Abstract
Background
To determine the optimal planning target volume (PTV) margins for adequate coverage by daily iterative cone-beam computed tomography (iCBCT)-guided online adaptive radiotherapy (oART) in postoperative treatment of endometrial and cervical cancer and the benefit of reducing PTV margins.
Methods
Fifteen postoperative endometrial and cervical cancer patients treated with daily iCBCT-guided oART were enrolled in this prospective phase 2 study. Pre- and posttreatment iCBCT images of 125 fractions from 5 patients were obtained as a training cohort, and clinical target volumes (CTV) were contoured separately. Uniform three-dimensional expansions were applied to the PTVpre to assess the minimum margin required to encompass the CTVpost. The dosimetric advantages of the proposed online adaptive margins were compared with conventional margin plans (7–15 mm) using an oART emulator in another cohort of 125 iCBCT scans. A CTV-to-PTV expansion was verified on a validation cohort of 253 fractions from 10 patients, and further margin reduction and acute toxicity were studied.
Results
The average time from pretreatment iCBCT to posttreatment iCBCT was 22 min. A uniform PTV margin of 5 mm could encompass nodal CTVpost in 100% of the fractions (175/175) and vaginal CTVpost in 98% of the fractions (172/175). The margin of 5 mm was verified in our validation cohort, and the nodal PTV margin could be further reduced to 4 mm if ≥ 95% CTV coverage was predicted to be achieved. The adapted plan with a 5 mm margin significantly improved pelvic organ-at-risk dosimetry compared with the conventional margin plan. Grade 3 toxicities were observed in only one patient with leukopenia, and no patients experienced acute urinary toxicity.
Conclusion
In the postoperative treatment of endometrial and cervical cancer, oART could reduce PTV margins to 5 mm, which significantly decrease the dose to critical organs at risk and potentially lead to a lower incidence of acute toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
For endometrial cancer and early-stage cervical cancer treated with surgery, adjuvant pelvic radiotherapy with or without chemotherapy is prescribed, depending on clinicopathological prognostic factors [1,2,3,4]. Previous studies have demonstrated that postoperative radiotherapy could decrease locoregional recurrence and improve survival for patients with high-risk factors [5, 6]. Compared with standard four-field radiotherapy, intensity-modulated radiation therapy (IMRT) has been widely used in pelvic radiotherapy for gynecologic malignancies, with greater conformity in treatment volumes, more homogeneous dose distribution and sharper dose reduction in adjacent organs at risk (OAR) [7,8,9]. However, the expansion of planning target volume (PTV) margins from IMRT to generate a large target volume needs to encompass both inter- and intrafractional variations, particularly large anatomical variations in the pelvic area, such as bladder and rectal filling. Postoperative pelvic radiotherapy with large target volumes may result in acute gastrointestinal and genitourinary toxicity [10, 11].
Recently, commercially available online adaptive radiotherapy (oART) has allowed automatic target volume delineation and per-fraction treatment plan reoptimization, potentially improving dosimetric outcomes [12, 13]. Further reduction of PTV margins may be possible by adapting the treatment to the patient’s daily anatomy. However, a certain intrafractional uncertainty should be considered for PTV expansions in oART. The time required to adapt anatomical variations and replan treatment cannot be ignored, especially the impact of the volumes of the bladder and rectum, which vary randomly during this time. A fast iterative cone-beam computed tomography (iCBCT)-guided daily oART technique showed great advantages in terms of both adaptive time and online workflow. Currently, it is not clear to what extent daily oART reduces PTV margins for postoperative cervical cancer and endometrial cancer, and the clinical benefits of reducing PTV margins have not been reported in previous studies.
We performed a prospective phase 2 study of daily oART for postoperative treatment of endometrial and cervical cancer to determine the optimal PTV margins for adequate coverage and to investigate the dosimetric and clinical benefits of reduced PTV margins.
