Abstract
Aims
Reirradiation of prostate cancer (PC) local recurrences represents an emerging challenge for current radiotherapy. In this context, stereotactic body radiation therapy (SBRT) allows the delivery of high doses, with curative intent. Magnetic Resonance guided Radiation Therapy (MRgRT) has shown promising results in terms of safety, feasibility and efficacy of delivering SBRT thanks to the enhanced soft tissue contrast and the online adaptive workflow. This multicentric retrospective analysis evaluates the feasibility and efficacy of PC reirradiation, using a 0.35 T hybrid MR delivery unit.
Methods
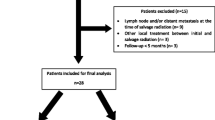
Patients affected by local recurrences of PC and treated in five institutions between 2019 and 2022 were retrospectively collected. All patients had undergone previous Radiation Therapy (RT) in definitive or adjuvant setting. Re-treatment MRgSBRT was delivered with a total dose ranging from 25 to 40 Gy in 5 fractions. Toxicity according to CTCAE v 5.0 and treatment response were assessed at the end of the treatment and at follow-up.
Results
Eighteen patients were included in this analysis. All patients had previously undergone external beam radiation therapy (EBRT) up to a total dose of 59.36 to 80 Gy. Median cumulative biologically effective dose (BED) of SBRT re-treatment was 213,3 Gy (103,1-560), considering an α/β of 1.5. Complete response was achieved in 4 patients (22.2%). No grade ≥ 2 acute genitourinary (GU) toxicity events were recorded, while gastrointestinal (GI) acute toxicity events occurred in 4 patients (22.2%).
Conclusion
The low rates of acute toxicity of this experience encourages considering MRgSBRT a feasibile therapeutic approach for the treatment of clinically relapsed PC. Accurate gating of target volumes, the online adaptive planning workflow and the high definition of MRI treatment images allow delivering high doses to the PTV while efficiently sparing organs at risk (OARs).
Similar content being viewed by others
Introduction
Prostate cancer (PC) is the most common malignancy in terms of incidence in men and currently represents the second leading cause of death [1].
Nevertheless, the possibility to early diagnose it and the recent advancements of treatment strategies are changing the framework of the management of this disease with promising outcomes [2].
The current treatment approach generally involves the use of surgery, androgen deprivation therapy (ADT) or radiation therapy (RT), either administered individually or in combination, depending on the stage of disease [3].
In particular, RT plays a well-standardized role in the radical, adjuvant or palliative treatment settings [4,5,6,7].
An emerging issue is the most appropriate management of disease in the case of a local relapse after previous radical treatment, as nearly one third of the patients experience biochemical or macroscopical relapse after primary treatment, of which 30–47% have previously undergone RT [8, 9].
The most appropriate approach in this clinical setting remains controversial also due to the limited number of evidence providing clear recommendations and consensus statements.
In this context, several treatment options have been investigated and ADT has traditionally been considered as a safe treatment modality [10]. Local treatment approaches including surgery and focal therapies have also been investigated with varying outcomes [11,12,13,14,15].
Considering recurrent PC reirradiation, High-Dose-Rate (HDR) Brachytherapy has historically been considered as a safe, even if minimally invasive, approach due to the possibility of delivering high doses to the target while avoiding healthy tissues [16]. Recently, Munoz et al. performed a systematic review regarding the efficacy and safety of PC reirradiation, demonstrating that this approach appears to be promising in terms of overall survival and biochemical disease control rates with no significant toxicity burden [17].
The risk of severe radiation-induced complications represents the main limiting factor for reirradiation, especially in the pelvic region with several dose-limiting organs at risk (OARs) such as bowel loops, sigmoid, bladder, urethra, rectum, cauda equina, nerves, and femoral heads [18, 19].
For this reason, it was deemed necessary to adopt delivery techniques that would allow the best achievable sparing of surrounding OARs while assuring the delivery of ablative radiation doses.
The introduction of intensity-modulated RT (IMRT) techniques has led to an increased precision in the delivery process by a rapid dose falloff, characterized by a steep dose gradient close to the target volumes. Furthermore, the implementation of stereotactic body radiation therapy (SBRT) assured the possibility to efficaciously shape the dose offering the advantage of delivering high doses even on very small target volumes or particularly close to radiosensitive OARs.
Previous experiences reported in literature demonstrate that the use of SBRT delivered with conventional linear accelerators or Cyberknife (CK; Accuray, Sunnyvale, CA, USA) is a technically feasible, effective and safe approach [20,21,22,23].
In more recent years, the introduction of hybrid magnetic resonance (MR) delivery units has made SBRT delivery feasible for lesions located in different anatomical sites, including PC [24,25,26,27]. MR-guided stereotactic RT (MRgSBRT) allows to combine the advantages of SBRT treatment with the use of MR imaging, improving visualization of the target and OARs and the possibility of performing gating and online adaptive treatment protocols [28]. The advantage of MRgSBRT in the management of prostate and prostate-bed reirradiation has recently been investigated, showing encouraging results in terms of feasibility, toxicity reduction and clinical outcomes [29, 30].
This retrospective multicentric analysis was designed in order to provide early results in terms of feasibility and effectiveness of MRgSBRT PC-reirradiation performed with a 0.35 T MRgRT unit.
