
In our series, the contralateral limb was more involved in LEA after major amputation than the minor. Some authors have reported similar data in the literature. Glaser et al. in the US found contralateral major amputation in 11.5% of patients and in 8.4% after a minor amputation at 5 years [36]. Huseynova et al. in Canada reported an incidence of contralateral major amputation of 4.8 and 2.2 after a minor amputation [37].
In our series, DM was the predominant risk factor of LEA, accounting for 64.61% of the patients. This percentage of DM has increased compared to 48.2% previously found at SOTH during 2000–2004 [20]. Patients with undertreated or not monitored DM, who often discover their disease with the limb problem stirring LEA, frequently undergo LEA complications; as found in previous studies done in Togo [21, 35].
The diabetic patients in our series had a significantly high hyperleukocytosis or leukopenia. However, there was no significant difference in infection rates between patients with and without DM in the early postoperative complications of LEA. In our setting, the first goal for patients with DM was to remove the gangrene and control infection. Therefore, the indication was major amputation, most in the leg, far from the foot infection. In contrast, many recent studies performed in Western countries reported a very low incidence of below-knee amputations (25.8–9.5%) and an increased number of foot amputations due to the high rate of revascularisation and endovascular surgeries [38]. In our setting, there were no revascularization surgeries, patients presented late, and, most of all, with a long duration from the indication of LEA to the surgery. The patients used to attempt to salvage the limb by all means, returning home for indigenous treatment before returning hospital for the LEA. In these conditions, the below-knee amputation was still the best option and should be preferable to serial amputations, overambitious desperate attempts to foot salvage [32, 33]. The CCI was below 2 in 89.19%, and the mortality rate was 7.14%; thus, the CCI also sustained the statement about the severity of the patient's disease, showing that the deaths are not directly related to the comorbidities or other health conditions the patients may have had [26, 27].
Our data missed post-histologic analysis of the amputated limb to reveal possible tumours. This deficiency could be due to a religious tradition, based on which, in case of death, patients’ families can request the amputated limbs for burial alone or with the whole patient’s body [39,40,41].
In our study, the mean duration of hospitalization was 36.3 days. In developing countries, the mean hospital stay for LEA was more than 2 weeks and longer than that in developed countries. The hospital admission time of the patients with LEA due to trauma was significantly longer than others. This long hospitalization time can be due to staged procedures in traumatic patients, from damage control surgery to the stump refinement [13, 14, 20, 33, 42].
The current pattern of LEA in Togo urges a multidisciplinary approach to improve soft tissue management after trauma, revascularization equipment, and post-surgery physiotherapy. It has also underscored the importance of a collaboration between in-hospital specialists and territory stakeholders. The involved specialists are orthopaedists, general or plastic or vascular surgeons, endocrinologists, cardiologists, geriatricians, internal medicine specialists, nutritionists, psychiatrists, prosthetics confectioners, physiotherapists and the territory stakeholders can be general practitioners and patients’ associations. This collaboration should focus on the lower limb pathologies leading to LEA early detection, and educational campaigns to prevent chronic cardiovascular diseases, especially DM.
Our findings have prompted the importance of organizing a structured surveillance system as earlier recommended by Abbas et al. [19] that considers the socio-economic context for comprehensive screening and the follow-up costs in charge of the patients.
Future studies about LEA in Togo should be prospective, focused on the profile of the subset of the patients with DM undergoing this disabling surgery, and leverage a specific database.
Conclusion
Notwithstanding an overall decreased incidence of LEAs at SOTH in Togo during 2010–2020 compared with previous decades, the still remarkable incidence in most cases correlates to chronic cardiovascular and cardiometabolic diseases, especially DM. This pattern of LEA imposes a multidisciplinary approach for screening programs, databases, a specific surveillance system, and clinical protocols and imaging monitoring.
Patients who must undergo LEA in Togo should benefit from an improved surgical approach and post-surgery physiotherapy. Moreover, sensitizing all the healthcare stakeholders and the population through dedicated educational campaigns for DM prevention can reduce the high rate of LEA.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKA:
-
Above knee amputation
- BKA:
-
Below knee amputation
- CCI:
-
Charlson comorbidity index
- CDC:
-
Centers for Disease Control and Prevention
- CI:
-
Confidence interval
- DM:
-
Diabetes mellitus
- F:
-
Fisher test
- Fig.:
-
Figure
- LEA:
-
Lower extremity amputation
- Nig.:
-
Nigeria
- OR:
-
Odds ratio
- P :
-
Cell value
- SD:
-
Standard deviation
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- SOTH:
-
Sylvanus Olympio Teaching Hospital
- US:
-
United States
- χ2 :
-
Chi 2 test
References
Mendelevich A, Kramer M, Maiarú M, Modica M, Ostolaza M, Peralta F. Amputations. A 5-year epidemiological study in Buenos Aires City. Medicina. 2015;75:384–6.