Methods and materials
Patient eligibility
Fifteen patients with postoperative endometrial and cervical cancer treated with daily iCBCT-guided oART were enrolled in this prospective nonrandomized phase 2 trial between September 2022 and February 2023. Eligible patients underwent surgical resection and had indications for adjuvant pelvic radiotherapy. The full eligibility and exclusion criteria are summarized in Supplementary Table A1. This study was reviewed and approved by the Institutional Review Board of Peking Union Medical College Hospital (No. K2416), and the registration code of the trial (ClinicalTrials.gov ID) is NCT05682950.
Target delineation and treatment
Delineation of clinical target volume (CTV) was performed according to the NRG Oncology/RTOG Consensus Guidelines [14], consisting of separate nodal CTV (CTV-N) and vaginal CTV (CTV-V) contours. The anterior border of CTV-V is the posterior aspect of the bladder wall, and the posterior border is the anterior rectal wall, including approximately the anterior one-third of the mesorectum, and the obturator nodal CTV is carved out of bladder, which does not take interfractional organ motion into account.
All patients received postoperative daily iCBCT-guided oART (Ethos, Varian Medical Systems, Palo Alto, USA), and a prescribed dose of 45 or 50.4 Gy in 25 or 28 fractions was applied to the PTV. Before simulation and each treatment fraction, patients were instructed to empty their bladder and rectum one hour and forty minutes before the appointment, followed by an intake of 450–500 ml water in 10 min according to their height and weight. The patients were fixed with a thermoplastic film and simulated in supine position, with their arms above their head or on their chest.
The CT-guided high-dose intracavitary brachytherapy was administered to the upper 1/3 or 1/2 of the vagina after oART, with a prescribed dose of 10 Gy in 2 fractions delivered to a depth of 0.5 cm below the vaginal mucosa.
iCBCT acquisition and registration
All enrolled patients underwent three iCBCT scans per oART fraction. The adaptive CTV was contoured based on the pretreatment iCBCT (iCBCT-1), which was acquired after patient enrollment. The second iCBCT (iCBCT-2) scan was obtained to verify the position of the target volume and OARs before treatment. The posttreatment iCBCT (iCBCT-3) scan was acquired immediately after treatment completion. The total time from the acquisition of the pretreatment iCBCT to the completion of the posttreatment iCBCT was recorded.
The iCBCT-3 scans were uploaded to the Ethos oART emulator, which was provided by Varian Medical Systems for remote simulation of an Ethos system with identical software and functions as the clinical version, and CTV-Npost and CTV-Vpost were contoured on each iCBCT. Then, iCBCT-3 scans with CTVpost were rigidly registered to the iCBCT-1 scans with PTVpre with respect to bony anatomy using an Eclipse treatment planning system (Varian Inc., Palo Alto, CA). The CTV delineation, iCBCT registration and target coverage evaluation were performed by the same physician and verified by a second expert radiation oncologist.
Target coverage evaluation from the training cohort
A total of 125 pre- and posttreatment iCBCT scans, corresponding to each fraction from 5 patients with conventional PTV margins (CTV-N expanded 10 mm in the superior-inferior direction and 7 mm in the anterior-posterior and lateral directions and CTV-V uniformly expanded 15 mm), were analyzed as a training cohort. Uniform three-dimensional planning margins of 5, 7, 10, 12, and 15 mm were added to the CTVpre to generate PTVpre, which were then projected onto each posttreatment iCBCT scan to assess the minimum planning margins required to encompass the CTVpost in the anterior-posterior, lateral, and superior-inferior directions.
Dosimetric evaluation
After determining the minimum adaptive margin expansion based on the training cohort, a separate cohort of 125 iCBCT scans from 5 postoperative endometrial and cervical cancer patients previously treated on Halcyon Linac (Varian Medical Systems, Palo Alto, USA) were evaluated to determine the dosimetric benefit of reduced online adaptive PTV margins. These iCBCT scans were uploaded to the Ethos oART emulator for daily adaptive replanning, and two reference plans on the same fraction were generated, adopting two margins and using the same planning templates. The adaptive margin plan, using the determined adaptive margin (5 mm), was compared with the conventional margin plan.
