Abstract
Background
While intensity modulated radiotherapy (IMRT) has been widely adopted for the treatment of anal cancer (AC), the added contour complexity poses potential risks. This study investigates the impact of contour variation on tumour control probability (TCP) when using IMRT for AC.
Methods
Nine Australian centres contoured a single computed tomography dataset of a patient with AC. The same optimised template-based IMRT planning protocol was applied to each contour set to generate nine representative treatment plans and their corresponding dose volume histograms. A geometric analysis was performed on all contours. The TCP was calculated for each plan using the linear quadratic and logitEUD model.
Results
The median concordance index (CI) for the bladder, head and neck of femur, bone marrow, small bowel and external genitalia was 0.94, 0.88, 0.84, 0.65 and 0.65, respectively. The median CI for the involved nodal, primary tumour and elective clinical target volumes were 0.85, 0.77 and 0.71, respectively. Across the nine plans, the TCP was not significantly different. Variation in TCP between plans increased as tumour cell load increased or radiation dose decreased.
Conclusions
When using IMRT for AC, contour variations generated from a common protocol within the limits of minor deviations do not appear to have a significant impact on TCP. Contouring variations may be more critical with increasing tumour cell load or reducing radiotherapy dose.
Similar content being viewed by others
Background
Squamous cell carcinoma (SCC) of the anal canal (AC) is an uncommon tumour with an increasing incidence [1,2,3]. The treatment standard is organ sparing chemo-radiotherapy (CRT) [4, 5], which achieves loco-regional control in 63–86% [6,7,8] of patients but carries a risk of significant acute and late toxicity [9].
In RTOG 0529, Intensity Modulated Radiotherapy (IMRT) was shown to reduce acute toxicity and cause fewer treatment interruptions compared with historical controls [10]. It also highlighted the increased complexity of IMRT. Remarkably, 81% of plans required revision and 46% required multiple revisions. Despite the provision of a contouring atlas, most revisions were required for incorrect contouring.
Contouring errors in IMRT based CRT increase loco-regional recurrence in head and neck SCC [11, 12]. This raises concerns for AC patients treated with IMRT by inexperienced clinicians. Indeed, population based data suggests AC patients treated at high volume centres have improved survival [13].
The Trans-Tasman Radiation Oncology Group (TROG) investigated IMRT as a new technology in the treatment of AC as part of their Assessment of New Radiation Oncology Technology and Treatments (ANROTAT) [14]. Their study compared the safety, clinical efficacy and cost effectiveness of IMRT with three-dimensional conformal radiation therapy (3DCRT).
The credentialing component of this study involved a benchmarking assessment performed by nine Australian centres. Each centre was provided a computed tomography (CT) dataset of a female patient with T3 N2 AC (primary greater than 50 mm with involved mesorectal and inguinal lymph nodes [LN]) to be treated with 54Gy in 30 fractions. Centres were asked to contour clinical target volumes (CTV) and organs at risk (OAR).
Having acquired these contours from TROG, we sought to describe CTV and OAR contour variability as well as model the impact of contour variation on tumour control probability (TCP) when using IMRT for AC.
Methods
Treatment planning
Each of the nine Australian centres participating in the TROG TRP11.A (ANROTAT) Study B contoured OAR and CTVs using a standard protocol (Table 1) and the Australian Gastrointestinal Trials Group (AGITG) contouring atlas [15].
Varian’s Eclipse treatment planning system was used to generate nine representative IMRT plans. An optimised template-based IMRT planning protocol was initially applied to each contour set. If dosimetric parameters were not met in all plans, the template was adjusted and re-applied to all plans until all target volume and OAR constraints were met. Planning Target Volume (PTV) coverage requirements and OAR constraints were based on ANROTAT guidelines (Appendix).
Geometric analysis
All observer generated contours were collated on a single copy of the benchmarking CT dataset. A reference contour was generated using the STAPLE [16] algorithm in the Computational Environment for Radiotherapy Research (CERR) [17]. In-house code was used to analyse the geometric variation in contours. Metrics of comparison included two volume overlap indices; concordance index (CI) and dice similarity coefficient (DSC).
Tumour control probability
Dose volume histogram (DVH) data was extracted from the treatment plans using CERR [17] and Matlab (The Mathworks, Natick, USA). From the DVHs, CompPlan [18] (open source software) calculated a TCP for each plan using a linear quadratic and logitEUD model (shown in Figs. 1 and 2). The models included a fraction-size effect correction.
For the linear quadratic model where n is the number of fractions and D’ is the total dose, an α/β ratio of 10Gy, α of 0.196 and N0 (number of initial clonogens) of 34,900 were used [19]. A sensitivity analysis investigated the effect on TCP of increasing N0 and decreasing prescription dose.
For the logitEUD model where vi is a voxel, γ50 is the slope of the dose response curve at D50 and Di is the total dose delivered to each voxel, D50 values (dose required for a 50% probability of tumor control at 2 years) for gross and microscopic disease were 42Gy and 30Gy, respectively [19]. Given these values were taken from curves fit to patients treated with CRT, the effect of concurrent chemotherapy was implicitly incorporated.
