Abstract
Background
In recent years, numerous investigations have been conducted to determine the clinical significance and critical functions of vascular endothelial growth factor (VEGF) in various malignant cancers. The purpose of this meta-analysis was to comprehensively evaluate the prognostic and clinicopathological value of VEGF in patients with osteosarcoma.
Methods
We performed a systematic literature retrieval of available databases. Odds ratios (ORs) or standard mean difference (SMD) for clinicopathological parameters, hazard ratios (HRs) for overall survival and disease-free survival were calculated to assess the correlation between VEGF expression and prognosis in patients with osteosarcoma.
Results
A total of 22 studies with 1144 patients were included in our study. Pooled analyses showed that VEGF overexpression predicted worse overall survival (HR, 2.42; 95% CI, 1.87–3.11, p < 0.001) and disease-free survival (HR, 2.604; 95% CI, 1.698–3.995, p < 0.001), respectively. Furthermore, investigation regarding osteosarcoma clinicopathologic characteristics suggested that high VEGF expression was significantly associated with metastasis (OR, 4.39; 95% CI, 2.77–6.95; p < 0.001), clinical stage (OR, 0.73; 95% CI, 0.62–0.87; p < 0.001), and microvessel density (SMD, 3.33, 95% CI,1.57–5.10, p < 0.001), but not associated with tumor location, gender, age, local recurrence, and chemotherapy response.
Conclusion
Our meta-analysis findings suggest that elevated VEGF expression may be a predictive biomarker for poor prognosis and adverse clinicopathological characteristics in patients with osteosarcoma.
Similar content being viewed by others
Introduction
Osteosarcoma is the most frequent malignant osteogenic tumor, mostly occurring in children and young adults [1]. Over the past decade, the clinic appliance of neoadjuvant reduced the size of the localized tumor and delayed the progression, significantly improving the 5-year survival rate of patients with low-grade osteosarcoma [2]. However, metastasis has been reported to be present in approximately 25% of newly diagnosed osteosarcoma patients, and the mortality rate in these patients remains extremely high at approximately 20% [2,3,4]. There is currently an absence of viable methods for the early diagnosis and treatment of osteosarcoma. Given this, further investigation of prognostic molecular biomarkers is critical for a better understanding of osteosarcoma's pathophysiology and the development of more effective treatment modalities.
Angiogenesis plays a vital role in tumor development as the growth of tumors relies on the perfusion of neovascular [5]. Vascular endothelial growth factor (VEGF) is a potent pro-angiogenic factor that regulates vascular endothelial cell proliferation, differentiation and migration [6]. Overexpression of VEGF has been reported to be attributed to the invasion and metastasis of a wide range of solid tumors [7,8,9]. To clarify the mechanism of VEGF in the advancement of osteosarcoma, the association between VEGF and prognosis features of osteosarcoma has been assessed. However, the prognostic and clinicopathological value of VEGF remains controversial [10, 11]. Previous relevant meta-analyses have been performed to define the clinical significance of VEGF expression in osteosarcoma. Nevertheless, these analyses were inconclusive as inconsistent results, limited involved studies, and the absence of a thorough evaluation of study quality and pooled results [12,13,14]. Therefore, this current study aimed to comprehensively and systematically assess the prognostic value of VEGF in 22 studies involving 1144 osteosarcoma patients.
Materials and methods
This study was conducted entirely in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15].
Search strategies
A comprehensive electronic literature search was performed in four databases: Web of Science, PubMed, Cochrane Library and Medline with no restrictions on language or publication date. The last search was conducted on September 12, 2021. The following terms were used to conduct the literature search: ("osteosarcoma" or "osteogenic sarcoma") and ("vascular endothelial growth factor" or VEGF). We additionally manually screened the references of identified articles to collect more studies.