Target coverage evaluation from the validation cohort and follow-up
A CTV-to-PTV uniform margin expansion of 5 mm was verified on a validation cohort of 253 pairs of iCBCT scans in 256 fractions from 10 enrolled patients treated with daily oART, and margins of 0, 1, 2, 3, 4, and 5 mm in each of the above directions were explored. The CTV-N was divided into upper, middle and lower portions for precise evaluation, and the boundaries were the bifurcation of the common iliac artery and the appearance of the piriformis muscle.
Acute toxicities, measured from the initiation of oART to 90 days after completion, of the patients from the validation cohort with reduced 5 mm PTV margins were studied. Since the beginning of the treatment, patients were interviewed weekly during the period of oART and followed up by evaluations at one-month intervals for three months after completing the treatment. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE 5.0). The workflow of this study is presented in Fig. 1.
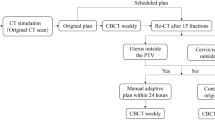
Workflow of this study to determine the optical PTV margins and explore the clinical and dosimetric advantages of reduced margins. The minimum adaptive margin expansion for the training cohort was determined by registering pre- and posttreatment iCBCT images with respect to bony anatomy. A separate cohort with a total of 125 fractions of iCBCT scans was uploaded to the oART simulator for dosimetric advantage evaluation with the minimum adaptive margins. The minimum adaptive expansion was verified in a validation cohort, and acute toxicity was studied
Statistical analysis
The statistical analysis was performed using SPSS (version 27.0; IBM Corp). The minimum PTVpre margins to encompass CTVpost were assessed using the chi-square test and compared with CTV 100% coverage. Data with a normal distribution were analyzed by t test, and data with a nonnormal distribution or heterogeneous variance were analyzed by nonparametric test. p values < 0.05 denoted a significant difference.
Results
Clinical characteristics of patients
Five postoperative endometrial cancer patients and ten cervical cancer patients with 381 fractions were enrolled in this prospective study and treated with daily oART. Three fractions for PTV margin analysis were excluded in the validation cohort due to posttreatment iCBCT loss. Supplementary Table A2 summarizes the clinicopathological characteristics of the patients.
Target coverage in the training cohort
The average time from iCBCT-1 to iCBCT-3 was 22 min 54 s (range: 17 min 24 s − 38 min 43 s). After the posttreatment iCBCT scan was rigidly registered pretreatment iCBCT scan with respect to bony anatomy, a uniform three-dimensional CTV-Npre to PTV-Npre margin of 5, 7, 10, 12, and 15 mm could cover CTV-Npost in all fractions (125/125, p > 0.05). A uniform margin of 5 mm could encompass 98% of the CTV-Vpost (123/125, p > 0.05), and a 7 mm anterior expansion was needed to cover the remaining two fractions. This 5 mm expansion was then used in the dosimetric evaluation and validation cohort.
Dosimetric outcomes
Dosimetric outcomes were assessed in a separate cohort of 125 daily fractions of 5 patients. Table 1 shows the complete list of dosimetry comparisons among the adapted plan with 5 mm margin oART (A-ART) and the adapted plan with conventional margin oART (A-CRT), and the target volume for both plans achieved clinically acceptable quality. Bladder dosimetry significantly improved with reduced margins (p < 0.05), including V40Gy (A-ART 20.78% vs. A-CRT 35.53%), V30Gy (A-ART 33.94% vs. A-CRT 52.1%), V20Gy (A-ART 54.06% vs. A-CRT 71.81%), V10Gy (A-ART 90.18% vs. A-CRT 91.95%), and Dmean (A-ART 0.99 Gy vs. A-CRT 1.22 Gy). The reduced margin also significantly decreased the dose to critical OARs, such as the rectum, bone marrow, left femur head, right femur head and bowel.