Methods
Patients affected by recurrent PC who underwent MRgSBRT reirradiation using a 0.35 T MR-linear accelerator (MRIdian, ViewRay, Mountain View, CA, USA) in five different institutions were considered for the analysis and their data were retrospective collected.
Patients were addressed to SBRT treatment following multidisciplinary discussion and signed specific informed consent to MRgSBRT treatment.
Magnetic Resonance Imaging (MRI) safety screening forms were administered to all patients before therapy.
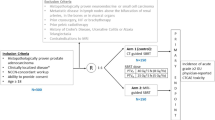
Inclusion criteria were: age > 18 years; radiological diagnosis of recurrent PC by means of MRI and F-choline- or Ga-PSMA-PET/TC due to a rising PSA value; previous RT treatment to prostate or prostate bed.
Clinical contraindications to MRI (e.g. claustrophobia, presence of non-MRI safe devices) or specific consent deny were considered as exclusion criteria. Patients’ characteristics are summarized in Table 1.
For the simulation procedure patients were required to perform bladder preparation by drinking 500 cc of water 30 min before the scan. An enema was required 3 h before the scan to guarantee rectal emptiness.
Two patients underwent prostate-rectal spacer implantation.
The same preparation approach was maintained for each treatment fraction.
Simulation scan was performed with patients in supine position using dedicated repositioning devices.
The irradiation field definition was performed using a 25-seconds MR true fast imaging with steady-state free precession (TRUFI) sequence; while a high resolution 175-seconds TRUFI sequence was acquired for the definition of target volumes and OARs. A non-contrast-enhanced simulation CT was acquired subsequently and fused with MRI simulation imaging in order to provide electron densities for the plan calculation.
Gross tumor volume (GTV) included the entire gross tumor relapse defined by means of MRI imaging or PET/CT. The clinical target volume (CTV) was considered equal to GTV and an isotropic 3–5 mm margin was added to GTV to generate the planning target volume (PTV).
The OARs considered were: rectum, anal canal, small intestine, bladder, urethra, femoral heads and penile bulb.
The plan objectives for target optimization were to have at least 95% of the PTV to be covered by 95% of the dose (V95% > 95%) and to avoid hot spots > 105% for treatments prescribed to mean dose according to International Commission on Radiation Units and Measurements (ICRU) 83 report [31].
For treatments normalized to a specific isodose the plan objectives were to have 100% of PTV covered by prescription isodose and to avoid hot spots > 140% according ICRU 91 report [32].
The minimal set of dose volume constraints used for OARs optimization were: for rectum, V10 Gy < 40%, V18 Gy < 20%; for bladder, V10 Gy < 25%, V18 Gy < 15%; for femoral heads, V24 < 10%; for penile bulb, V24 < 50%; for small intestine, V18 < 5 cm3 and for urethra Dmax value < 120% of prescribed dose, according to current evidences [33, 34].
Clinical evaluations were performed during the treatment, 30 days after RT course completion and then every three months.
Acute and late toxicities were assessed using Common Terminology Criteria for Adverse Events (CTCAE) v4.0 and v5.0 [35, 36].
Toxicity incidence, local control (LC), distant progression free survival (DPFS), biochemical recurrence free survival (BRFS) and overall survival (OS) rates were registered. Actuarial outcomes results were analyzed through the Kaplan-Meier method; log-rank tests were used to evaluate subgroups differences. Prism version 8.31 for macOS software (1994–2019 GraphPad Software, La Jolla California USA, www.graphpad.com) was used to perform statistical analyses.
Results
Data from 18 patients who underwent MRgSBRT re-treatment for prostate or prostate bed recurrences were collected. The patients were treated in five different institutions: 6 patients were enrolled at Fondazione Policlinico Universitario “A. Gemelli” IRCCS in Rome; 5 patients were enrolled at Ospedale San Pietro Fatebenefratelli in Rome, Italy and University Hospital of Munich (LMU), while both Mater Olbia Hospital and Heidelberg University Hospital enrolled 1 patient.
The median time between EBRT course and relapse was 10 years (range 5–17), while median age at recurrence time was 77 years (range 60–86).
In 9 patients (50%) relapse occurred after adjuvant treatment following radical prostatectomy, while intraprostatic relapse occurred in 9 patients (50%).
Reirradiation consisted of a MRgSBRT treatment delivered with a target mean normalization according to ICRU 83 report in 6 patients (33.3%) or isodose normalization between 80 and 86% according ICRU 91 report in 12 patients (66.7%).
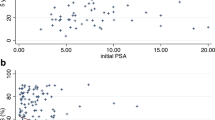
A nominal dose less or equal than 30 Gy was prescribed in 6 patients (33.3%) while a dose higher than 30 Gy was prescribed in 12 patients (66.7%). Median biologically effective dose (BED) of MRgSBRT re-treatment was 213,3 Gy (103,1-560), considering an α/β of 1.5.
All patients were treated on alternate days with a median number of fractions of 5 (range 4–6); in 5 patients (27.8%) an online adaptive treatment was performed when deemed necessary by the treating physician.
OARs dose constraints and delivered doses are reported in Table 2.