Selection criteria
The eligible articles were selected in accordance with the following criteria: (1) patients were diagnosed with osteosarcoma pathologically; (2) the relationship between VEGF expression and clinicopathological characteristics or prognosis were investigated; (3) the expression VEGF was determined on samples of tumor tissue. Articles were excluded according to the following criteria: (1) studies were published in the form of conference abstracts, letters, case reports, expert opinions, reviews, or sequence data; (2) focused on tumor cell lines or animal experiments; (3) patients did not confirm the diagnosis of osteosarcoma; (4) when study comprised overlapping patient cohort. Two independent authors determined whether studies were eligible. Any discrepancies were settled by consensus following a discussion.
Data extraction and quality assessment
Two independent investigators carefully reviewed all eligible publications to extract interested data. The following data were collected, including (1) first author, publication year, patient source; (2) number of patients, age, gender, VEGF assay method, antibody type, source and dilution of immunohistochemistry (IHC), and cutoff value; (3) tumor stage at diagnosis, metastasis, local recurrence, tumor location, chemotherapy response, microvessel intensity (MVD), hazard ratio (HR) of VEGF expression and corresponding 95% CI.
If a study stated both univariate and multivariate survival results, the HRs from the multivariate analyses were used. When the survival results were not given explicitly while a Kaplan–Meier curve was present, the HRs with 95% CIs were retrieved using Engauge Digitizer 11.0 software and Tierney’s reported method [16].
Each involved study’s quality was assessed using the Newcastle–Ottawa Scale (NOS) by two independent reviewers [17]. The scale judges the quality of studies from three main aspects: the selection of the groups, comparability, and exposure, with a maximum of nine points. Articles with a NOS score of more than six were considered to be of high quality.
Statistical analysis
The statistical analysis in this study was performed by STATA 14.0 (Stata Corporation, College Station, TX, USA). We estimate the pooled HRs for survival results, the pooled odds ratio (OR) for the clinicopathological characteristics (age, gender, stage, metastasis, local recurrence, response to chemotherapy). The continuous variables are described as standard mean difference (SMD). The statistical between-study heterogeneity was assessed by the Chi-squared test and the Higgins I2 statistic. Significant heterogeneity was defined as a p > 0.10 or I2 > 50%. A fixed-effects model was utilized when there was no significant heterogeneity. Otherwise, a random-effects model was utilized. The potential publication bias was estimated by using Begg’s funnel plot and Egger’s test. Additionally, we performed sensitivity analyses to assess the stability of the pooled outcomes. p < 0.05 was considered statistically significant.
Results
Search results
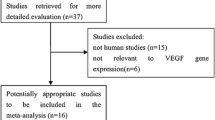
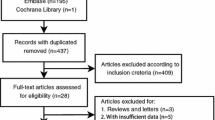
A total of 2075 articles were identified from four online databases. After removing 640 duplicates, the remaining 1435 records were systematically evaluated by the titles and abstracts. Among these articles, 165 articles were excluded for irrelevant studies, 245 articles involved non-human experiments, 492 articles were conference abstracts, case reports, letters, and reviews, and 501 articles were not related to VEGF or osteosarcoma. After assessing the entire text of the remaining 32 studies, 10 articles were excluded for insufficient data. Finally, 22 studies with a total of 1144 osteosarcoma patients were included in this study [10, 11, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The detailed flowchart of the study filtrating process is shown in Fig. 1.
Study characteristics and quality assessment
The summarized characteristics of the included study are shown in Table 1. Among them, 11 studies focused on the prognostic significance, 22 studies analyzed the correlation between VEGF expression and clinicopathological characteristics. All of the eligible research was published between 1999 and 2020, and it was written in English, with a patient population ranging from 25 to 153. Additionally, immunochemical staining (IHC) was the most often employed technique to measure VEGF expression (21/22, 95.5%), with 95.2% of the studies using IHC having defined the cutoff value of VEGF expression. Each article used the tissue as the sample. In terms of study quality, all of the eligible studies were high quality with a NOS score greater than 6 points. Other information about the involved studies is shown in Table 1.
