Abstract
Propose
To evaluate the advantage of the manual adaptive plans comparing to the scheduled plans, and explored clinical factors predicting patients suitable for adaptive strategy.
Methods and materials
Eighty two patients with weekly online cone-beam computed tomography (CBCT) were enrolled. The re-CT simulation was performed after 15 fractions and a manual adaptive plan was developed if a significant deviation of the planning target volume (PTV) was found. To evaluate the dosimetric benefit, D98, homogeneity index (HI) and conformity index (CI) for the planning target volume (PTV), as well as D2cc of the bowel, bladder, sigmoid and rectum were compared between manual adaptive plans and scheduled ones. The clinical factors influencing target motion during radiotherapy were analyzed by chi-square test and logistic regression analysis.
Results
The CI and HI of the manual adaptive plans were significantly superior to the scheduled ones (P = 0.0002, 0.003, respectively), demonstrating a better dose coverage of the target volume. Compared to the scheduled plans, D98 of the manual adaptive plans increased by 3.3% (P = 0.0002), the average of D2cc to the rectum, bladder decreased 0.358 Gy (P = 0.000034) and 0.240 Gy (P = 0.03), respectively. In addition, the chi-square test demonstrated that age, primary tumor volume, and parametrial infiltration were the clinical factors influencing target motion during radiotherapy. Multivariate analysis further identified the large tumor volume (≥ 50cm3, OR = 3.254, P = 0.039) and parametrial infiltration (OR = 3.376, P = 0.018) as the independent risk factors.
Conclusion
We found the most significant organ motion happened after 15 fractions during treatment. The manual adaptive plans improved the dose coverage and decreased the OAR doses. Patients with bulky mass or with parametrial infiltration were highly suggested to adaptive strategy during definitive radiotherapy due to the significant organ motion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locally advanced cervical cancer (LACC), which is defined as stage IB3-IVA according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) staging system, accounts for more than 80% of all cases in patients with cervical cancer (Sung et al. 2021). Concurrent chemoradiotherapy is the standard of care for locally advanced cervical cancer (Cohen et al. 2019; Monk et al. 2007). However the tumor regression and deformation combined with organ motion during the course of fractionated RT remain challenges in clinical practice of IMRT use.
Often a significant reduction of cervix tumor is observed during the 5 weeks of radiotherapy (RT) (Mayr et al. 2002, 1996). Besides the tumor regression, previous studies also demonstrated the intrafraction movements of pelvic organ, especially the movements of uterus, the filling status of bladder and rectum might result in variations in position and shape of the clinical target volume (CTV), and eventually cause geographical miss of the target and unnecessary volumes of organs-at-risk (OARs) included into high dose regions (Beadle et al. 2009; Chan et al. 2008; Lee et al. 2007). The most effective way to date is online adaptive radiotherapy (ART), which uses an imaging feedback loop to correct the deviation caused by tumor shrinkage and organ motion and modify the treatment plan accordingly (Shelley et al. 2021; Hall et al. 2022; Yan et al. 1997).
Despite the real-time online ART has provided improved clinical benefits (Tan et al. 2019), this technique needs intensive clinical effort, facing big challenges with clinical implementation, which make its utilization relatively limited (Qin et al. 2015), especially in low- and middle-income countries where cervical cancer has a greater prevalence. The time spent throughout the online ART workflow has been always an important consideration especially in a busy radiation oncology clinic. Radiation oncologists spend more time (usually more than 30 min per fraction) on verification of the recontouring and approving the replanning at each treatment fraction, while the patient was lying on the LINAC treatment couch during the whole process which would cause further changes of the bladder and rectum filling. In addition, all adaptive plans are automatically created and cannot be adjusted manually to better meet the whole team needs.
