Abstract
Purpose
This study implemented a piecewise volumetric modulated arc therapy (P-VMAT) for realizing whole-brain radiation therapy (WBRT) with simultaneous integrated boost (SIB) for multiple brain metastases (> 40 metastases) with a conventional C-arm linear accelerator.
Materials and methods
This study retrospectively analyzed 10 patients with multiple brain metastases (40–120 metastases, median 76), who underwent WBRT and SIB using helical tomotherapy (HT). The prescribed doses were 40 Gy/20 f and 60 Gy/20 f for WBRT and SIB, respectively. Corresponding new HT plans were designed with P-VMAT using 7 arcs. For each arc, the collimator was rotated to 45°, and the field width was limited to 2.5 cm with 0.5 cm overlap with adjacent arcs. Thus, each arc covered only one section of the brain target volume. A conventional dual arc VMAT (DA-VMAT) plan was also designed. HT, P-VMAT, and DA-VMAT plans were compared using dose distribution reviews and dosimetric parameters. ArcCHECK phantom measurements were performed for verification of P-VMAT plans.
Results
No significant differences in the mean coverage of the whole-brain target and metastases were observed between HT and P-VMAT (p > 0.05). The conformity index for the whole-brain target improved with P-VMAT compared with HT (p < 0.05). Furthermore, the volume of 44 Gy V44 (110% of prescribed dose for WBRT) received for whole-brain significantly reduced with P-VMAT from 38.2 ± 12.9% to 23.3 ± 9.4% (p < 0.05), and the maximum dose for organs at risks such as the hippocampus, optical nerve, optical chiasm, and spinal cord declined with P-VMAT (p < 0.05). Unlike HT and P-VMAT, DA-VMAT was clinically unacceptable because V44 in the whole-brain was too high (54.7 ± 8.2%). The mean absolute dose gamma passing rate for P-VMAT plans was 97.6 ± 1.1% (3%/3 mm criterion, 10%).
Conclusions
P-VMAT is favorable for WBRT and SIB for multiple brain metastases. It provides comparable coverage of whole-brain target and SIB, with better conformity, lower V44, and better dose sparing of organs at risk compared with HT. Furthermore, results show that DA-VMAT fails clinical practice even for a relatively large number of brain metastases with a high degree of plan complexity. The patient specific verification demonstrates the feasibility of P-VMAT for clinical application.
Similar content being viewed by others
Introduction
Brain metastases are common intracranial tumors in approximately 20–40% of patients with cancer [1] while multiple brain metastases accounted for about 70% [2]. Whole-brain radiation therapy (WBRT) is a standard modality for treating brain metastases [3]. However, the median survival is about 3–6 months for WBRT [4]. Stereotactic radiosurgery (SRS) or stereotactic fractionated radiotherapy (SRT) is a favorable approach for treating few metastases. WBRT is concerned with learning and memory function decline and no benefit of survival [5, 6]. Nevertheless, SRS alone has a high rate of remote disease progression, and developing new lesions increases with the increased number of brain metastases [7]. With the development of intensity-modulated radiotherapy (IMRT), WBRT with simultaneous integrated boost (SIB) or sequential SRS/SRT were introduced and proved to be effective for treating brain metastases [8, 9]. Comparing with WBRT followed by sequential SRS/SRT, WBRT with SIB has better dose distribution to spare normal tissue and organs at risk (OAR) and a reduced treatment period [9]. It was reported that for 43 patients with metastases ranging from 3 to 36, the median survival time was 21.3 months treated by WBRT + SIB using helicon tomotherapy (HT) which was obviously longer than WBRT alone [10]. Therefore, for radiation therapy of many brain metastases, WBRT plus SIB is also recommended to increase local control [10].