Genitourinary (GU) acute Grade 1 toxicity events occurred in 2 patients (11.1%); 4 patients suffered from gastrointestinal (GI) toxicity with Grade 1 and Grade 2 events in 1 (5.6%) and 3 (16.8%) patients, respectively. Multivariate analysis showed no correlation between any grade toxicity events and dose prescription such as for OARs dose values exceeding suggested constraints values. All patients completed the foreseen treatment schedule without interruptions.
Median follow-up time was 4 months (range 1–39) and late toxicity assessment was performed in 13 patients (83.3%).
Late Grade 1 GU toxicity was seen in 3 patients (16.8%), no Grade 2 or greater GU toxicity occurred; 3 (16.8%) patients showed Grade 1 late GI toxicity while Grade 2 event was observed in 1 (5.6%) patient.
1-year and 2-years LC rates of 88.9% and 66.7%, respectively, with 16 patients (88.9%) being free from local failure in the considered time frame. Complete response was observed in 4 patients (22.2%), while partial response and stable disease were observed in 4 patients (22.2%) and 8 patients (44.4%), according to RECIST version 1.1 criteria [37].
Following reirradiation, 2 patients (11.1%) underwent local failure at 4 and 24 months, respectively, both in the prostate bed. No significant differences were found at dose prescription subgroup analysis. BRFS occurred in 2 patients (11.1%) with a 1-year rate of 69.64% and a median PSA value of 0.8 ng/ml (range < 0.001–131), both patients with biochemical failure showed also distant failure.
Distant progression was shown in 5 patients (27.8%) with 1-year and 2-years DPFS rates of 53.1%, no significant correlation to prescribed dose was shown in subgroup analysis. Only 1 patient (5.6%) died of non-cancer related event (sepsis) five months after reirradiation. The 1-year and 2-years percentage for investigated clinical outcomes are summarised in Table 3.
Discussion
This multicentric experience represents a preliminary analysis in terms of feasibility and efficacy of MRgSBRT reirradiation in a cohort of 18 previously irradiated patients with PC local relapse, using a 0.35 T hybrid LINAC. The low incidence of high-grade toxicity and the good results in terms of efficacy, although related to a modest number of patients, would seem to encourage the use of this technique.
The occurrence of PC relapse after local treatments is still relevant despite the increasing accuracy and technological advancement of multimodal treatments. The new imaging modalities also provide high accuracy for local relapse diagnosis, also in absence of needle biopsy [38, 39].
The best treatment strategy in this setting is still unclear and no consensus has been reached about the best approach, especially considering the availability of different local therapy options such as salvage cryotherapy, high-intensity focused ultrasound ablation (HIFU), HDR brachytherapy, and salvage radical prostatectomy [11,12,13,14,15,16, 40].
Regardless these alternatives, observation and androgen-deprivation therapy have historically been the preferred option and the number of patients treated with local salvage therapies still remains low, despite the curative potential of this approach and the advantage of postponing the eventual use of systemic therapies [10, 41].
The use of RT in this setting could represent a valid alternative to surgery, which is an approach not devoid of sequelae impacting on the quality of life of the patient, such as urinary incontinence, impotence and anorectal dysfunction [12, 14, 42].
Considering the implementation of modern radiotherapy modalities reirradiation could be considered as a feasible and safe approach [17, 43,44,45,46,47].
Interesting preliminary results suggest that more than 50% of patients who have undergone salvage reirradiation are biochemically relapse-free with very low rates of severe toxicity [48].
The role of HDR-BT has been widely assessed, recent systematic reviews considering the evidence reported in literature has showed good results in terms of toxicity and oncological outcomes [17, 49]. HDR-BT showed promising results in terms of biochemical control in different experiences with a 2-year median BRFS of 74%, with limited occurrence of G3 toxicity.
Literature data showed that SBRT represents the second therapeutic strategy used in local re-treatments, after brachytherapy. In particular image-guided radiotherapy, combined with HDR-BT and SBRT, allows a higher sparing of the surrounding OARs, with a sharper gradient of dose, while still maintaining ablative dose [16, 50].
The optimal dose prescription for prostate reirradiation is not yet standardized. Corkum et al. recently performed a meta-analysis in order to describe the oncologic and toxicity outcomes for salvage reirradiation with EBRT and SBRT with a median reirradiation dose in EQD2 of 77.1 Gy (α/β = 1.5), with 92% patients receiving SBRT [51]. The authors observed an increase in local control and biochemical relapse free survival for higher EQD2 doses, even though high rates of GU and GI toxicities occurred. Partial prostate re-RT appears promising as it showed the decrease of toxicity with no apparent negative impact on disease control outcomes [51].
Precise knowledge on long-term recovery of occult radiation injury in various OARs is essential and literature data showed that accepted cumulative reirradiation dose should not exceed 120 Gy for bladder and 70–100 Gy for rectum [52, 53].
The adoption of a hydrogel rectal spacer could be a possible strategy to reduce the dose to healthy organs. Hamstra et al. performed a single-blind phase III trial of IGRT-IMRT with the adoption of a device called SpaceOAR, a FDA–approved hydrogel intended to create a rectal–prostate space, providing strong evidence for the benefit of its use in prostate irradiation in terms of rectal and urinary morbidity [54].