It would be beneficial to find an alternative workflow to avoid recontouring for every adaptive plan. A number of studies showed the largest uterus motion happened in the 2nd or 3rd week of treatment (Lee et al. 2007), and the most of tumor regression occurs about 21 days, or after 30.8 Gy of treatment (Lee et al. 2004, and Bunt et al. 2006). Thus, we proposed an alternative offline ART, the manual adaptive radiotherapy, which only required one more CT simulation after 15 fractions during treatment, and the manual adaptive plan was created by conventional treatment planning systems. The offline ART course could be more easily implemented in clinics.
The goal of our study was to verify the appropriate time for manual adaptive planning during the fractionated treatment, compare the dosimetric outcomes of manual adaptive plans to the scheduled plans, and explore the clinical factors to guide patient selection for ART.
Materials and methods
Patients and treatment
Consecutive patients with intact cervical cancer undergoing definitive treatment at our department who agreed to undergo twice CT simulation scans during treatment participated in this study. Between August 2019 and November 2022, 82 patients with histologically confirmed cervical cancer were included. Staging was performed according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) classification. The characteristic of patients, including age, BMI, FIGO stage, tumor size, histological type, and courses of concurrent chemotherapy, were recorded.
Patients underwent CT simulation for treatment planning (initial CT) in supine position with custom immobilization and 3-mm slice thickness. All patients were treated on a linear accelerator equipped with kilo-voltage CBCT (kV-CBCT) with IMRT using seven to nine coplanar fields and 6-MV photons. Treatment was planned using the Eclipse Treatment Planning System version 13.0 (Varian Medical Systems, Palo Alto, CA). In accordance with our treatment protocol all the patients received a combination of external beam radiation therapy (EBRT) with concurrent cisplatin of 40 mg/m2 weekly, followed by high-dose-rate brachytherapy 6 Gy per fraction for 5 fractions.
The delineation of the clinical target volume and organs-at-risk is in accordance with RTOG consensus guidelines. The dose given to PTV was 45–50.4 Gy (25 or 28 fractions of 1.8 Gy), five fractions a week. Simultaneous integrated boost (SIB) of the parametrium and positive lymph nodes were applied if needed. And all patients received the high-dose-rate brachytherapy 6 Gy per fraction for 5 fractions. The total dose achieved 85–90 Gy (EQD2, biologically equivalent dose of 2 Gy per fraction).
All patients received at least a weekly cone-beam computed tomography (CBCT) scan throughout the whole EBRT course. The image datasets including one simulation CT for treatment planning (initial CT) and weekly CBCT images at the time of treatment per patient were collected. For each patient, the weekly CBCT images were projected rigidly to the initial CT image using matching of the bony structures to evaluate the uterine motion during radiation treatment. To minimize internal organ motion caused by bladder, patients were asked to empty bladder first and then to drink 500 ml of water 1 h before CT simulation as well as daily radiation treatments. To maintain an empty rectum, patients were asked to have a bowel movement or to use glycerin enema within 4 h before simulation and each of the radiation treatments.
Manual adaptive radiotherapy
Figure 1 showed the workflow procedure of the manual adaptive planning during radiotherapy. All patients underwent a second CT simulation scan (re-CT) after 15 fractions during treatment. The re-CT images were fused to the initial CT image set using bone matching, and the original PTV were copied to the re-CT images. The thresholds for re-plan action after 15 fractions of treatment were 1) if uterus was outside the original PTV or 2) if cervix/upper vagina was outside the original PTV. In this case, a new treatment plan (the manual adaptive plan) with the re-contoured target volume and OARs based on re-CT scan of the remaining fractions was calculated. Similarly, the delineation of the clinical target volume and organs-at-risk is in accordance with RTOG consensus guidelines.
The new plan utilizes the same standards and prescribed dose as the original plan, even being created by the same physicist, the primary target coverage objectives were as follows, more than 99% of the PTV volume to be covered by 95% of the prescription dose.