WBRT with SIB has employed several techniques, such as IMRT, volumetric modulated arc radiotherapy (VMAT), and HT are common choices [11, 12]. However, conventional IMRT or dual arc VMAT (DA-VMAT) delivered with a C-arm linear accelerator treated a limited number of brain metastases. Brain metastases vary between tens or over 100 for some patients. The complexity of plan optimization increases with increased metastases and is difficult to solve using the inverse optimizer in the treatment planning system for IMRT or VMAT. For intracranial SRS/SRT, non-coplanar beams/arcs are commonly used as non-coplanar beams/arcs increase the conformity and dose fall-off outside the target area [13]. For WBRT, it was also reported by a few publications that WBRT had the benefit of reducing the dose delivering to some OARs such as parotid or hippocampus [14, 15]. Nevertheless, non-coplanar technique is still not a common treatment modality for WBRT as it will largely increase the treatment time for WBRT with 10–20 fractions. HT is clinically used for treating large-scale brain metastases like with WBRT + SIB in our institution. However, HT is not available in many radiotherapy centers. Meanwhile, when treating large-scale brain metastases, sparing normal brain tissue should be improved to protect the neurocognition in patients [16].
Most studies reported the use of WBRT + SIB with 1–3 brain metastases [17, 18]. The radiotherapy technology of WBRT + SIB with a large number of brain metastases (> 40) is not reported. Exploring the feasibility of treating a large number of brain metastases with conventional C-arm linac, a new technique called piecewise volumetric modulated arc therapy (P-VMAT) was applied in this study. Recently, the technique was proposed by our group for WBRT with hippocampal sparing to improve plan quality and better spare hippocampus [19, 20]. This study designed P-VMAT plans for WBRT + SIB with a large number of brain metastases and compared it with HT. DA-VMAT plan was also optimized for each patient for comparison.
Materials and methods
Patient information
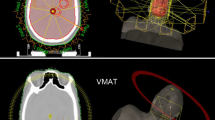
This retrospective study was approved by the review ethical board of our institute, and informed consent was waived. Ten patients with multiple brain metastases treated using HT were included in the study. The mean patient age was 48.7 (range 27–71) years. Table 1 shows the number of brain metastases varying between 40 and 120 (median 76). Patients were scanned by contrast-enhanced computed tomography (CT) with a brilliant CT big bore (Philips Healthcare, Best, Netherlands) using 2 mm thick slices, and fused with contrast-enhanced magnetic resonance imaging (MRI) scanner (Philips Healthcare, Best, Netherlands) with the same thickness. Gross target volume (GTV) was defined as the enhanced metastatic region in MRI T1 enhanced sequence, excluding edema area, numbered GTV1, GTV2 etc. for each metastasis. And GTV is the combination of all metastases. The clinical target volume (CTV) of the brain (CTV-brain) comprised the whole-brain. The planning target volume PTV-brain was generated by expanding a 5 mm margin to CTV-brain, and hippocampus with a 3 mm margin expanding in three dimensions was excluded from PTV-brain. A typical target volume is shown in Fig. 1. The total volume of all GTV for each patient varied from 3.7 to 45.1 cc (mean 17.4 ± 13.3 cc), and the volume for PTV-brain ranged from 1443.1 to 2068.8 cc (mean 1762.1 ± 198.1 cc). The OARs were contoured on the CT images, which included lens, hippocampus, optical nerve, optical chiasm, spinal cord, brain stem and pituitary.
Treatment planning
HT
HT was used for WBRT with SIB for treatment. Patient plans were designed with a Hi-Art planning station (version 5.1) for tomotherapy (Tomotherapy Inc, Madison, Wisconsin). An iterative inverse treatment planning algorithm was utilized for HT planning. The dynamic jaw was used with a field width of 2.51 cm, and a modulation factor of 2.6. The value of pitch was 0.287 with a fine calculation grid of 0.264 cm × 0.264 cm.
P-VMAT
The Pinnacle treatment planning system (version 9.1, Philips Healthcare, Eindhoven, Netherlands) redesigned all plans using a 6 MV X-ray delivered using an Elekta Versa HD accelerator (Elekta Oncology Systems, Crawley, UK). The optimization algorithm for VMAT planning was SmartArc. The multi-leaf collimator (MLC) module was Agility with 80 pairs of leaves and leaf width was 5 mm at the isocenter. The dose calculation grid was 0.4 cm × 0.4 cm × 0.4 cm. P-VMAT had seven full arcs with a collimator rotating to 45° and a couch angle of 0° using dynamic jaws. As shown in Fig. 2, the maximum field width was limited to 2.5 cm for each arc, similar to HT. The target volume was covered by seven piecewise arcs from the top to the bottom with 0.5 cm overlap regions between every two adjacent arcs to ensure uniform dose coverage in the overlap regions.