Kotha VS, Ragothaman K, Walters E, Attininger C, Steinberg JS. Lower extremity amputations in at-risk patients. Clin Podiatr Med Surg. 2019;36:483–98.
Li L, Reinhardt JD, Zhang X, Pennycott A, Zhao Z, Zeng X, Li J. Physical function, pain, quality of life and life satisfaction of amputees from the 2008 Sichuan earthquake: a prospective cohort study. J Rehabil Med. 2015;47:466–71.
Pierce RO Jr, Kernek CB, Ambrose TA. The plight of the traumatic amputee. Orthopedics. 1993;16:793–7.
Sinha R, Van Den Heuvel WJA, Arokiasamy P. Factors affecting quality of life in lower limb amputees. Prosthet Orthot Int. 2011;35:90–6.
Banza LN, Mkandawire NC, Harrison WJ. Amputation surgery in children: an analysis of frequency and cause of early wound problems. Trop Dr. 2009;39:129–32.
Beckles V, McCahill JL, Stebbins J, Mkandawire N, Church JCT, Lavy C. The African disability scooter: efficiency testing in paediatric amputees in Malawi. Disabil Rehabil Assist Technol. 2016;11:247–50.
Buckley CM, O’Farrell A, Canavan RJ, Lynch AD, De La Harpe D, Bradley CP, Perry IJ. Trends in the incidence of lower extremity amputations in people with and without diabetes over a 5-year period in the Republic of Ireland. PLoS ONE. 2012;7:e41492.
Fortington LV, Rommers GM, Postema K, van Netten JJ, Geertzen JHB, Dijkstra PU. Lower limb amputation in Northern Netherlands: unchanged incidence from 1991–1992 to 2003–2004. Prosthet Orthot Int. 2013;37:305–10.
Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM, Holt PJ. Lower extremity amputations: a review of global variability in incidence. Diabet Med. 2011;28:1144–53.
Varma P, Stineman MG, Dillingham TR. Epidemiology of limb loss. Phys Med Rehabil Clin N Am. 2014;25:1–8.
Imam B, Miller WC, Finlayson HC, Eng JJ, Jarus T. Incidence of lower limb amputation in Canada. Rev Canadienne Santé Publique. 2017;108:e37480.
Tobome SR, Hodonou AR, Dadjo AY, Ahononga BC, Haoudou R, Gayito RC, Adeniran FAS, Boukari AK, AllodE AS, Caronna R, Priuli G. Amputations de membre dans un hôpital de zone du Nord-Bénin: à propos de 122 cas. Med Afr Noire. 2015;62:165–72.
Kouassi KJE, Yao LB, Sery BJLN, M’Bra K, Assere Y, Kodo M. Causes d’amputation majeure de membre. Rev Int Sc Méd. 2016;18:265–8.
Sarfo-Kantanka O, Sarfo FS, Kyei I, Agyemang C, Mbanya JC. Incidence and determinants of diabetes-related lower limb amputations in Ghana, 2010–2015-a retrospective cohort study. BMC Endocr Disord. 2019;19(1):27.
Grudziak J, Mukuzunga C, Melhado C, Young S, Banza L, Cairns B, et al. Etiology of major limb amputations at a tertiary care centre in Malawi. Malawi Med J. 2019;31(4):244–8.
Khan MZ, Smith MTD, Brice JL, Kong VY. Evolving indications for lower limb amputations in South Africa offer opportunities for health system improvement. WJG. 2020;44:1436–43.
Ugwu E, Adeleye O, Gezawa I, Okpe I, Enamino M, Ezeani I. Predictors of lower extremity amputation in patients with diabetic foot ulcer: findings from MEDFUN, a multi-center observational study. J Foot Ankle Res. 2019;12:34.
Abbas ZG, Boulton AJM. Diabetic foot ulcer disease in African continent: “from clinical care to implementation”: review of diabetic foot in last 60 years-1960–2022. Diabetes Res Clin Pract. 2022;183:109155. https://doi.org/10.1016/j.diabres.2021.109155.
Abalo A, Sanon G, Walla A, Akakpo A, Dossim A. Amputations de membres inférieurs: aspects épidémiologiques, facteurs étiologiques et aspects évolutifs. J Rech Sci. 2011;13:61–7.
Amouzou KS, Kouevi-Koko TE, Domtse KM, Abalo A, Dossim A. Leg ulcers in tertiary public hospital in a sub-Saharan African country (Togo). Wound Healing South Afr. 2017;10:20–4.
World Health Organization. Country cooperation strategy at a glance: Togo.1 May 2016.
World Medical Association. World medical association declaration of Helsinki ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
van Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, for the STROBE initiative. The strengthening the reporting of observational studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson comorbidity index: a critical review of clinimetric properties. Psychother Psychosom. 2022;91(1):8–35. https://doi.org/10.1159/000521288.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Lewis TL, Mason L, Gordon D, Ray R. The Clavien–Dindo complication classification modified for foot and ankle orthopaedic surgery. Foot Ankle Surg. 2022;28(6):800–2. https://doi.org/10.1016/j.fas.2022.03.006.