Within our population this solution was adopted only in two patients (11.1%), with good tolerance during RT sessions and without substantial differences in terms of GI toxicity compared with those who did not use it.
Other strategies to improve the reproducibility of patient positioning, such as the supine over prone set-up position, endorectal balloons, bladder-filling protocols and bladder ultrasound image guided radiation therapy (IGRT) have also been used in order to standardize organs’ volume and consequently manage internal organ motion during pelvic irradiation, although their clinical benefit still remains uncertain [55].
Historically, cone beam computed tomography (CBCT) has been largely employed during RT and SBRT treatment in order to ensure correct patient positioning, provide anatomical information during RT and to overcome the uncertainties arising from organ motion, but soft tissues are challenging to locate with standard CBCT-image-guidance techniques.
Bladder and rectum also suffer from inter-fraction volume variation with potentially significant effects on the cumulative dose received: while volumetric dose received by the bladder decreases as its volume increases, the inverse effect was observed for the rectum [56]. The robotic Cyberknife technique with the use of fiducial marker implantation has also been shown to be a safe approach for reirradiation, assuring a high accuracy of target positioning [22, 34].A recent experience on 64 patients treated with cyberknife modality showed a 2-year LC rate of were 75%, with 1-year LC rate for BED ≥ 130 Gy 85% [22]. The analysis of toxicity profile demonstrated the safety of the procedure as only 1 patient showed grade 3 late GU toxicity.
The availability of MR-hybrid RT devices has allowed the introduction of MR-guided IGRT that can overcome the uncertainties associated with x-rays based IGRT [25, 27]. MR-guidance provides excellent visualization of soft tissues, especially for lung, pelvic and abdominal neoplasms [24,25,26,27].
During treatment delivery a cine-MRI is acquired in a sagittal plane in in order to automatically gate treatment beams. The contours of target volume and a boundary structure are automatically deformed and transferred onto different cine MR frames. During the treatment session, the beam automatically shuts off, if a user-defined percentage of the volume of interest is outside of the user-defined boundary [57]. This method has increased delivery precision during both free-breathing and gated treatments with decreased toxicity for close tissues [58]. Interim analysis of MIRAGE phase III randomized trial have also shown a statistically significant reduction in acute grade ≥ 2 GU toxicity with MRI-guidance versus CT-guidance in the context of prostate SBRT [59].
The implementation of MRgSBRT in the PC reirradiation has also been hypothesized by other groups, who described encouraging results in terms of feasibility, toxicity reduction and clinical outcomes [29, 30].
Our experience reports the preliminary results in terms of MRgSBRT PC-reirradiation performed with 0.35 T hybrid units in five different institutions.
MRI sequences can reduce the daily uncertainties in identifying the exact interface between the posterior part of the prostate gland and the anterior rectal wall or between the prostate apex and the penile bulb, allowing a better definition of the daily critical structures and consequently the possible reduction of PTV margins [60, 61].
Our results are in line with those already reported in the literature in terms of local control, as in our cohort a 1-year control rate of 88.9% was shown [62].
As for acute toxicity rates, only grade 1 GU toxicities events were observed in our cohort, and only 3 patients (16.7%) suffered from grade 2 GI toxicities, which are comparable results to the published data [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56].
Salvage SBRT reirradiation for locally recurrent PC offers a satisfactory tumor control: an Italian mono-institutional study on 64 patients demonstrated 1-year biochemical progression-free survival rate and clinical progression-free survival rate of 85 and 90%, respectively, with excellent toxicity profile [22].
Our results are also comparable to recent experience of PC reirradiation by means of MRgSBRT. Alongi et al. reported in a preliminary report of 22 patients grade 2 GI and GU acute toxicity events in 4 patients (18%) and a BRFS 1-year rate of 85.9% [29].
Similarly, Michalet et al. showed low rates of acute grade 1 GI toxicity rates with no grade 2 events and no grade 2 GI acute toxicity events at 3 months FUP. In the cohort of 37 patients at 1 year follow up, the BRFS rate was 65% [30].
SBRT treatments delivered with MRgRT have been investigated for abdominal and pelvic neoplasms, supporting the opportunity to perform online treatment plan adaptation, by optimizing the dose distribution on a daily basis under MRI guidance with reduced PTV margins [24,25,26,27].
Henke et al. described the first experience of stereotactic adaptive MRgSBRT in the upper abdominal malignancies’ scenario, demonstrating that this approach is feasible and safe, and also allows to perform a dose escalation and simultaneous OARs sparing. [63]
Finally, it should be noted that this study has some limitations. First, the limited number of patients, mainly related to the fact that the number of centers equipped with MRgRT units is still very low. Secondly the heterogeneous range of prescription doses and the limited observation period could represent biases that should be considered in defining the correlation with clinical outcomes and toxicity.
Nevertheless, we can state that MRgSBRT seems to offer the opportunity to overcome the traditional limitations of prostatic reirradiation, ensuring precise delivery of the high doses and OARs sparing through an accurate target visualization and the application of cutting-edge gating and online adaptive replanning protocols.
Longer follow-up and a larger number of patients are necessary to evaluate reirradiation effectiveness and optimal patient selection criteria in order to identify the population most suitable for this innovative and promising therapeutic approach.