DA-VMAT
DA-VMAT plans use two full arcs with opposite rotational directions (clockwise and counterclockwise) without limiting the field width for comparison. The collimator and couch angle were the same to P-VMAT.
Plan optimization
The optimization parameters were similar among HT, P-VMAT, and DA-VMAT. It covered 95% of every metastasis with a prescribed dose of 60 Gy delivering in 20 fractions and covered 95% of the PTV-brain with 40 Gy/20 f. A metastasis close to critical OARs, such as brain stem was de-escalated to 50 Gy for preventing the occurrence of neurologic deficit or brain stem necrosis. For OARs, the maximum dose (Dmax) for the lens was less than 9 Gy. The Dmax for the hippocampus was less than 40 Gy if it was not adjacent to metastases. If the hippocampus was close to metastases, the dose constrain for the maximum dose of hippocampus could be loosen to ensure the coverage of metastases. The Dmax for the spinal cord was less than 40 Gy, and that for the brain stem was less than 54 Gy. The Dmax for optical nerve, optical chiasm, and pituitary was lower than 110% of the prescribed dose. The maximum dose in PTV-brain excluding GTV was set lower than 44 Gy.
Plan evaluation
The representative dosimetric parameters were evaluated for all plans. The conformity indices (CI) of PTV-brain were calculated using Paddick’s formula [21]: CI = (TVPV)2/(TV × PV), where TVPV is the absolute volume in PTV-brain covered by the prescription dose 40 Gy, TV is the absolute volume of PTV-brain and PV is the absolute volume covered by the prescription dose 40 Gy inside the body of patient. CI values range between 0 and 1 and CI close to 1 indicated better conformity. The homogeneity index (HI) was defined as [22]: HI = D5%/D95%, where D5% and D95% are doses covering 5% and 95% of the target volume, respectively, and a smaller HI indicates a better homogeneity. HI was calculated only for GTV since SIB was in PTV-brain, which nullified HI for evaluating homogeneity in the PTV-brain. Hence, V44 (the proportion of volume receiving 110% prescribed dose in PTV-brain) indicated homogeneity in PTV-brain. The coverage of GTV V60 (the proportion of volume receiving 60 Gy in GTV) and the coverage of PTV-brain V40 (the proportion of volume receiving 40 Gy in PTV-brain) were evaluated for all plans. Both maximum dose (Dmax) and mean dose (Dmean) of the lens, hippocampus, optical nerve, optical chiasm, spinal cord, brain stem, and pituitary were recorded from the evaluation tool in treatment planning system. Monitor unit (MU) for each plan was also recorded, and treatment delivery time was counted by a dry run with the accelerator.
All data were statistically analyzed with SPSS (version 19.0, IBM, New York, USA). An independent sample test was used for analyzing parameters with normal distribution; otherwise, a nonparametric Wilcoxon signed-rank test was used for a statistical test. A value of p < 0.05 was considered statistically significant.
Plan verification
A 3D diode array ArcCHECK phantom (Sun Nuclear Corporation, Melbourne, USA) were used for patient specific verification of P-VMAT plans. The data was analyzed with SNC patient software (v8.2, Sun Nuclear Corporation, Melbourne, USA). The criterion for the absolute dose gamma passing rate is 3%/3 mm with threshold of 10%.
Results
Figure 3 shows an example of dose distribution for a patient with 107 brain metastases planning with HT, P-VMAT, and DA-VMAT separately. From the figure, the volume covered by 4500 cGy in PTV-brain was obviously smaller for HT and P-VMAT comparing to DA-VMAT. Thus, the DA-VMAT plan quality was unacceptable for clinical practice because the volume covered by 4500 cGy was too large in normal brain tissue. In Fig. 4, a comparison of the dose-volume histogram also shows that the homogeneity in PTV-brain was best for P-VMAT compared with HT and DA-VMAT.