VEGF expression and prognostic significance
The survival data, including overall survival (OS) or disease-free survival (DFS), were analyzed in 11 studies among eligible studies. Due to the lack of evident heterogeneity detected (I2 = 0.00%, p = 0.894), a fixed-effects model was utilized. The result showed that the elevated VEGF expression was associated with poor overall survival (HR, 2.42; 95% CI, 1.87–3.11, p < 0.001). To further find out the potential sources of heterogeneity, we undertook a subgroup analysis stratified by ethnicity, publication date, testing isoform, antibody type, positive rate, HR resource, sample size, and NOS score. As shown in Table 2, each subgroup presented a significant association with overall survival. Besides, disease-free survival was also extracted in studies. The fixed-effect model was employed to calculate the pooled HR (I2 = 0.00%, p = 0.485). Results reveal that the elevated VEGF expression predicted poor disease-free survival (HR, 2.604; 95% CI, 1.698–3.995, p < 0.001).
VEGF expression and clinicopathological features
The correlation between VEGF expression and clinicopathological values, including age, gender, metastasis, local recurrence, tumor stage, response to chemotherapy, and MVD, was investigated. A fixed-effects or a random-effects model was employed based on the heterogeneity results of each parameter. The detailed information is shown in Table 3. Under the fixed-effects model, overexpression of VEGF was significantly related to a higher rate of osteosarcoma metastasis (OR, 4.39; 95% CI, 2.77–6.95; p < 0.001). The random-effects model showed that the overexpression of VEGF was significantly related to a higher clinical stage (OR, 0.73; 95% CI, 0.62–0.87; p < 0.001). Besides, VEGF expression showed a significant correlation with microvessel density (MVD) according to the results of the random-effects model (SMD, 3.33, 95% CI,1.57–5.10, p < 0.001). However, we failed to find a significant relationship between overexpression of VEGF and gender, tumor location, local recurrence, age, and response to chemotherapy (Fig. 2).
Publication bias and sensitivity analysis
Publication bias was measured by using Begg's funnel plot and Egger's tests. As shown in Figs. 3B and 4B, there was no publication bias for overall survival (Begg's test, p = 0.436; and Egger's test, p = 0.745) and disease-free survival (Begg's test, p = 0.089; and Egger's test, p = 0.198).
We performed a sensitivity analysis of overall survival and disease-free survival to investigate the influence of each study on the pooled HR. As Figs. 3C and 4C show, we did not find any significant alteration in the pooled HR when omitting any single study sequentially, demonstrating that the analyses were stable and credible.
Discussion
As the most frequent primary osteogenic tumor, osteosarcoma is characterized as aggressive cancer with a high risk of distant metastasis. Although immune checkpoint inhibitors have recently revolutionized the treatment of a wide range of solid malignancies, they have demonstrated limited efficacy in osteosarcoma [38,39,40]. Therefore, the identification of other biomarkers related to the prognosis of osteosarcoma is crucially essential to the development of new potential therapeutic targets.
Angiogenesis is essential for the proliferation and metastasis of tumor cells [5]. In the past decades, VEGF has been the most studied biomarker of tumor neovascularization for its crucial significance in angiogenesis and vasculogenesis [41]. Through binding to tyrosine kinases receptors, the VEGF signaling pathways play an important role in a variety of physiological and pathological processes. In the process of tumorigenesis, the transcription of several hypoxia-related genes induces the expression of VEGF, mainly via VEGFR-2, to activate angiogenesis [42]. High levels of VEGF expression are linked to endothelial barrier disruption in pathological tumor conditions, promoting cancer distant metastasis [43, 44]. Furthermore, VEGF is involved in regulating the immune response of tumors. A variety of innate immune cells have been reported to secrete VEGF in the tumor microenvironment to reduce the immune response of immune cells to tumor tissue [45,46,47]. The upregulated VEGF expression was also reported to actively participate in tumor escape from immune surveillance by suppressing the proliferation of T-cells and increasing the exhaustion of T-cells [48, 49].