Data Availability
Datasets used and analyzed for this study could be provided upon reasonable request from corresponding author.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. https://doi.org/10.3322/caac.21708.
Cellini F, Tagliaferri L, Frascino V, Alitto AR, Fionda B, Boldrini L, Romano A, Casà C, Catucci F, Mattiucci GC, et al. Radiation therapy for prostate cancer: what’s the best in 2021. Urol J. 2022;89:5–15. https://doi.org/10.1177/03915603211042335.
National Comprehensive Cancer Network. Prostate cancer (Version 2.2021). (2021) https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, Tombal B, Gillessen S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1119–34. https://doi.org/10.1016/j.annonc.2020.06.011.
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62. https://doi.org/10.1016/j.eururo.2020.09.042.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer. Part II-2020 update: treatment of relapsing and metastatic prostate Cancer. Eur Urol. 2021;79:263–82. https://doi.org/10.1016/j.eururo.2020.09.046.
Sanda MG, Chen RC, Crispino T, Freedland S, Greene K, Klotz LH, Makarov DV, Reston J, Rodrigues G, Sandler HM et al. Clinically localized prostate cancer: AUA/ASTRO/SUO GUIDELINE. Prostate Cancer (2017)56.
Fuller DB, Wurzer J, Shirazi R, Bridge SS, Law J, Mardirossian G. High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol. 2015;5:e615–23. https://doi.org/10.1016/j.prro.2015.04.009.
Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. https://doi.org/10.1016/j.ijrobp.2006.04.029.
Priore GD, Hoffman S. Timing of androgen-deprivation therapy in prostate cancer. Lancet Oncol. 2017;18:e633. 1470-2045(17)30774-X.
Chade DC, Eastham J, Graefen M, Hu JC, Karnes RJ, Klotz L, Montorsi F, van Poppel H, Scardino PT, Shariat SF. Cancer Control and Functional Outcomes of Salvage Radical Prostatectomy for Radiation-recurrent prostate Cancer: a systematic review of the literature. Eur Urol. 2012;61:961–71. https://doi.org/10.1016/j.eururo.2012.01.022.
Philippou Y, Parker RA, Volanis D, Gnanapragasam VJ. Comparative oncologic and toxicity outcomes of Salvage Radical Prostatectomy Versus Nonsurgical Therapies for Radiorecurrent prostate Cancer: a Meta–regression analysis. Eur Urol Focus. 2016;2:158–71. https://doi.org/10.1016/j.euf.2015.09.004.
Parekh A, Graham PL, Nguyen PL. Cancer Control and Complications of Salvage Local Therapy after failure of Radiotherapy for prostate Cancer: a systematic review. Semin Radiat Oncol. 2013;23:222–34. https://doi.org/10.1016/j.semradonc.2013.01.006.
Ingrosso G, Becherini C, Lancia A, Caini S, Ost P, Francolini G, Høyer M, Bottero M, Bossi A, Zilli T, et al. Nonsurgical salvage local therapies for radiorecurrent prostate Cancer: a systematic review and Meta-analysis. Eur Urol Oncol. 2020;3:183–97. https://doi.org/10.1016/j.euo.2018.12.011.
Valle LF, Lehrer EJ, Markovic D, Elashoff D, Levin-Epstein R, Karnes RJ, Reiter RE, Rettig M, Calais J, Nickols NG, et al. A systematic review and Meta-analysis of local salvage therapies after radiotherapy for prostate Cancer (MASTER). Eur Urol. 2021;80:280–92. https://doi.org/10.1016/j.eururo.2020.11.010.
Chatzikonstantinou G, Zamboglou N, Rödel C, Zoga E, Strouthos I, Butt SA, Tselis N. High-dose-rate brachytherapy as salvage modality for locally recurrent prostate cancer after definitive radiotherapy: a systematic review. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2017;193:683–91. https://doi.org/10.1007/s00066-017-1157-2.
Munoz F, Fiorica F, Caravatta L, Rosa C, Ferella L, Boldrini L, Fionda B, Alitto AR, Nardangeli A, Dionisi F, et al. Outcomes and toxicities of re-irradiation for prostate cancer: a systematic review on behalf of the Re-Irradiation Working Group of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Cancer Treat Rev. 2021;95:102176. https://doi.org/10.1016/j.ctrv.2021.102176.
Abusaris H, Hoogeman M, Nuyttens JJ, Re-Irradiation. Outcome, cumulative dose and toxicity in patients retreated with stereotactic Radiotherapy in the abdominal or pelvic region. Technol Cancer Res Treat. 2012;11:591–7. https://doi.org/10.7785/tcrt.2012.500261.
Catucci F, Alitto AR, Masciocchi C, Dinapoli N, Gatta R, Martino A, Mazzarella C, Fionda B, Frascino V, Piras A et al. Predicting Radiotherapy Impact on Late Bladder Toxicity in Prostate Cancer Patients: An Observational Study. Cancers (2021) 13:E175. doi: https://doi.org/10.3390/cancers13020175.