Dosimetric evaluation of target volume
All plans were normalized to cover 95% of GTV and PTV-brain with the prescribed dose. The average dosimetric parameters of GTV and PTV-brain planning with three techniques are shown in Table 2 and statistical analyses are illustrated in Table 4. The mean coverage was about 95% for both GTV and PTV-brain, and no significant difference among HT, P-VMAT, and DA-VMAT (p > 0.05). Meanwhile, P-VMAT had the highest CI of 0.911 ± 0.031, compared with HT and DA-VMAT. Regarding hot spot V44 in PTV-brain, it was measured 23.3 ± 9.4% for P-VMAT and significantly better than HT (38.2 ± 12.9%, p < 0.05) and DA-VMAT (54.7 ± 8.2%, p < 0.05). The V44 in PTV-brain for DA-VMAT was high and shows a poor dose homogeneity in the brain. The mean HI of GTV for HT was 1.092 ± 0.031, better than P-VMAT (p < 0.05) and DA-VMAT (p < 0.05).
OARs
The dosimetric parameters and statistical analyses for OARs are illustrated in Tables 3 and 4, separately. No significant difference exists between HT and P-VMAT for the Dmax of both the left and right lens (p > 0.05). Sparing of the lens was better for DA-VMAT compared with P-VMAT (p < 0.05). Compared with HT, both Dmax and Dmean were lower for the hippocampus, optical nerve, optical chiasm, pituitary, and spinal cord with P-VMAT (p < 0.05). Moreover, P-VMAT spared most OARs better than DA-VMAT (p < 0.05), except for the Dmean of the left optical nerve and the Dmax of the spinal cord (p > 0.05) shown in Tables 3 and 4. Furthermore, all dosimetric constraints for HT and P-VMAT were clinically acceptable.
MU and treatment times
The average MU values were 5564 ± 364 MU, 4161 ± 379 MU, and 1737 ± 185 MU for HT, P-VMAT, and DA-VMAT, respectively. The average time for treatment delivery was longer (497 ± 34 s) delivering with P-VMAT compared with (230 ± 21 s) for DA-VMAT (p < 0.05) and (393 ± 25 s) for HT (p < 0.05).
Plan verification results
The mean absolute dose gamma passing rate for P-VMAT plans was 97.6 ± 1.1% (3%/3 mm criterion, 10%), and the passing rates for all P-VMAT plans were larger than 95% which fulfill the requirements for clinical practice.
Discussions
P-VMAT is an alternative to HT for treating a large number of brain metastases with comparable or improved plan quality. Conventional VMAT using 7 arcs was also tried without limiting the field width to compare with P-VMAT which limited the field width for each arc. However, the plan quality did not improve compared with DA-VMAT and not fulfill the dose constraints or the treatment planning system had no solution. It means that the improvement of plan quality for P-VMAT was not because the number of arcs increase, but for limiting the field width for each arc. Thus, the reason for the superior P-VMAT can be explained as follows:
A local gradient-based optimization method was adopted in the algorithm named SmartArc for Pinnacle [23, 24]. In principle, the optimization results achieved with P-VMAT is a subset of conventional VMAT using dynamic jaws without limiting the field width. However, due to the constraints of delivery time, machine-specific parameters, limited motion speed of jaw positions and optimization algorithm, it is difficult for the treatment planning system to get a solution similar to P-VMAT without indicating jaw positions. For partial target volume covered by each arc, the modulation ability was improved as the motion of the MLC was limited to a small range. Hence, it is beneficial for P-VMAT by indicating jaw positions for partial regions manually. HT is similar to a CT scanner with a linac replacing the X-tube [25]. The target volume is irradiated using a fan beam with rotating linac continuously moving the treatment couch. With this special helical design, HT can achieve comparable or better plan quality than conventional VMAT, which is used for a limited number of brain metastases [26]. Several studies compared HT and VMAT for different sites, and mixed results were reported [27,28,29]. However, these studies show that HT has a larger lower dose-volume than VMAT, which showed a relatively slow dose fall-off for HT. For treating a larger number of brain metastases with WBRT + SIB, the dose gradient varied swiftly in the target volume. Therefore, a better plan quality could be achieved with P-VMAT compared with HT, when similar field width (2.5 cm) was used.