Recently, it has been implicated that high expression of VEGF mediates metastasis and progression in many malignancies [7,8,9]. Several meta-analyses have previously assessed the clinical significance of VEGF expression in patients with osteosarcoma [12,13,14]. Nevertheless, Han et al. focused on the part of the clinicopathological characteristics of VEGF [12]. Researches on the prognostic effect of VEGF expression had inconsistent results and did not pay attention to the quality evaluation, heterogeneity, and sensitivity analysis [13, 14]. Moreover, these researches were published 5 years ago. Limited to the relatively small number of studies, the conclusion was not robust, and some crucial clinicopathological features were not evaluated. Here, we conducted a comprehensive literature search to combine all relevant studies related to VEGF expression's prognostic and clinicopathological value.
In the present meta-analysis study, we pooled 22 studies on VEGF expression in the prognosis or clinicopathology of osteosarcoma patients. In terms of survival data, our findings revealed that overexpression of VEGF was associated with poor overall survival and disease-free survival. The analyses did not find significant heterogeneity or obvious publication bias, and sensitivity analysis showed our results were robust and reliable. Therefore, we supported the hypothesis that elevated VEGF expression predicted poor DFS. In terms of clinicopathological characteristics, similar to previous reports, VEGF overexpression was related to a higher tumor grade and rate of metastasis but not associated with gender, age, tumor location, local recurrence, clinical stage and response to chemotherapy [12]. The results indicated that high levels of VEGF expression predict metastasis and an advanced stage of osteosarcoma. Additionally, previous meta-analyses had not assessed the association between VEGF and MVD. In our study, VEGF overexpression had a marked effect on promoting vascularization in osteosarcoma. However, the results should be interpreted cautiously as only limited studies were included in the analyses, and more related research is needed.
This meta-analysis has some limitations. Firstly, the methods for identifying and evaluating VEGF expression varied among the eligible studies. Although most of these studies applied IHC, the varied antibodies and dilutions utilized may have contributed to heterogeneity. In addition, there were discrepancies in the definition of VEGF positive. The staining methods, the details of the IHC scoring criteria, and cutoff values varied across the included studies. Secondly, the correlation between VEGF expression and some clinicopathological characteristics of osteosarcoma, such as tumor size, were not analyzed in our study due to the insufficient studies using the same criteria of tumor size. Furthermore, when the results of the multivariate survival analysis were reported, the survival data were extracted directly. When not stated in the original articles, the HRs with their corresponding 95% CIs were calculated through the reconstruction of survival curves, which may affect the robustness of the pooled overall survival and disease-free survival. In order to eliminate bias, more precise data extraction methods or better study quality were needed. Lastly, although this study comprised more than 1000 osteosarcoma patients, future studies with larger sample sizes are necessary to further elucidate the association between VEGF and prognosis and clinicopathological characteristics.
Conclusion
This meta-analysis indicated that elevated VEGF expression was correlated with adverse osteosarcoma clinicopathological features and poor prognosis. Our results suggest that VEGF is a predictive biomarker in patients with osteosarcoma. However, further large-scale, prospective research is required to validate our results.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- VEGF:
-
Vascular endothelial growth factor
- HR:
-
Hazard ratios
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- OR:
-
Odds ratio
- CI:
-
Confidence intervals
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- IHC:
-
Immunochemical staining
- MVD:
-
Microvessel density
References
Sadykova LR, Ntekim AI, Muyangwa-Semenova M, Rutland CS, Jeyapalan JN, Blatt N, Rizvanov AA. Epidemiology and risk factors of osteosarcoma. Cancer Invest. 2020;38:259–69. https://doi.org/10.1080/07357907.2020.1768401.
Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004 data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–43. https://doi.org/10.1002/cncr.24121.
He H, Ni J, Huang J. Molecular mechanisms of chemoresistance in osteosarcoma. Oncol Lett. 2014;7:1352–62. https://doi.org/10.3892/ol.2014.1935.
Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, Gobel U, Helmke K, Jundt G, Kabisch H, Kevric M, Klingebiel T, Kotz R, Maas R, Schwarz R, Semik M, Treuner J, Zoubek A, Winkler K. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23:559–68. https://doi.org/10.1200/jco.2005.04.063.
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. https://doi.org/10.1038/nm0603-669.
Wang F, Peng L, Wang Y, Liu X. A meta-analysis of vascular endothelial growth factor for nasopharyngeal cancer prognosis. Front Oncol. 2018. https://doi.org/10.3389/fonc.2018.00486.
Xia H, Shen J, Chen S, Huang H, Xu Y, Ma H. Overexpression of VEGF-C correlates with a poor prognosis in esophageal cancer patients. Cancer Biomark. 2016;17:165–70. https://doi.org/10.3233/cbm-160627.
Zong S, Li H, Shi Q, Liu S, Li W, Hou F. Prognostic significance of VEGF-C immunohistochemical expression in colorectal cancer: a meta-analysis. Clin Chim Acta. 2016;458:106–14. https://doi.org/10.1016/j.cca.2016.04.037.
Kong X, Xu L, Cao X. Correlations of expressions of IMP3 and VEGF with stage of osteosarcoma, microvascular density and pulmonary metastasis. J BUON Off J Balkan Union Oncol. 2020;25:2438–43.
Baptista AM, de Franca Camargo AF, Filippi RZ, Mendes C, de Oliveira CRG, de Azevedo Neto RS, de Camargo OP. Correlation between the expression of VEGF and survival in osteosarcoma. Acta Ortopedica Bras. 2014;22:250–5. https://doi.org/10.1590/1413-78522014220500978.
Han G, Wang Y, Bi W, Jia J, Wang W, Xu M. Effects of vascular endothelial growth factor expression on pathological characteristics and prognosis of osteosarcoma. Clin Exp Med. 2016;16:577–84. https://doi.org/10.1007/s10238-015-0382-1.
Yu X-W, Wu T-Y, Yi X, Ren W-P, Zhou Z-b, Sun Y-q, Zhang C-q. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumor Biol. 2014;35:155–60. https://doi.org/10.1007/s13277-013-1019-1.
Zhuang Y, Wei M. Impact of vascular endothelial growth factor expression on overall survival in patients with osteosarcoma: a meta-analysis. Tumor Biol. 2014;35:1745–9. https://doi.org/10.1007/s13277-014-1692-8.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (Lond, England). 2021;88:105906. https://doi.org/10.1016/j.ijsu.2021.105906.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P., Stewart, L.A., Group P-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1.https://doi.org/10.1186/2046-4053-4-1
Mohamed FEZA, Khalil EZI, Toni NDM. Caveolin-1 expression together with VEGF can be a predictor for lung metastasis and poor prognosis in osteosarcoma. Pathol Oncol Res. 2020;26:1787–95. https://doi.org/10.1007/s12253-019-00755-5.
Wu H, Zhang J, Dai R, Xu J, Feng H. Transferrin receptor-1 and VEGF are prognostic factors for osteosarcoma. J Orthop Surg Res. 2019;14:296. https://doi.org/10.1186/s13018-019-1301-z.
Liu Y, Zhang F, Zhang Z, Wang D, Cui B, Zeng F, Huang L, Zhang Q, Sun Q. High expression levels of Cyr61 and VEGF are associated with poor prognosis in osteosarcoma. Pathol Res Pract. 2017;213:895–9. https://doi.org/10.1016/j.prp.2017.06.004.
Lei P, Ding D, Xie J, Wang L, Liao Q, Hu Y. Expression profile of Twist, vascular endothelial growth factor and CD34 in patients with different phases of osteosarcoma. Oncol Lett. 2015;10:417–21. https://doi.org/10.3892/ol.2015.3246.