Vavassori A, Jereczek-Fossa BA, Beltramo G, De Cicco L, Fariselli L, Bianchi LC, Possanzini M, Bergantin A, DeCobelli O, Orecchia R. Image-guided robotic Radiosurgery as Salvage Therapy for locally recurrent prostate Cancer after External Beam Irradiation: Retrospective Feasibility Study on six cases. Tumori J. 2010;96:71–5. https://doi.org/10.1177/030089161009600112.
Detti B, Bonomo P, Masi L, Doro R, Cipressi S, Iermano C, Bonucci I, Franceschini D, Di Brina L, Baki M, et al. CyberKnife stereotactic radiotherapy for isolated recurrence in the prostatic bed. World J Urol. 2016;34:311–7. https://doi.org/10.1007/s00345-015-1613-5.
Jereczek-Fossa BA, Rojas DP, Zerini D, Fodor C, Viola A, Fanetti G, Volpe S, Luraschi R, Bazani A, Rondi E, et al. Reirradiation for isolated local recurrence of prostate cancer: Mono-institutional series of 64 patients treated with salvage stereotactic body radiotherapy (SBRT). Br J Radiol. 2019;92:20180494. https://doi.org/10.1259/bjr.20180494.
Fuller D, Wurzer J, Shirazi R, Bridge S, Law J, Crabtree T, Mardirossian G. Retreatment for local recurrence of Prostatic Carcinoma after prior therapeutic irradiation: efficacy and toxicity of HDR-Like SBRT. Int J Radiat Oncol Biol Phys. 2020;106:291–9. https://doi.org/10.1016/j.ijrobp.2019.10.014.
Tetar SU, Bruynzeel AME, Oei SS, Senan S, Fraikin T, Slotman BJ, van Moorselaar RJA, Lagerwaard FJ. Magnetic resonance-guided stereotactic radiotherapy for localized prostate Cancer: final results on patient-reported outcomes of a prospective phase 2 study. Eur Urol Oncol. 2021;4:628–34. https://doi.org/10.1016/j.euo.2020.05.007.
Corradini S, Alongi F, Andratschke N, Belka C, Boldrini L, Cellini F, Debus J, Guckenberger M, Hörner-Rieber J, Lagerwaard FJ, et al. MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiat Oncol. 2019;14:92. https://doi.org/10.1186/s13014-019-1308-y.
Boldrini L, Corradini S, Gani C, Henke L, Hosni A, Romano A, Dawson L. MR-Guided Radiotherapy for Liver Malignancies. Front Oncol. 2021;11:616027. https://doi.org/10.3389/fonc.2021.616027.
Corradini S, Alongi F, Andratschke N, Azria D, Bohoudi O, Boldrini L, Bruynzeel A, Hörner-Rieber J, Jürgenliemk-Schulz I, Lagerwaard F, et al. ESTRO-ACROP recommendations on the clinical implementation of hybrid MR-linac systems in radiation oncology. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021;159:146–54. https://doi.org/10.1016/j.radonc.2021.03.025.
Bruynzeel AME, Tetar SU, Oei SS, Senan S, Haasbeek CJA, Spoelstra FOB, Piet AHM, Meijnen P, van der Jagt MABB, Fraikin T, et al. A prospective single-arm phase 2 study of stereotactic magnetic resonance guided adaptive Radiation therapy for prostate Cancer: early toxicity results. Int J Radiat Oncol Biol Phys. 2019;105:1086–94. https://doi.org/10.1016/j.ijrobp.2019.08.007.
Cuccia F, Rigo M, Figlia V, Giaj-Levra N, Mazzola R, Nicosia L, Ricchetti F, Trapani G, De Simone A, Gurrera D, et al. 1.5T MR-Guided daily adaptive stereotactic body radiotherapy for prostate re-irradiation: a preliminary report of toxicity and clinical outcomes. Front Oncol. 2022;12:858740. https://doi.org/10.3389/fonc.2022.858740.
Michalet M, Riou O, Valdenaire S, Debuire P, Ailleres N, Draghici R, Charissoux M, Moscardo CL, Farcy-Jacquet M-P, Fenoglietto P, et al. Magnetic resonance–guided reirradiation for local recurrence within the prostate or in the prostate Bed: preliminary results of a prospective Registry Study. Adv Radiat Oncol. 2021;6:100748. https://doi.org/10.1016/j.adro.2021.100748.
ICRU Report 83, Prescribing, Recording, and Reporting Intensity-Modulated Photon-Beam Therapy (IMRT) – ICRU. https://www.icru.org/report/prescribing-recording-and-reporting-intensity-modulated-photon-beam-therapy-imrticru-report-83/ [Accessed June 10, [Accessed June 10, 2022].022]
Wilke L, Andratschke N, Blanck O, Brunner TB, Combs SE, Grosu A-L, Moustakis C, Schmitt D, Baus WW, Guckenberger M. ICRU report 91 on prescribing, recording, and reporting of stereotactic treatments with small photon beams: Statement from the DEGRO/DGMP working group stereotactic radiotherapy and radiosurgery. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2019;195:193–8. https://doi.org/10.1007/s00066-018-1416-x.
D’Agostino GR, Di Brina L, Mancosu P, Franzese C, Iftode C, Franceschini D, Clerici E, Tozzi A, Navarria P, Scorsetti M. Reirradiation of locally recurrent prostate Cancer with Volumetric Modulated Arc Therapy. Int J Radiat Oncol. 2019;104:614–21. https://doi.org/10.1016/j.ijrobp.2019.02.041.