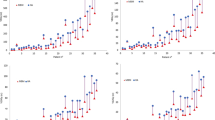
Several studies have reported declines in functions of learning and memory associated with irradiation of the hippocampus during WBRT [30, 31]. Results show that P-VMAT better spares the hippocampus compared with HT. The improved modulation ability of P-VMAT to treat volume of high dose gradient range is an advantage. Homogeneity is a common index for evaluating plan quality. With P-VMAT, the hot spot volume V44 in PTV-brain was only 23.3 ± 9.4%, which was significantly improved compared with HT (38.2 ± 12.9%, p < 0.05) and DA-VMAT (54.7 ± 8.2%, p < 0.05). This improvement supports the use of conventional linac for treating large-scale brain metastases since the plan quality of DA-VMAT was unacceptable for clinical practice. Figures 5 and 6 show that the V44 in PTV-brain varies with the number and volume of GTV. Therefore, V44 in PTV-brain tends to increase with an increase in the number and volume of GTV. However, it depends on the distribution, location, and relation to OARs, etc. of GTV in PTV-brain, which impacts the complexity for optimization.
Gamma knife are dedicated for radiosurgery of brain metastases, and it can not be used for whole brain radiotherapy [32]. CyberKnife with linac mounting on robotic arm are also commonly utilized for radiosurgery of metastases, but due to small aperture of collimator, it is also not suitable for the WBRT + SIB [33].
Seven arcs were used for P-VMAT with a 2.5 cm field width in this study to compare with HT as similar filed width 2.51 cm was used for HT. Actually, different numbers of arcs can be utilized for P-VMAT. About 3–7 arcs compromise the dose constraints and treatment efficiency according to our experience, however, the plan quality improves with more arcs and narrower field width for each arc. If the number of arcs are more than 7, it is usually hard for the treatment planning system Pinnacle to find a solution. On the other hand, if the number of arcs are smaller than 3, the plan quality can not be improved comparing to DA-VMAT. To ensure the coverage of prescribed dose at the joint, there must be overlapping between adjacent arcs. Actually, there are indeed some requirements for linacs, the plan quality is better with movable jaws comparing to fixed jaws and as the jaw positions of some linacs were limited to maximum 2 cm cross the central axis in Y direction, so the number of arcs were limited to max. 4 for these machines. Nevertheless, 3 or 4 arcs are sufficient for most clinical cases according to our experience.
The treatment accuracy also needs to be considered for P-VMAT. For intracranial tumor, the setup error is relatively small (within 2–3 mm) [34]. Moreover, the MLC and jaws position accuracy are also very crucial to the treatment reproducibility, which are required to be less than 1 mm. Collimator angle of 90° was tried for P-VMAT, but the dose verification gamma passing rate was relatively low measured by ArcCHECK phantom (3%/3 mm criterion < 90%). This may because that the setup error of MLC positions overlapped between adjacent subareas when the collimator angle is 90°. With collimator angle of 45°, P-VMAT plans can fulfill the requirement of gamma passing rate (3%/3 mm criterion > 95%) for clinical use. Therefore, collimator angle of 45° was utilized for planning in this study. Therefore, reasonable quality assurance procedures are very important for P-VMAT.
Conclusions
For WBRT with SIB, P-VMAT is suitable for treating a large number of brain metastases with conventional linac and is an alternative for HT. Compared with HT, P-VMAT provides comparable coverage of whole-brain target and GTV with better conformity, lower V44, and better dose sparing of the hippocampus, optical nerve, optical chiasm, and spinal cord. DA-VMAT was not suitable for clinical use. The ArcCHECK phantom measurements shows the feasibility of P-VMAT for clinical treatment.
Availability of data and materials
All data in this study is available if it is required.