Target coverage in the validation cohort
The average time from iCBCT-1 to iCBCT-3 was 22 min 47 s (range: 18 min 15 s -26 min 46 s). Figure 2 shows the CTVpost and PTVpre contours superimposed onto the images after pre- and posttreatment iCBCT matching to exemplify the required minimum margin. Using a uniform 5-mm expansion, 100% (253/253, p > 0.05) of CTV-Npost was covered, and 99% (250/253, p > 0.05) of CTVpost was covered. The three fractions of two patients not fully covered needed the following minimum margins to cover the difference: 7 mm in the anterior direction; 6 mm in the anterior, posterior, left directions; 8 mm in the posterior direction, and 7 mm in the left direction. After further isocentric reduction of the PTV margins to 4 mm, the percentage of CTV-N coverage was greater than 91.3% (231/253) in six directions. This was not statistically significant (p > 0.05) if ≥ 95% CTV coverage was predicted to be achieved. The margins to encompass the CTVpost in the anterior-posterior, lateral, and superior-inferior directions are shown in Table 2.
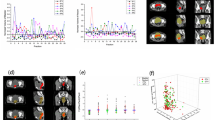
Example images of axial (a and b) and sagittal (c and d) slices of a patient from the validation cohort. The CTVpost (red) and PTVpre (blue) contours were superimposed onto the images after pre- and posttreatment iCBCT matching with respect to bony anatomy. After 100% coverage with a 5 mm margin (a and c), margins of 0 (CTVpre, yellow), 1 (white), 2 (magenta), 3 (orange), 4 (green), and 5 (PTVpre, blue) mm in the three-dimensional direction were explored (b and d)
Acute toxicities
The follow-up data from ten patients with reduced 5 mm margins were complete. No patient experienced distant metastasis or pelvic recurrence at the last follow-up. Table 3 shows the acute toxicities. No urinary or skin toxicities were observed in this study. Leukopenia (6/10 patients) was the most common hematologic toxicity, while diarrhea (4/10 patients) and nausea (3/10 patients) were the most common gastrointestinal disorders. One patient had mild vaginal discharge, and one had slightly increased alanine aminotransferase levels. Toxicities were relieved in all but one patient with grade 1 diarrhea at one month after the end of oART. No toxicity was reported at the three-month and six-month follow-up.
Discussion
To our knowledge, this is the first prospective clinical study to evaluate PTV margins for postoperative treatment of endometrial and cervical cancer with daily oART. The PTV margins and OAR volumes were correlated with the severity of irradiation toxicity [15, 16]. Traditional PTV margins for postoperative endometrial and cervical cancer have been large, and the recommendation is to have a uniform expansion of 5 to 10 mm for CTV-N and an expansion of 6 to 8 mm for CTV-V if using an ITV with daily CBCT guidance [14]. In the NRG/Radiation Therapy Oncology Group (RTOG) 1203 trial, a uniform PTV expansion of 7 mm was used on the basis of the ITV determined by emptying and filling the bladder [17]. Image-guided irradiation therapy (IGRT) allows real-time monitoring of the target and OAR but cannot conduct interfractional interventions, such as modifying the target volume influenced by bladder and rectum filling. In contrast, daily oART may potentially allow for reduced CTV-to-PTV margins by adapting to the deforming operative bed. However, the extent and value of margin reduction must be determined and validated in clinical trials.