Monitoring uterine motion during external beam radiotherapy
In the median plane (sagittal plane through the midline of the body), we measured “line A” (Fig. 2) from the superior border of the fundus uteri to the superior border of the pubic symphysis, and measured “line B” (Fig. 2) from the anterior border of the uterus to the anterior border of the pubic symphysis in weekly CBCT images as well as the initial CT image per patient. The initial position of uterus was normalized to 0. We calculated ΔA and ΔB to evaluate the movement of uterus, and eventually got value “C” for comparison.
The definition of the distance to the boundary of the uterus in the median plane (sagittal plane through the midline of the body) on magnetic resonance images (an example of 1 patient, on a T2-weighted magnetic resonance image). A means the distance from the superior border of the fundus uteri to the superior border of the pubic symphysis. B means the distance from the anterior border of the uterus to the anterior border of the pubic symphysis
The ΔA, ΔB and C was calculated as follows,
“A0” means the distance from the superior border of the fundus uteri to the superior border of the pubic symphysis in initial CT image.
“B0” means the distance from the anterior border of the uterus to the anterior border of the pubic symphysis in initial CT image.
“n” represents the number of CBCT sessions.
The volumes of cervix gross tumor were computed by eclipse software.
Dose data acquisition
The original CT and the re-CT (15 fractions after) are fused and registered in the Varian Eclipse planning system according to the bone structure, and then the original plan is loaded onto the re-CT to generate the plan, named scheduled plan. To compare the planning target volume coverage between the manual adaptive plan and scheduled plan, the conformity index (CI) and homogeneity index (HI) were calculated. The values of dosimetric objectives obtained from the dose-volume histograms (DVHs) were compared. For the PTV, the dosimetric parameters included the mean dose (Dmean), the dose received by 98% of the target volume (D98), the dose received by 2% of the target volume (D2), homogeneity index (HI) and conformity index (CI). For the OARs, such as bowel, bladder, sigmoid colon and rectum, the dosimetric parameters referred to the maximum dose to 2 cc’s (D2cc). All these dosimetric parameters were compared between the manual adaptive plan and scheduled plan in the remaining radiation fractions after 3 weeks of treatment.
The HI was calculated as follows (Wu et al. 2003),
where D2, D98 and Dmean were defined as mentioned above.
The CI was calculated as follows (Paddick. 2000),
Vt, ref is the target volume covered by the reference isodose line, Vt is the volume of the PTV, and Vref is the total volume covered by the reference isodose line.
Statistical analysis
The SPSS software (version 19, SPSS Inc., Chicago, IL, USA) was conducted for the statistical analysis. Kruskal–Wallis with Dunn’s multiple comparisons test were used to analyze uterine motion during EBRT, and t-tests were used to compare the accumulated doses to the targets and OARs from the two planning scenarios. In addition, the chi-square test (or Fisher’s exact test when appropriate), univariate and multivariate logistic regression analysis were performed to evaluate the clinical factors influencing target motion. We defined p < 0.05 as statistically significant. This study was approved by the Medical Ethics Committee of the Ruijin Hospital (approval number:2024153). Written informed consent was not required because of the retrospective nature of the study.
Result
Baseline characteristics of patients
Table S1 showed the clinical characteristics of these 82 LACC patients. The median age was 57 years (range 34–85 years) at time of diagnosis. And disease was restaged according to the 2018 FIGO staging system. The mean clinical tumor volume was 66.8cm3 (range 12–279.9cm3). All patients underwent a second CT simulation scan (re-CT) after 15 fractions during treatment, and 41 patients completed the remaining fractions using a new treatment plan (the manual adaptive plan) due to significant deviation of the PTV. All patients completed EBRT followed by brachytherapy, while 66 patients received concurrent platinum-based chemotherapy.