Abbreviations
- P-VMAT:
-
Piecewise volumetric modulated arc therapy
- WBRT:
-
Whole-brain radiation therapy
- SIB:
-
Simultaneous integrated boost
- HT:
-
Helical tomotherapy
- DA-VMAT:
-
Dual arc volumetric modulated arc therapy
- V44 :
-
The proportion of volume receiving 44 Gy
- SRS:
-
Stereotactic radiosurgery
- SRT:
-
Stereotactic fractionated radiotherapy
- IMRT:
-
Intensity-modulated radiotherapy
- OAR:
-
Organs at risk
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- GTV:
-
Gross target volume
- CTV:
-
Clinical target volume
- PTV:
-
Planning target volume
- MLC:
-
Multi-leaf collimator
- CI:
-
Conformity indices
- TVPV:
-
The absolute volume in PTV covered by the prescription dose
- TV:
-
The absolute volume of PTV
- PV:
-
The absolute volume covered by the prescription dose
- HI:
-
Homogeneity index
- D5% :
-
Dose covering 5% of the target volume
- D95% :
-
Dose covering 95% of the target volume
- Dmax :
-
Maximum dose
- Dmean :
-
Mean dose
- MU:
-
Monitor unit
References
Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–40.
Franchino F, Rudà R, Soffietti R. Mechanisms and therapy for cancer metastasis to the brain. Front Oncol. 2018;24(8):161.
Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–304.
Jeene PM, de Vries KC, van Nes JGH, et al. Survival after whole brain radiotherapy for brain metastases from lung cancer and breast cancer is poor in 6325 Dutch patients treated between 2000 and 2014. Acta Oncol. 2018;57(5):637–43.
Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–91.
Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–44.
Chang WS, Kim HY, Chang JW, et al. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg. 2010;113(Suppl):73–8.
Pokhrel D, Sood S, McClinton C, et al. Treatment planning strategy for whole-brain radiotherapy with hippocampal sparing and simultaneous integrated boost for multiple brain metastases using intensity-modulated arc therapy. Med Dosim. 2016;41(4):315–22.
Prokic V, Wiedenmann N, Fels F, et al. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: a planning study on treatment concepts. Int J Radiat Oncol Biol Phys. 2013;85(1):264–70.
Ma YC, Xiao JP, Bi N, et al. Whole-brain irradiation with simultaneous integrated boost by helical tomotherapy for multiple brain metastases: dosimetric and clinical analyses. Chin J Radiat Oncol. 2018;27(5):435–40 (in Chinese).
Gutiérrez AN, Westerly DC, Tomé WA, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys. 2007;69(2):589–97.
Jiang A, Sun W, Zhao F, et al. Dosimetric evaluation of four whole brain radiation therapy approaches with hippocampus and inner ear avoidance and simultaneous integrated boost for limited brain metastases. Radiat Oncol. 2019;14:46.
Nicosia L, Figlia V, Mazzola R, et al. Repeated stereotactic radiosurgery (SRS) using a non-coplanar mono-isocenter (HyperArc™) technique versus upfront whole-brain radiotherapy (WBRT): a matched-pair analysis. Clin Exp Metastasis. 2020;37(1):77–83.
Krayenbuehl J, Di Martino M, Guckenberger M, et al. Improved plan quality with automated radiotherapy planning for whole brain with hippocampus sparing: a comparison to the RTOG 0933 trial. Radiat Oncol. 2017;12(1):161.
Park J, Park JW, Yea JW. Non-coplanar whole brain radiotherapy is an effective modality for parotid sparing. Yeungnam Univ J Med. 2019;36(1):36–42.
Zhou L, Liu J, Xue J, et al. Whole brain radiotherapy plus simultaneous in-field boost with image guided intensity-modulated radiotherapy for brain metastases of non-small cell lung cancer. Radiat Oncol. 2014;9:117.
Rodrigues G, Yartsev S, Yaremko B, et al. Phase I trial of simultaneous in-field boost with helical tomotherapy for patients with one to three brain metastases. Int J Radiat Oncol Biol Phys. 2011;80(4):1128–33.
Rodrigues G, Yartsev S, Tay KY, et al. A phase II multi-institutional study assessing simultaneous in-field boost helical tomotherapy for 1–3 brain metastases. Radiat Oncol. 2012;7:42.
Fu Q, Chen DQ, Yan H, et al. Treatment planning of volumetric modulated arc therapy and positioning optimization for hippocampal-avoidance prophylactic cranial irradiation. J Appl Clin Med Phys. 2021;22(5):15–23.