In a previous similar experiment, the predicted margins were estimated by equations comparing the overlap of CTVpost and PTVpre to evaluate adequate coverage [18, 19]. Using equations to calculate the predicted margin may yield better coverage, but there may be some areas in each fraction that are not covered, and whether this missed area has a clinical impact is unclear. In this prospective study, we adopted more stringent judgment criteria to assess target coverage, according to Jhingran et al. [20], evaluating the PTV margins with 100% morphological overlap of CTV coverage. In addition, unlike most previous weekly adaptation studies [17, 18], our department appointed designated physicians, physicists and therapists to participate in this phase 2 study concerning daily adaptation. Given that, we anticipate that the current study would be more accurate. As this was the first clinically implemented online adaptive workflow with postoperative cervical and endometrial cancer with no defined PTV margins, we started with a conventional margin (7–15 mm) in the training cohort. Then, posttreatment iCBCT scans were uploaded to the oART emulator to determine the expansion margins in the validation cohort.
In previous studies, CTV-V has received more research attention and is considered to be more prone to variation than CTV-N due to the daily changes in the operative bed caused by the adjacent bladder and rectum pushing boundaries. Jhingran et al. [21] evaluated vaginal vault variations during a 5-week course of postoperative radiotherapy of endometrial and cervical cancer through measured movement of vaginal markers, and they found that the maximum variations were largest in the anterior-posterior and superior-inferior directions, with a median of 1.46 cm and 1.2 cm, respectively, and the median maximum movement was 0.59 in the right-left direction. Jürgenliemk-Schulz et al. [22] reported that the CTV-V position changed after hysterectomy for cervical or endometrial cancer during the course of radiotherapy. Homogenous CTV-to-PTV margins that allowed complete coverage of 90% and 95% of CTV-V were 0.9–1.1 cm and 2.3–1.5 cm, respectively, and CTV-V was not completely covered in 53.3% and 20.0% of fractions with homogenous CTV–PTV margins of 1.0 and 1.5 cm, respectively. These studies performed offline adaptive analysis using weekly MR or CT scans during treatment, which could not represent the intrafractional changes and were therefore larger than what we reported in our study. In contrast, our results confirmed that daily oART could correct these variations and significantly reduce PTV margins; specifically, the borders of CTV-V were the posterior bladder wall and the anterior rectal wall, which does not take interfractional organ motion into account. The missed CTV-V area occurred mostly in the anterior-posterior direction for fractions with 5-mm margins, which is consistent with the maximum anterior–posterior shift reported above.
Yen et al. [19] first performed CTV delineation on pre- and posttreatment CBCT scans to analyze the PTV margin for the cervical lesion region by using CBCT-guided oART, and they recommended that a 5 mm CTV expansion was sufficient for cervical lesions without complex circumstances. However, they did not explore margins for CTV-N. Our results indicated that the PCTV-N margins could be further reduced to 4 mm (p > 0.05) if ≥ 95% CTV coverage was predicted to be achieved. However, the PTV-N margin could not be further reduced, which may be inconsistent with our initial assumption that CTV-N is close to the pelvis and that the ITV could be corrected after iCBCT registration with respect to bony anatomy, resulting in further reductions in CTV-N expansion margins. These discrepancies could be from a residual rotational error, which could not be ignored in daily CBCT-guided radiotherapy in the study by Laursen and colleagues [23], and Ethos Linac from our study is equipped with a three-dimensional treatment couch that could correct limited rotational error. Furthermore, the observer bias for CTV contour inevitably increased as the PTV margin decreased. Our results also indicated that the PTV margins in the anterior-posterior direction of the middle CTV-N and lateral direction of the lower CTV-N were smaller than those in other directions in the same regions. Given that the presacral nodal CTV was not contoured when the piriformis muscle appeared, the vertical displacement of the image easily leads to missed CTV in the anterior-posterior direction with small PTV margins after pretreatment iCBCT was rigidly matched to posttreatment iCBCT with respect to bony anatomy. Another potential explanation is that the degree of relaxation of the piriformis muscle changed before and after treatment, which affected the delineation of CTV. In the lower CTV-N portion, the obturator nodal CTV is affected by intrafractional bladder filling, which may account for the larger lateral expansion margins of the lower CTV-N.