Di Franco R, Borzillo V, Scipilliti E, Ametrano G, Serra M, Arrichiello C, Savino F, De Martino F, D’Alesio V, Cammarota F et al. Reirradiation of Locally Recurrent Prostate Cancer with Cyberknife® System or Volumetric Modulated Arc Therapy (VMAT) and IGRT-Clarity®: Outcomes, Toxicities and Dosimetric Evaluation. Cancers (2022) 14:3187. doi: https://doi.org/10.3390/cancers14133187.
Common. Terminology Criteria for Adverse Events (CTCAE). (2009)79.
Common. Terminology Criteria for Adverse Events (CTCAE). (2017)155.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Trabulsi EJ, Rumble RB, Jadvar H, Hope T, Pomper M, Turkbey B, Rosenkrantz AB, Verma S, Margolis DJ, Froemming A, et al. Optimum imaging strategies for advanced prostate Cancer: ASCO Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38:1963–96. https://doi.org/10.1200/JCO.19.02757.
Piras A, Laudicella R, Boldrini L, D’Aviero A, Sanfratello A, La Rocca A, Scurria S, Salamone G, Alongi P, Angileri T et al. The Added Value of [18F]Choline PET/CT in Low-Risk Prostate Cancer Staging: A Case Report. Life (2022) 12:1728. doi: https://doi.org/10.3390/life12111728.
Mouraviev V, Spiess PE, Jones JS. Salvage Cryoablation for locally recurrent prostate Cancer following primary Radiotherapy. Eur Urol. 2012;61:1204–11. https://doi.org/10.1016/j.eururo.2012.02.051.
Tran H, Kwok J, Pickles T, Tyldesley S, Black PC. Underutilization of local salvage therapy after radiation therapy for prostate cancer11Funding: UBC Summer Student Research Program. Urol Oncol Semin Orig Investig. 2014;32:701–6. https://doi.org/10.1016/j.urolonc.2013.12.014.
Nguyen PL, D’Amico AV, Lee AK, Warren Suh W. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer. 2007;110:1417–28. https://doi.org/10.1002/cncr.22941.
Das S, Patro K, Mukherji A. Recovery and tolerance of the organs at risk during re-irradiation. J Curr Oncol. 2018;1:23. https://doi.org/10.4103/jco.jco_2_17.
Lee J, Shin I-S, Kim WC, Yoon WS, Koom WS, Rim CH. Reirradiation with intensity-modulated radiation therapy for recurrent or secondary head and neck cancer: Meta-analysis and systematic review. Head Neck. 2020;42:2473–85. https://doi.org/10.1002/hed.26264.
Lukovic J, Bourque J-M, Abdel-Wahab M. A systematic review on the role for reirradiation in locally recurrent rectal cancer. J Radiat Oncol. 2015;4:141–8. https://doi.org/10.1007/s13566-015-0188-5.
Maddalo M, D’Angelo E, Fiorica F, Argenone A, Scricciolo M, Cozzi S, Nardangeli A, Dionisi F, Costantino G, Vagge S, et al. Thoracic re-irradiation with 3D-conformal or more advanced techniques: a systematic review of treatment safety by the re-irradiation Study Group of the Italian Association of Radiation and Oncology AIRO. Crit Rev Oncol Hematol. 2021;167:103500. https://doi.org/10.1016/j.critrevonc.2021.103500.
Caravatta L, Fiorica F, Rosa C, Boldrini L, Alitto AR, Nardangeli A, Dionisi F, Bianco L, Munoz F, Lupattelli M, et al. Role of upper abdominal reirradiation for gastrointestinal malignancies: a systematic review of cumulative dose, toxicity, and outcomes on behalf of the Re-Irradiation Working Group of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2020;196:1–14. https://doi.org/10.1007/s00066-019-01519-5.
Créhange G, Roach M, Martin É, Cormier L, Peiffert D, Cochet A, Chapet O, Supiot S, Cosset J-M, Bolla M et al. Salvage reirradiation for locoregional failure after radiation therapy for prostate cancer: Who, when, where and how? Cancer/Radiothérapie (2014) 18:524–34. doi: https://doi.org/10.1016/j.canrad.2014.07.153.
Zhong J, Slevin F, Scarsbrook AF, Serra M, Choudhury A, Hoskin PJ, Brown S, Henry AM. Salvage Reirradiation Options for locally recurrent prostate Cancer: a systematic review. Front Oncol. 2021;11:681448. https://doi.org/10.3389/fonc.2021.681448.
Mbeutcha A, Chauveinc L, Bondiau P-Y, Chand M-E, Durand M, Chevallier D, Amiel J, Kee DLC, Hannoun-Lévi J-M. Salvage prostate re-irradiation using high-dose-rate brachytherapy or focal stereotactic body radiotherapy for local recurrence after definitive radiation therapy. Radiat Oncol. 2017;12:49. https://doi.org/10.1186/s13014-017-0789-9.