Xu YJ, Miao JJ, Liu QF, Huang P, Ma P, Chen XY, Men K, Xiao JP, Dai JR. Longitudinal grouping of target volumes for volumetric-modulated arc therapy of multiple brain metastases. Front Oncol. 2021;11: 578934.
Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Suppl 3):S219–22.
Wang X, Zhang X, Dong L, et al. Effectiveness of noncoplanar IMRT planning using a parallelized multiresolution beam angle optimization method for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:594–601.
Bzdusek K, Friberger H, Eriksson K, et al. Development and evaluation of an efficient approach to volumetric arc therapy planning. Med Phys. 2009;36(6):2328–39.
Unkelbach J, Bortfeld T, Craft D, et al. Optimization approaches to volumetric modulated arc therapy planning. Med Phys. 2015;42(3):1367–77.
Mackie TR. History of tomotherapy. Phys Med Biol. 2006;51:R427-453.
Hauswald H, Bernhardt D, Krug D, et al. Whole-brain helical tomotherapy with integrated boost for brain metastases in patients with malignant melanoma - final results of the BRAIN-RT trial. Cancer Manag Res. 2019;11:4669–76.
Nichols GP, Fontenot JD, Gibbons JP, et al. Evaluation of volumetric modulated arc therapy for postmastectomy treatment. Radiat Oncol. 2014;9:66.
Clemente S, Wu B, Sanguineti G, et al. SmartArc-based volumetric modulated arc therapy for oropharyngeal cancer: a dosimetric comparison with both intensity-modulated radiation therapy and helical tomotherapy. Int J Radiat Oncol Biol Phys. 2011;80:1248–55.
Rong Y, Tang G, Welsh JS, et al. Helical tomotherapy versus single-arc intensity-modulated arc therapy: a collaborative dosimetric comparison between two institutions. Int J Radiat Oncol Biol Phys. 2011;81:284–96.
Grosu AL, Frings L, Bentsalo I, et al. Whole-brain irradiation with hippocampal sparing and dose escalation on metastases: neurocognitive testing and biological imaging (HIPPORAD) - a phase II prospective randomized multicenter trial (NOA-14, ARO 2015–3, DKTK-ROG). BMC Cancer. 2020;20(1):532.
Oskan F, Ganswindt U, Schwarz SB, et al. Hippocampus sparing in whole-brain radiotherapy. A review. Strahlenther Onkol. 2014;190(4):337–41.
Lee CK, Lee SR, Cho JM, et al. Therapeutic effect of gamma knife radiosurgery for multiple brain metastases. J Korean Neurosurg Soc. 2011;50(3):179–84.
Nishizaki T, Saito K, Jimi Y, et al. The role of cyberknife radiosurgery/radiotherapy for brain metastases of multiple or large-size tumors. Minim Invasive Neurosurg. 2006;49(4):203–9.
Pramanik S, Ray DK, Bera S, et al. Analysis of setup uncertainties and determine the variation of the clinical target volume (CTV) to planning target volume (PTV) margin for various tumor sites treated with three-dimensional IGRT couch using KV-CBCT. J Radiat Oncol. 2020;9:25–35.
Acknowledgements
Not applicable.
Funding
This work is supported by the National Natural Science Foundation of China (Grant No. 11875320) and the National Key Projects of Research and Development of China [2017YFC0107501].
Author information
Authors and Affiliations
Contributions
YX: designed the study, treatment planning, collected the data, wrote paper; YX: treatment planning, data analysis; KM: reviewed treatment plans; JX: provided patient’s data, contouring; JD: supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the review board of Cancer Hospital, Chinese Academy of Medical Sciences, and informed consent was waived.
Consent for publication
All authors approved the publication of this manuscript by journal Radiation Oncology.
Competing interests
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, Y., Xu, Y., Men, K. et al. Application of piecewise VMAT technique to whole-brain radiotherapy with simultaneous integrated boost for multiple metastases. Radiat Oncol 17, 86 (2022). https://doi.org/10.1186/s13014-022-02059-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02059-6