Analysis of inter-fraction motion during EBRT
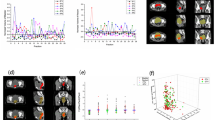
Table S2 summarized the motion of the uterus during the EBRT treatment period. The initial position of uterus was normalized to 0. As shown in the Table S2, the maximum movement of the uterus happened three weeks after treatment started, the median value “C” and the maximum value “C”were 1.811 and 4.517, respectively. Kruskal–Wallis with Dunn’s multiple comparisons test showed that the median uterus movement after three weeks (3rd CBCT) was greater compared with the movement after one week (1st CBCT, P < 0.0001) and two weeks (2nd CBCT, P = 0.0045) after the treatment started. Additionally, the median uterus movement after three weeks and four weeks has no significant differences. Figure 3 showed the inter-fraction motion of uterus (Value C) of all the patients.
Target volume and OARs dose data analyses
Figure 4a showed the mean CI of scheduled plan was 0.7711 ± 0.1382, which was lower than that of the manual adaptive plan 0.8586 ± 0.0354 (P = 0.0002). Similarly, the mean HI of the scheduled plan was 0.1764 ± 0.0779, which was higher than that of the manual adaptive plan 0.1469 ± 0.0776 (P = 0.003). Additionally, as shown in the Fig. 4b, PTV D98 of manual adaptive plan was 2287.6 ± 59.8 cGy, which was higher than that of the scheduled plan (2212.9 ± 117.67 cGy, P = 0.0002). That is to say, the dose conformity, dose homogeneity and target coverage of PTV in manual adaptive plan is better than the scheduled plan (see Fig. 4c). There is no difference in D2 and Dmean between the two groups (Table S3).
Comparison of the planning target volume coverage (PTV CI, HI, and D98) between the scheduled plan and manual adaptive plan. a Point plots of CI and HI for scheduled plan and manual adaptive plan, student’s t test, n = 41. b Point plots of accumulated dose to 98% volume for scheduled plan and manual adaptive plan, student’s t test, n = 41. c Comparison of the isodose distribution images of transverse for scheduled plan and manual adaptive plan of one patient. ***p < 0.001
Table 1 depict the differences in the D2cc of the organs-at-risk between the manual adaptive plan and scheduled plan. The D2cc of the rectum, bladder for the remaining fractions of manual adaptive plan was 2426.53 ± 67.66 cGy and 2502.75 ± 13.18 cGy, which was lower than that of scheduled plan, 2462.37 ± 74.37 cGy (P = 0.000034) and 2526.7 ± 14.32 cGy (P = 0.03), respectively. There is no difference in D2cc to the sigmoid and bowel between the two groups. The DVH lines for the two plans were displayed in Fig. S1 (an example of 1 patient).
Relationship between clinicopathological characteristics and replanning in LACC patients
Table 2 showed the clinicopathological characteristics of patients treated with scheduled plan or manual adaptive plan. Chi-square test showed that compared with the scheduled plan group, patients in the manual adaptive plan group were younger (age ≤ 60, 73.2% vs. 48.8%, P = 0.024) and with larger primary tumor volume (≥ 50cm3, 82.9% vs. 58.5%, P = 0.015). In addition, parametrial infiltration was found more in manual adaptive plan than in scheduled plan one, 75.6% (31/82) vs. 51.2% (21/82), P = 0.022.
In univariate analysis, the age (HR 2.864; 95%CI 1.138–7.209; P = 0.026), primary tumor volume (HR 3.440; 95%CI 1.236–9.576; P = 0.018), and parametrial infiltration (HR 2.952; 95%CI 1.154–7.556; P = 0.024) were the clinical factors significantly influencing target motion during EBRT. The multivariate analysis found that the tumor volume ≥ 50cm3 (HR 3.254; 95%CI 1.062–9.969; P = 0.039) and parametrial infiltration (HR 3.376; 95%CI 1.229–9.270; P = 0.018) were independent risk factors for replanning in patients with LACC (Table 3).