The implementation of reduced margins in our study resulted in a significantly lower OAR dose. This is consistent with previous studies that have demonstrated the dosimetric advantages of reduced margins compared with larger conventional margins [13, 24, 25]. These dosimetric advantages ultimately need to be reflected in clinical advantages. When compared with previous studies [17, 26, 27], the acute complication rates observed in our enrolled patients were more satisfactory, with no grade 3–4 adverse gastrointestinal complications and no urinary complications, correlating well with the improvement in bladder dosimetry. However, the improvement of grade 1 and 2 gastrointestinal toxicity and hematological toxicity was not obvious, which was not consistent with the significantly reduced dose to the corresponding OAR. This may be related to the resulting high toxicity rates from the majority of enrolled patients receiving concurrent chemotherapy or prior chemotherapy. Furthermore, although the posterior border was the anterior rectal wall and interfractional organ motion was not considered when the observer delineated CTV-V, the residual uterosacral ligaments and approximately the anterior one-third of the mesorectum still needed to be contoured. After expansion with a 5 mm PTV margin, most of the rectum was still irradiated.
Given that the coverage of the para-aortic lymph node chain required the use of extended iCBCT and was time-consuming, patients who had indications for treatment with extended-field pelvic radiotherapy were not enrolled in this study, so the PTV-N margins for the para-aortic nodal CTV were not evaluated. In addition, considering the implementation efficiency and time span of this clinical trial, a separate cohort of 125 iCBCT scans from Halcyon Linac was uploaded to the oART simulator for dosimetric advantage evaluation after determining the minimum margin required to encompass the CTVpost instead of using the iCBCT scans from the validation cohort. Furthermore, the PTV expansions in oART data presented in this study were based on 378 fractions from 15 patients. This is sufficient for evaluating PTV margins but far from sufficient to assess irradiation toxicities, which need to be studied in larger populations.
Conclusion
In summary, to our knowledge, this is the first prospective phase 2 study to evaluate PTV margins for postoperative oART of endometrial and cervical cancer. The oART margins could be reduced to 5 mm symmetrically while maintaining excellent dosimetric coverage, and oART with these reduced margins could significantly decrease the dose to OARs and lead to a lower incidence of acute toxicity. Furthermore, PTV-N expansion could be further narrowed with strict bladder and bowel preparations. Currently, the PTV margins reduction could only be safely accomplished with daily oART, and requires favorable logistics to accommodate prolonged treatment time on couch. The irradiation toxicity of oART will need to be validated prospectively in a larger cohort.
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- CTCAE:
-
National Cancer Institute Common Terminology Criteria for Adverse Events
- CTV-N:
-
Nodal clinical target volume
- CTV-V:
-
Vaginal clinical target volume
- iCBCT:
-
Iterative cone-beam computed tomography
- IMRT:
-
Intensity-modulated radiation therapy
- ITV:
-
Internal target volume
- OAR:
-
Organ at risk
- oART:
-
Online adaptive radiotherapy
- PTV:
-
Planning target volume
References
Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet Mar. 2010;6(9717):816–23. https://doi.org/10.1016/s0140-6736(09)62163-2
Creutzberg CL, Nout RA. The role of radiotherapy in endometrial cancer: current evidence and trends. Curr Oncol Rep Dec. 2011;13(6):472–8. https://doi.org/10.1007/s11912-011-0191-y