Zhao H, Wu Y, Chen Y, Liu H. Clinical significance of hypoxia-inducible factor 1 and VEGF-A in osteosarcoma. Int J Clin Oncol. 2015;20:1233–43. https://doi.org/10.1007/s10147-015-0848-x.
Becker RG, Galia CR, Morini S, Viana CR. Immunohistochemical expression of VEGF and HER-2 proteins in osteosarcoma biopsies. Acta Ortopedica Bras. 2013;21:233–8. https://doi.org/10.1590/s1413-78522013000400010.
Lammli J, Fan M, Rosenthal HG, Patni M, Rinehart E, Vergara G, Ablah E, Wooley PH, Lucas G, Yang S-Y. Expression of vascular endothelial growth factor correlates with the advance of clinical osteosarcoma. Int Orthop. 2012;36:2307–13. https://doi.org/10.1007/s00264-012-1629-z.
Chen Y, Yang Y, Yuan Z, Wang C, Shi Y. Predicting chemosensitivity in osteosarcoma prior tochemotherapy: an investigational study of biomarkers with immunohistochemistry. Oncol Lett. 2012;3:1011–6. https://doi.org/10.3892/ol.2012.604.
Zhou Q, Zhu Y, Deng Z, Long H, Zhang S, Chen X. VEGF and EMMPRIN expression correlates with survival of patients with osteosarcoma. Surg Oncol. 2011;20:13–9. https://doi.org/10.1016/j.suronc.2009.09.002.
Lin F, Zheng SE, Shen Z, Tang LN, Chen P, Sun YJ, Zhao H, Yao Y. Relationships between levels of CXCR4 and VEGF and blood-borne metastasis and survival in patients with osteosarcoma. Med Oncol (Northwood, London, England). 2011;28:649–53. https://doi.org/10.1007/s12032-010-9493-4.
Lugowska I, Wozniak W, Klepacka T, Michalak E, Szamotulska K. A prognostic evaluation of vascular endothelial growth factor in children and young adults with osteosarcoma. Pediatr Blood Cancer. 2011;57:63–8. https://doi.org/10.1002/pbc.23021.
Abdeen A, Chou AJ, Healey JH, Khanna C, Osborne TS, Hewitt SM, Kim M, Wang D, Moody K, Gorlick R. Correlation between clinical outcome and growth factor pathway expression in osteogenic sarcoma. Cancer. 2009;115:5243–50. https://doi.org/10.1002/cncr.24562.
Mizobuchi H, García-Castellano JM, Philip S, Healey JH, Gorlick R. Hypoxia markers in human osteosarcoma: an exploratory study. Clin Orthop Relat Res. 2008;466:2052–9. https://doi.org/10.1007/s11999-008-0328-y.
Huang Y, Lin Z, Zhuang J, Chen Y, Lin J. Prognostic significance of alpha V integrin and VEGF in osteosarcoma after chemotherapy. Onkologie. 2008;31:535–40. https://doi.org/10.1159/000151685.
Park HR, Min K, Kim HS, Jung WW, Park YK. Expression of vascular endothelial growth factor-C and its receptor in osteosarcomas. Pathol Res Pract. 2008;204:575–82. https://doi.org/10.1016/j.prp.2008.01.015.
Charity RM, Foukas AF, Deshmukh NS, Grimer RJ. Vascular endothelial growth factor expression in osteosarcoma. Clin Orthop Relat Res. 2006;448:193–8. https://doi.org/10.1097/01.blo.0000205877.05093.c9.
Oda Y, Yamamoto H, Tamiya S, Matsuda S, Tanaka K, Yokoyama R, Iwamoto Y, Tsuneyoshi M. CXCR4 and VEGF expression in the primary site and the metastatic site of human osteosarcoma: analysis within a group of patients, all of whom developed lung metastasis. Mod Pathol Off J US Can Acad Pathol. 2006;19:738–45. https://doi.org/10.1038/modpathol.3800587.
Jung ST, Moon ES, Seo HY, Kim JS, Kim GJ, Kim YK. Expression and significance of TGF-β isoform and VEGF in osteosarcoma. Orthopedics. 2005;28:755–60.
Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, Higashino F, Mezawa F, Okada F, Ishii S. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res Off J Am Assoc Cancer Res. 2000;6:572–7.
Lee YH, Tokunaga T, Oshika Y, Suto R, Yanagisawa K, Tomisawa M, Fukuda H, Nakano H, Abe S, Tateishi A, Kijima H, Yamazaki H, Tamaoki N, Ueyama Y, Nakamura M. Cell-retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma. Eur J Cancer (Oxford, England: 1990). 1999;35:1089–93. https://doi.org/10.1016/s0959-8049(99)00073-8.
Serra M, Hattinger CM. The pharmacogenomics of osteosarcoma. Pharmacogenomics J. 2017;17:11–20. https://doi.org/10.1038/tpj.2016.45.
Chawla, S.P., Van Tine, B.A., Pollack, S., Ganjoo, K.N., Elias, A.D., Riedel, R.F., Attia, S., Choy, E., Okuno, S.H., Agulnik, M., von Mehren, M., Livingston, M.B., Keedy, V.L., Verschraegen, C.F., Philip, T., Bohac, G.C., Yurasov, S., Lu, H.L., Chen, M., Maki, R.G. A phase II randomized study of CMB305 and atezolizumab versus atezolizumab in NY-ESO-1(+) soft tissue sarcoma: Analysis of immunogenicity, tumor control, and patient survival. J Clin Oncol. 2019; 37
Tawbi HA, Burgess M, Bolejack V. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial (vol 18, pg 1493, 2017). Lancet Oncol. 2018;19:E8.
Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. https://doi.org/10.1038/nrd.2015.17.
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. https://doi.org/10.1038/nrc1093.
Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. 2013;3:211–211. https://doi.org/10.3389/fonc.2013.00211.
Weis S, Cui JH, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–9. https://doi.org/10.1083/jcb.200408130.
Bruno A, Focaccetti C, Pagani A, Imperatori AS, Spagnoletti M, Rotolo N, Cantelmo AR, Franzi F, Capella C, Ferlazzo G, Mortara L, Albini A, Noonan DM. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia. 2013;15:133-U191. https://doi.org/10.1593/neo.121758.
Wheeler KC, Jena MK, Pradhan BS, Nayak N, Das S, Hsu C-D, Wheeler DS, Chen K, Nayak NR. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE. 2018. https://doi.org/10.1371/journal.pone.0191040.
Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, Sukhatme VP, Karumanchi SA, Kopcow HD. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol. 2013;190:3939–48. https://doi.org/10.4049/jimmunol.1202582.
Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet A-L, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8(+) T cells in tumors. J Exp Med. 2015;212:139–48. https://doi.org/10.1084/jem.20140559.
Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Ioannou K, Ziogas AC, Rodolakis A, Vlahos G, Thomakos N, Haidopoulos D, Terpos E, Antsaklis A, Dimopoulos MA, Bamias A. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer. 2012;107:1869–75. https://doi.org/10.1038/bjc.2012.468.
Funding
This work was funded by National Natural Science Foundation of China (81873998).
Author information
Authors and Affiliations
Contributions
CZ and XL involved in conceptualization. CZ and LW involved in methodology. CZ involved in writing—original draft. XL involved in writing—review and editing. LW and HL involved in statistical analysis. CX and RZ involved in data search and extraction. XL involved in supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethic approval and consent for publication
This study obtained the wavier of consent by the hospital ethics committee of The First Affiliated Hospital of Chongqing Medical University, Chongqing, China. All the authors listed in this article agree to the publication of the paper.
Competing interests
The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., Wang, L., Xiong, C. et al. The role of vascular endothelial growth factor as a prognostic and clinicopathological marker in osteosarcoma: a systematic review and meta-analysis. J Orthop Surg Res 16, 738 (2021). https://doi.org/10.1186/s13018-021-02888-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-021-02888-3