Discussion
Substantial uterus motion and tumor shrinkage during radiotherapy significantly influence target coverage and OAR doses in LACC patients (Oh et al. 2014). There are previous studies demonstrating that oART can improve the target coverage and also reduce the dose of RT delivered to organs at risk in a variety of tumor sites (Hall et al. 2022). Online Adaptive Radiation Therapy (oART) is a radiation therapy technique that adjusts the treatment plan during the radiation therapy process based on changes in the patient's anatomical structure. This technology relies on advanced image-guided systems, such as cone-beam computed tomography (CBCT), to capture real-time images of the patient before each treatment fraction. If significant changes in the patient's anatomical structure are detected, such as tumor shrinkage, displacement, or shape alteration, the doctors can adjust the patients’ target volume and treatment plan by oART technology to ensure the accuracy and effectiveness of the treatment. During the online ART treatment, additional consideration regarding staffing and workflow are needed during each treatment, for example, it requires a radiation oncologist, medical physicist and radiation therapists to spend additional time at the machine daily (Shelley et al. 2021; Hall et al. 2022).
Thus, in our study, we introduced an alternative offline ART, the manual adaptive radiotherapy, which only required one more CT simulation during the definitive EBRT for cervical cancer. Our study analyzed the uterus mobility based on the weekly CBCT images, and found that the greatest variation in uterus position happened after 15 fractions of treatment. Thus, we recommend the patients underwent re-CT after 15 fractions during the manual adaptive radiotherapy, and ran the manual adaptive plan for the remaining fractions if significant deviation of the original PTV (1. if uterus was outside the PTV or 2. if cervix/upper vagina was outside the PTV). The new plan utilizes the same standards and prescribed dose as the original plan, even being created by the same physicist. We furthermore evaluated the potential dosimetric benefits compared with the scheduled plans, and explored the clinicopathological factors guiding patient selection for oART as well (for those have great variation in their uterus were suggested to underwent oART which will create a new plan according to their anatomical changes daily).
The key of offline ART is to determine the appropriate time for the replanning. A number of studies showed that the location of the uterus changes significantly in LACC patients during the EBRT course (Bunt et al.2008, Taylor et al. 2008, Jadon et al.2014), with the largest motion happened in the 2nd or 3rd week of treatment (Lee et al. 2007). In addition, it is reported that most of the tumor regression occurs during the first 3–4 weeks of treatment, with a mean tumor volume reduction of 50% after about 21 days, or after 30.8 Gy (Lee et al. 2004, and Bunt et al. 2006). Consistent with these findings, our study analyzed the uterus mobility based on the weekly CBCT images, and found that the largest range of uterus movement happened after 15 fractions of treatment. All patients in our study underwent re-CT simulation after 15 fractions, and half of them completed the remaining fractions using a new treatment plan (the manual adaptive plan), due to the significant changes in target volume. Several studies have analyzed the dosimetric results on target coverage and dose to OARs when various adaptive procedures adopted. Creating an adaptive plan based on weekly CT/MRI images improved the dosimetric parameters by increasing the target coverage and decreasing the dose to OARs, such as the D2cc and V45Gy (Yock et al.2021; Stewart et al. 2010). And the dosimetric parameters of adaptive plans based on re-CT simulation images after 15 fractions in our study were not inferior to those based on weekly CT/MRI images. We also found PTV D98 of the manual adaptive plans increased by 3.3% (2287.6 cGy vs. 2212.9 cGy, P = 0.0002), which was higher than that of 2.5% in the study with automated weekly replanning (Stewart et al.2010).
For the OARs, Yock et al. described the decrease in the D2cc of the OAR resulting from the adaptive replan for cervical cancer patients. In their study, the D2cc to the bladder, bowel, rectum, and sigmoid colon for each fraction changed an average of − 0.02 Gy (SD = 0.09, p = 0.017), − 0.08 Gy (SD = 0.06, p < 0.001), − 0.07 Gy (SD = 0.07, p < 0.001), and − 0.04 Gy (SD = 0.05, p < 0.001) respectively (Yock et al. 2021). Consistent with the previous studies, ours showed that compared to the scheduled plans, the average of D2cc to the rectum and bladder in the manual adaptive plans decreased 0.358 Gy (P < 0.001) and 0.240 Gy (P = 0.03), respectively. However, there is no difference in D2cc to the sigmoid or bowel between the two plans, indicating the manual adaptive plan, which depends on numerous interrelated considerations regarding plan reoptimization, may not universally bring dosimetric benefits for all organs in all situations. On the contrary, Oh et al. analyzed the dosimetric effects of various adaptation strategies for cervix cancer. They observed dose reduction to the OAR was not at the level of clinically meaningful in most cases, though target shrinkage was observed (Oh et al. 2014). Therefore, the dosimetric advantages of manual adaptive plans in patients with LACC needed further validation in large-scale studies.