Perrucci E, Cerrotta A, Macchia G, et al. Postoperative treatment of intermediate-risk early stage cervical cancer: results of a survey from the gynecology study group in the AIRO Gyn and MITO groups. Crit Rev Oncol Hematol Jun. 2022;174:103704. https://doi.org/10.1016/j.critrevonc.2022.103704
Huang H, Feng YL, Wan T, et al. Effectiveness of sequential chemoradiation vs concurrent chemoradiation or radiation alone in adjuvant treatment after hysterectomy for cervical cancer: the STARS phase 3 randomized clinical trial. JAMA Oncol Mar. 2021;1(3):361–9. https://doi.org/10.1001/jamaoncol.2020.7168
Wortman BG, Creutzberg CL, Putter H, et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer Oct. 2018;119(9):1067–74. https://doi.org/10.1038/s41416-018-0310-8
Li L, Kou X, Feng X, Zhang M, Chao H, Wang L. Postoperative external beam irradiation with and without brachytherapy in pelvic node-positive IB1-IIA2 cervical cancer patients: a retrospective clinical study. Radiat Oncol Sep. 2015;17:10:189. https://doi.org/10.1186/s13014-015-0495-4
Kwak YK, Lee SW, Kay CS, Park HH. Intensity-modulated radiotherapy reduces gastrointestinal toxicity in pelvic radiation therapy with moderate dose. PLoS ONE. 2017;12(8):e0183339. https://doi.org/10.1371/journal.pone.0183339
Alongi F, Fiorino C, Cozzarini C, et al. IMRT significantly reduces acute toxicity of whole-pelvis irradiation in patients treated with post-operative adjuvant or salvage radiotherapy after radical prostatectomy. Radiother Oncol Nov. 2009;93(2):207–12. https://doi.org/10.1016/j.radonc.2009.08.042
Small W Jr., Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys Jun. 2008;1(2):428–34. https://doi.org/10.1016/j.ijrobp.2007.09.042
Vandecasteele K, Tummers P, Makar A, et al. Postoperative intensity-modulated arc therapy for cervical and endometrial cancer: a prospective report on toxicity. Int J Radiat Oncol Biol Phys Oct. 2012;1(2):408–14. https://doi.org/10.1016/j.ijrobp.2011.12.020
Zhang G, He F, Miao L, Wu H, Zhang Y, Fu C. Post-operative small pelvic intensity-modulated radiation therapy for early-stage cervical cancer with intermediate-risk factors: efficacy and toxicity. Jpn J Clin Oncol May. 2021;28(6):905–10. https://doi.org/10.1093/jjco/hyab047
de Jong R, Visser J, van Wieringen N, Wiersma J, Geijsen D, Bel A. Feasibility of Conebeam CT-based online adaptive radiotherapy for neoadjuvant treatment of rectal cancer. Radiat Oncol Jul. 2021;23(1):136. https://doi.org/10.1186/s13014-021-01866-7
Shelley CE, Bolt MA, Hollingdale R, et al. Implementing cone-beam computed tomography-guided online adaptive radiotherapy in cervical cancer. Clin Transl Radiat Oncol May. 2023;40:100596. https://doi.org/10.1016/j.ctro.2023.100596
Small W Jr., Bosch WR, Harkenrider MM, et al. NRG Oncology/RTOG Consensus guidelines for delineation of clinical target volume for intensity modulated pelvic radiation therapy in postoperative treatment of endometrial and cervical cancer: an update. Int J Radiat Oncol Biol Phys Feb. 2021;1(2):413–24. https://doi.org/10.1016/j.ijrobp.2020.08.061
Fiorino C, Alongi F, Perna L, et al. Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat Oncol Biol Phys Sep. 2009;1(1):29–35. https://doi.org/10.1016/j.ijrobp.2008.10.086
Wang D, Yin Y, Zhou Q, et al. Dosimetric predictors and lyman normal tissue complication probability model of hematological toxicity in cervical cancer patients with treated with pelvic irradiation. Med Phys Jan. 2022;49(1):756–67. https://doi.org/10.1002/mp.15365
Klopp AH, Yeung AR, Deshmukh S, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG oncology-RTOG 1203. J Clin Oncol Aug. 2018;20(24):2538–44. https://doi.org/10.1200/jco.2017.77.4273