Corkum MT, Mendez LC, Chin J, D’Souza D, Boldt RG, Bauman GS. A novel salvage option for local failure in prostate Cancer, Reirradiation using External Beam or Stereotactic Radiation Therapy: systematic review and Meta-analysis. Adv Radiat Oncol. 2020;5:965–77. https://doi.org/10.1016/j.adro.2020.04.022.
Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000;10:200–9. https://doi.org/10.1053/srao.2000.6593.
Armstrong S, Hoskin P. Complex clinical decision-making process of Re-Irradiation. Clin Oncol. 2020;32:688–703. https://doi.org/10.1016/j.clon.2020.07.023.
Hamstra DA, Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi RA, et al. Continued benefit to rectal separation for prostate Radiation Therapy: final results of a phase III trial. Int J Radiat Oncol. 2017;97:976–85. https://doi.org/10.1016/j.ijrobp.2016.12.024.
Slevin F, Beasley M, Speight R, Lilley J, Murray L, Henry A. Overview of patient preparation strategies to manage internal organ motion during radiotherapy in the pelvis. J Radiother Pract. 2020;19:182–9. https://doi.org/10.1017/S1460396919000530.
Pearson D, Gill SK, Campbell N, Reddy K. Dosimetric and volumetric changes in the rectum and bladder in patients receiving CBCT-guided prostate IMRT: analysis based on daily CBCT dose calculation. J Appl Clin Med Phys. 2016;17:107–17. https://doi.org/10.1120/jacmp.v17i6.6207.
Votta C, Cusumano D, Boldrini L, Dinapoli N, Placidi L, Turco G, Antonelli MV, Pollutri V, Romano A, Indovina L, et al. Delivery of online adaptive magnetic resonance guided radiotherapy based on isodose boundaries. Phys Imaging Radiat Oncol. 2021;18:78–81. https://doi.org/10.1016/j.phro.2021.05.005.
Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl Radiat Oncol. 2019;18:98–101. https://doi.org/10.1016/j.ctro.2019.04.007.
Kishan AU, Lamb J, Casado M, Wang X, Ma TM, Low D, Sheng K, Yang Y, Gao Y, Basehart V, et al. Magnetic resonance imaging-guided versus computed tomography-guided stereotactic body radiotherapy for prostate cancer (MIRAGE): interim analysis of a phase III randomized trial. J Clin Oncol. 2022;40:255–5. https://doi.org/10.1200/JCO.2022.40.6_suppl.255.
Corradini S. ESTRO-ACROP recommendations on the clinical implementation of hybrid MR-linac systems in radiation oncology. Radiother Oncol (2021)9.
Alongi F, Rigo M, Figlia V, Cuccia F, Giaj-Levra N, Nicosia L, Ricchetti F, Sicignano G, De Simone A, Naccarato S, et al. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat Oncol. 2020;15:69. https://doi.org/10.1186/s13014-020-01510-w.
Loi M, Di Cataldo V, Simontacchi G, Detti B, Bonomo P, Masi L, Desideri I, Greto D, Francolini G, Carfora V, et al. Robotic stereotactic retreatment for biochemical control in previously irradiated patients affected by recurrent prostate Cancer. Clin Oncol. 2018;30:93–100. https://doi.org/10.1016/j.clon.2017.11.007.
Henke L, Kashani R, Robinson C, Curcuru A, DeWees T, Bradley J, Green O, Michalski J, Mutic S, Parikh P, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126:519–26. https://doi.org/10.1016/j.radonc.2017.11.032.
Funding
All authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Conception and design: L.B., G.C.M., V.V., P.G. and M.A.G. Data Collection: VA.V., A.D., A.C., D.M. Analysis and Interpretation of Data: A.R., G.C., S.C., V.D.L., A.R.A., F.C., A.D., C.T., J.HR., V.F. Manuscript Writing: L.B., A.D., V.V., V.D.L. Manuscript editing: L.B., S.C., J.HR., A.C. Final approval: all authors.
Corresponding author
Ethics declarations
Ethical approval and Consent to participate
The study was conducted according to the guidelines of the declaration of Helsinki. Patients enrolled signed an informed consent according to departments regulations and ethical committee guidelines.
Consent for publication
Patients enrolled signed an informed consent for data collection and publication, according to the study design requirements and to departments regulations.
Competing interests
Luca Boldrini has active research and consultation agreements with Varian Medical Systems, ViewRay Inc and IBA and received speaker honoraria for scientific presentations and travel reimbursements.Stefanie Corradini received research grants from Elekta, ViewRay and Brainlab and speaker fees/travel support from Elekta, ViewRay, C-RAD, Roche and Brainlab.Juliane Hörner-Rieber received speaker fees and travel reimbursement from ViewRay Inc. as well as travel reimbursement from IntraOP Medical and Elekta Instrument AB outside the submitted work. JHR further reports grants from IntraOP Medical and Varian Medical Systems outside the submitted work.Vincenzo Valentini has received departmental research grants from Varian Medical Systems, ViewRay Inc., Elekta, Merck-Serono, Roche.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Boldrini, L., Romano, A., Chiloiro, G. et al. Magnetic resonance guided SBRT reirradiation in locally recurrent prostate cancer: a multicentric retrospective analysis. Radiat Oncol 18, 84 (2023). https://doi.org/10.1186/s13014-023-02271-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02271-y