Unfortunately, financial and human resources remain barriers for the introduction of online adaptive radiotherapy in most low- and middle-income countries where cervical cancer has a greater prevalence, although the online ART improved CTV D98 and reduced normal tissue dose. In our study, we meant to find out who would benefit the most from the adaptive radiotherapy with daily replanning to save the time as well as human resources spending on the ART for all the patients. Thus, it’s necessary to identify patients who experience greater organ mobility during EBRT. In our study, the manual adaptive replanning workflow started when the uterus position observed beyond the PTV margin by re-CT after 15 fractions. We tried to explore the clinicopathological factors that may influence organ mobility during EBRT to establish a prediction model selecting patients the most suitable for the ART, which has not been reported in previous studies. The patients in our study with bulky mass (≥ 50cm3, OR = 3.254, P = 0.039) or with parametrial infiltration (OR = 3.376, P = 0.018) were highly suggested to receive adaptive replanning during definitive radiotherapy. Data from our study revealed that 82.9% of patients with tumor volume ≥ 50 cm3 and 75.6% of patients with parametrial infiltration received raplanning. However, to date, there has been no study on the relationship between clinicopathological factors and organ mobility during EBRT. Therefore, further studies are needed to verify the value of these factors for predicting ART in patients with LACC.
The limitations of this study are the CBCT-guided ART and the limited sample size. However, kV CBCT is currently the most commonly available for online volumetric imaging, and in most cases differentiating target from surrounding tissues was not difficult (Khan et al. 2012). The MRI-guided online ART system has the potential advantages such as excellent soft-tissue definition and more accurate target delineation compared to CBCT-guided ART (Fields et al. 2016). And the results from our study need to be confirmed in a large sample of prospective studies. Despite these limitations, our study determined the appropriate time of manual adaptive replanning and further revealed that the tumor volume and parametrial infiltration may be two factors that predict the need for online ART during the EBRT for LACC patients, which has great significance.
In summary, we found the most significant organ motion happened after 15 fractions during treatment. The manual adaptive plan ran only once during the whole treatment time improved the dose coverage and decreased the volume of organs at risk in patients with LACC. Patients with bulky mass or with parametrial infiltration were highly suggested to receiving adaptive radiotherapy.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Beadle BM, Jhingran A, Salehpour M et al (2009) Cervix regression and motion during the course of external beam chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys 73:235–241
Chan P, Dinniwell R, Haider MA et al (2008) Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: a cinematic-MRI point-of-interest study. Int J Radiat Oncol Biol Phys 70:1507–1515
Cohen PA, Jhingran A, Oaknin A (2019) Cervical cancer. Lancet 393:169–182
Fields EC, Weiss E (2016) A practical review of magnetic resonance imaging for the evaluation and management of cervical cancer. Radiat Oncol 2(11):15
Hall WA, Paulson E, Li XA et al (2022) Magnetic resonance linear accelerator technology and adaptive radiation therapy: an overview for clinicians. CA Cancer J Clin 72:34–56
Jadon R, Pembroke CA, Hanna CL et al (2014) A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol (R Coll Radiol) 26:185–196
Khan A, Jensen LG, Sun S et al (2012) Optimized planning target volume for intact cervical cancer. Int J Radiat Oncol Biol Phys 83:1500–1505
Lee CM, Shrieve DC, Gaffney DK (2004) Rapid involution and mobility of carcinoma of the cervix. Int J Radiat Oncol Biol Phys 58:625–630