Morgan HE, Wang K, Yan Y, et al. Preliminary evaluation of PTV margins for online adaptive radiation therapy of the prostatic fossa. Pract Radiat Oncol Dec. 2022;10. https://doi.org/10.1016/j.prro.2022.11.003
Yen A, Choi B, Inam E, et al. Spare the bowel, don’t spoil the target: optimal margin assessment for online cone beam adaptive radiation therapy (OnC-ART) of the cervix. Pract Radiat Oncol Mar-Apr. 2023;13(2):e176–83. https://doi.org/10.1016/j.prro.2022.10.009
Tyagi N, Lewis JH, Yashar CM, et al. Daily online cone beam computed tomography to assess interfractional motion in patients with intact cervical cancer. Int J Radiat Oncol Biol Phys May. 2011;1(1):273–80. https://doi.org/10.1016/j.ijrobp.2010.06.003
Jhingran A, Salehpour M, Sam M, Levy L, Eifel PJ. Vaginal motion and bladder and rectal volumes during pelvic intensity-modulated radiation therapy after hysterectomy. Int J Radiat Oncol Biol Phys Jan. 2012;1(1):256–62. https://doi.org/10.1016/j.ijrobp.2010.08.024
Jürgenliemk-Schulz IM, Toet-Bosma MZ, de Kort GA, et al. Internal motion of the vagina after hysterectomy for gynaecological cancer. Radiother Oncol Feb. 2011;98(2):244–8. https://doi.org/10.1016/j.radonc.2010.10.026
Laursen LV, Elstrøm UV, Vestergaard A, et al. Residual rotational set-up errors after daily cone-beam CT image guided radiotherapy of locally advanced cervical cancer. Radiother Oncol Nov. 2012;105(2):220–5. https://doi.org/10.1016/j.radonc.2012.08.012
Åström LM, Behrens CP, Storm KS, Sibolt P, Serup-Hansen E. Online adaptive radiotherapy of anal cancer: normal tissue sparing, target propagation methods, and first clinical experience. Radiother Oncol Nov. 2022;176:92–8. https://doi.org/10.1016/j.radonc.2022.09.015
Al-Mamgani A, Kessels R, Janssen T, et al. The dosimetric and clinical advantages of the GTV-CTV-PTV margins reduction by 6 mm in head and neck squamous cell carcinoma: significant acute and late toxicity reduction. Radiother Oncol Mar. 2022;168:16–22. https://doi.org/10.1016/j.radonc.2022.01.013
Hasselle MD, Rose BS, Kochanski JD, et al. Clinical outcomes of intensity-modulated pelvic radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys Aug. 2011;1(5):1436–45. https://doi.org/10.1016/j.ijrobp.2010.04.041
Yuce Sari S, Guler OC, Gultekin M, Onal HC, Yildiz F. Adjuvant small pelvic radiotherapy in patients with cervical cancer having intermediate risk factors only - is it sufficient? Oncol Res Treat. 2017;40(9):523–7. https://doi.org/10.1159/000476037
Acknowledgements
We acknowledge the technical support from Varian, a Siemens Healthineers company, for providing the oART emulator and equipment necessary to carry out the experiments described in this study.
Funding
The National High Level Hospital Clinical Research Funding (2022-PUMCH-B-052, No.2022-PUMCH-B-116) supported this work.
Author information
Authors and Affiliations
Contributions
WG was responsible for data collection and drafted the manuscript; WG and WZ were major contributors in writing the manuscript. YJ and ZF designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board of Peking Union Medical College Hospital (No. K2416).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Table A1
Study inclusion and exclusion criteria. Table A2 Clinicopathological characteristics
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, G., Wang, Z., Guo, Y. et al. Evaluation of PTV margins with daily iterative online adaptive radiotherapy for postoperative treatment of endometrial and cervical cancer: a prospective single-arm phase 2 study. Radiat Oncol 19, 2 (2024). https://doi.org/10.1186/s13014-023-02394-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02394-2