Macrotrends LLC. Togo population 1950–2022 [Internet]. c2010–2022. https://www.macrotrends.net/countries/TGO/togo/population. Accessed 20 Sept 2022.
Yang Z, Xu C, Zhu YG, Li J, Wu ZX, Zou JW, Xue BB, Miao DM, Shang L, Zhao GY. Radical treatment of severe open fractures of extremities by orthoplastic surgery: a 10-year retrospective study. J Orthop Surg Res. 2021;16(1):340. https://doi.org/10.1186/s13018-021-02479-2.
Amouzou K, Kouevi-Koko TE, Ayouba G, Bakriga B, Abalo A. A free gracilis muscle flap for foot resurfacing, the first microsurgical case in a Sub-Saharan African country, Togo. Niger J Plast Surg. 2019;15(2):44.
Ouchemi C, Abdoul T, Dionadji M, Chaib MAA, Okim AM, Maide MK. Les amputations majeures des membres à l’hôpital général de référence nationale de N’djamena. Tchad Eur Sc J. 2016;12:270–80.
Ahmad N, Thomas GN, Gill P, Chan C, Torella F. Lower limb amputation in England: prevalence, regional variation and relationship with revascularisation, deprivation and risk factors. A retrospective review of hospital data. J R Soc Med. 2014;107:483–9.
Djibril AM, Mossi EK, Djagadou AK, Balaka A, Tchamdja T, Moukaila R. Pied diabétique: aspects épidémiologique, diagnostique, thérapeutique et évolutif à la Clinique Médico-chirurgicale du CHU Sylvanus Olympio de Lomé. Pan Afr Med J [En ligne]. 2018;4:5.
Glaser JD, Bensley RP, Hurks R, Dahlberg S, Hamdan AD, Wyers MC, et al. Fate of the contralateral limb after lower extremity amputation. J Vasc Surg. 2013. https://doi.org/10.1016/j.jvs.2013.06.055.
Huseynova K, Sutradhar R, Booth GL, Huang A, Ray JG. Risk of contralateral lower limb amputation and death after initial lower limb amputation: a population based study. Heliyon. 2018;4:e00836. https://doi.org/10.1016/j.heliyon.2018.e00836.
Ponkilainen VT, Vuorlaakso M, Kaartinen I, Kiiski J, Saarinen E, Huttunen TT, Paloneva J, Mattila VM. The development of lower limb amputations in Finland from 1997 to 2018: A nationwide retrospective registry study. Eur J Vasc Endovasc Surg. 2022;63(1):138–46. https://doi.org/10.1016/j.ejvs.2021.09.030.
Malay DS. Editor’s introduction to Chaplain Ehman’s comments on amputation and limb burial. J Foot Ankle Surg. 2020;59:449.
Van Damme H, Limet R. Amputation in diabetic patients. Clin Podiatr Med Surg. 2007;24:569–82.
Ehman JW. Patient requests for burial of amputated limbs. J Foot Ankle Surg. 2020;59:450–1.
Boffeli TJ, Thompson JC. Partial foot amputations for salvage of the diabetic lower extremity. Clin Podiatr Med Surg. 2014;31:103–26.
Acknowledgements
The authors of this article are grateful to Doctor Ede Osita, MBBS (Nigeria), FWACS, FMCOrtho, a consultant orthopaedic surgeon at National Orthopaedic Hospital Enugu, who helped in the data analysis and provided statistical assistance.
Funding
None.
Author information
Authors and Affiliations
Contributions
TEKK, KSA designed, made the conception of the study and contributed in drafting the article. TEKK, DYEL helped in acquisition, analysis and interpretation of data. TEKK, KSA, SA, AS, AA helped in revising it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors confirm that any patient, service user, or participant (or that person’s parent or legal guardian) included in this clinical trial has given written consent to the inclusion of material pertaining to themselves, that they acknowledge that they cannot be identified via the paper; and that we have fully anonymized them. The composition of the Ethical Committee of SOTH is currently changing according to the scientific guidelines. For this study, we defend the protocol in the staff of Professor Anani Abalo and Professor Ekoue David Dosseh. Professor Abalo is the author of the epidemiologic study about LEA in Togo published 10 years ago. Professor Abalo thought an updated analysis of the current status of the disease in the country is mandatory. We include the written declaration of Prof Abalo.
Consent for publication
All the patients provided written consent to be enrolled in the present study and the publication of the relative article.
Competing interests
They authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kouevi-Koko, T.E., Amouzou, K.S., Sogan, A. et al. Lower extremity amputations (LEAs) in a tertiary hospital in Togo: a retrospective analysis of clinical, biological, radiological, and therapeutic aspects. J Orthop Surg Res 18, 155 (2023). https://doi.org/10.1186/s13018-023-03628-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-03628-5