Lee JE, Han Y, Huh SJ et al (2007) Interfractional variation of uterine position during radical RT: weekly CT evaluation. Gynecol Oncol 104:145–151
Mayr NA, Magnotta VA, Ehrhardt JC et al (1996) Usefulness of tumor volumetry by magnetic resonance imaging in assessing response to radiation therapy in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 35:915–924
Mayr NA, Taoka T, Yuh WT et al (2002) Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys 52:14–22
Monk BJ, Tewari KS, Koh WJ (2007) Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol 25:2952–2965
Oh S, Stewart J, Moseley J, Kelly V et al (2014) Hybrid adaptive radiotherapy with on-line MRI in cervix cancer IMRT. Radiother Oncol 110:323–328
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical Note J Neurosurg 93(Suppl 3):219–222
Qin A, Sun Y, Liang J, Yan D (2015) Evaluation of online/offline image guidance/adaptation approaches for prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys 91:1026–1033
Shelley CE, Barraclough LH, Nelder CL et al (2021) Adaptive radiotherapy in the management of cervical cancer: review of strategies and clinical implementation. Clin Oncol (R Coll Radiol) 33:579–590
Stewart J, Lim K, Kelly V et al (2010) Automated weekly replanning for intensity-modulated radiotherapy of cervix cancer. Int J Radiat Oncol Biol Phys 78:350–358
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249
Tan LT, Tanderup DK, Kirisits DC et al (2019) Image-guided adaptive radiotherapy in cervical cancer. Semin Radiat Oncol. 29:284–298
Taylor A, Powell ME (2008) An assessment of interfractional uterine and cervical motion: implications for radiotherapy target volume definition in gynaecological cancer. Radiother Oncol 88:250–257
van de Bunt L, van der Heide UA, Ketelaars M et al (2006) Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer: the impact of tumor regression. Int J Radiat Oncol Biol Phys 64:189–196
van de Bunt L, Jurgenliemk-Schulz IM, de Kort GA et al (2008) Motion and deformation of the target volumes during IMRT for cervical cancer: what margins do we need? Radiother Oncol 88:233–240
Wu Q, Mohan R, Morris M et al (2003) Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys 56:573–585
Yan D, Vicini F, Wong J et al (1997) Adaptive radiation therapy. Phys Med Biol 42:123–132
Yock AD, Ahmed M, Ayala-Peacock D et al (2021) Initial analysis of the dosimetric benefit and clinical resource cost of CBCT-based online adaptive radiotherapy for patients with cancers of the cervix or rectum. J Appl Clin Med Phys 22:210–221
Funding
This study was supported in part by the Scientific and Technological Innovation Action Plan of Shanghai Science and Technology Committee (No. 22Y31900103), Shanghai Pujiang Program (No. 2019PJD029), and Shanghai Sailing Program (No. 21YF1437500).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Collection and assembly of data: Yi-Wei Wang, Min Chen and Wen-Tong Shen. Data analysis and interpretation: All authors. Manuscript writing: Yi-Wei Wang, and Hao-Ping Xu. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest associated with this manuscript.
Ethical approval
This retrospective study was performed using data from anonymized patients who received radiotherapy treatment between August 2019 and November 2022. Because of the nature of retrospective design and patient anonymization, the Institutional Ethics Committee of the affiliated Ruijin hospital of Shanghai Jiaotong University School of Medicine approved the retrospective study.
Consent to participate
The need for written informed consent was waived due to the retrospective nature of the study.
Consent for publication
All authors have consented to publication of the results presented in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, YW., Chen, M., Shen, WT. et al. The clinical practice and dosimetric outcome of the manual adaptive planning during definitive radiotherapy for cervical cancer. J Cancer Res Clin Oncol 150, 280 (2024). https://doi.org/10.1007/s00432-024-05809-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05809-z