Results
Geometric analysis
Table 2 shows the results of the OAR geometric analysis. There was close contour agreement for the bladder (median concordance index [CI] 0.94), head and neck of femur (CI 0.88) and bone marrow (CI 0.84). Greater contour variation was seen in the small bowel (CI 0.65) and external genitalia (CI 0.65).
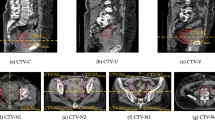
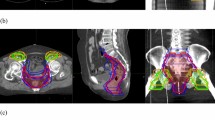
Table 3 shows the results of the tumour volume geometric analysis. Increasing contour variation was seen in CTV54n (CI 0.85), CTV54p (CI 0.77) and CTV45 (CI 0.71), respectively. The CTV45 and CTV54p contours are shown in Fig. 3.
Tumour control probability
The TCP for CTVs are shown in Table 3. Using the linear quadratic model, the median TCP for CTV45, CTV54p and CTV54n was 0.84 (standard deviation [SD] 0.18), 0.94 (SD 0.11) and 1.00 (SD 0.00), respectively. Using the logitEUD model, the median TCP for CTV45, CTV54p and CTV54n was 0.60 (SD 0.04), 0.52 (SD 0.02) and 0.53 (SD 0.02).
Sensitivity analysis of the linear quadratic model revealed that the TCP reduced as tumour cell load increased (Fig. 4a) but variation in TCP between plans increased (Fig. 4b). Likewise, as radiation dose decreased, TCP reduced (Fig. 4c) and variation in TCP between plans increased (Fig. 4d).
Sensitivity analyses. a: Tumour control probability with changing N0, b: variation in tumour control probability between plans with changing N0, c: tumour control probability with changing dose, d: variation in tumour control probability between plans with changing dose. TCP = Tumour Control Probability, SD = Standard Deviation, N0 = number of initial clonogens, Gy = Gray, CTV54p = Primary clinical target volume to receive 54Gy, CTV54n = Nodal clinical targe volume to receive 54Gy, CTV45 = Elective clinical target volume to receive 45Gy
Discussion
Organ at risk contour variation
Our results reveal while there was good inter-observer agreement for the bladder (CI: 0.94), head and neck of femur (CI 0.88) and bone marrow (CI: 0.84), there was significant contour variation in the small bowel (CI 0.65) and external genitalia (CI: 0.65).
For the small bowel, inclusion of the large bowel, iliac vessels, ovaries, peritoneum and muscle were common contouring errors. The lack of oral contrast may have contributed to these inaccuracies. Oral contrast delivered 1 h prior to CT simulation is usually recommended to improve small bowel identification [20].
Controversy persists regarding the optimal bowel contouring technique. No consensus was reached by RTOG [20] and QUANTEC [21] list dose constraints for both bowel loops and a bowel bag. While a bowel bag accounts for the mobility of viscera and simplifies OAR contouring, it may have limitations for post-surgical patients and in capturing inferior motion [22].
Misidentification of the small (60%) and large (45%) bowel was also common in plans submitted to RTOG 0529 [23]. However, accuracy of the external genitalia contours was not reported. In fact the RTOG pelvic OAR contouring guidelines do not include instructions for contouring external genitalia [20] and no consensus guidelines exist. While United Kingdom guidelines have been proposed [24], until consensus guidelines are formalised, variation in external genitalia contours is likely to persist.
For the development and implementation of evidenced based OAR dose constraints, consistent contouring is imperative [25, 26]. This is particularly true in AC IMRT where the proximity of OAR and tumour volumes often requires a compromise between adequate coverage and safety.
Tumour volume contour variation
Despite being provided gross tumour volumes (GTV), variations in the protocoled CTV expansions were seen: CTV54p (CI 0.77), CTV54n (CI 0.85) and CTV45 (CI 0.71). Unsurprisingly, the greatest variation was seen in the CTV45 volumes. These are the largest and the least influenced by the GTV. Incorrect elective nodal contouring was also common in RTOG 0529 where errors were seen in the mesorectum (55%), pre-sacral (43%), inguinal (33%) and iliac nodal groups (31%).
The greatest discrepancies in the CTV45 volumes were in the anterior direction with variable expansion into the uterus and bladder. The AGITG guidelines recommend an internal margin expansion of 1 cm into the bladder and uterus to account for bladder volume variation [15].
The greatest discrepancies in the CTV54p volumes were also in the anterior direction with variable expansion into the vagina and genitalia. Some have highlighted the risk of genital relapse when IMRT is used to achieve genital sparing [27]. While this has been challenged more recently [28], we emphasise the priority of adequate tumour coverage over genital sparing and stress the importance of accurate multi-modality baseline imaging to help optimally define the extent of disease.
Contouring atlases for anorectal volumes have been shown to reduce inter-observer variability [29], however, atlases were available to observers in both the RTOG and TROG studies. Perhaps this variation can be attributed to clinicians coming to terms with a new technique.
A literature review identified guidelines, teaching, auto-contouring and multi-modality imaging as interventions that reduce inter-observer variability in contour delineation [30]. Online teaching and contouring workshops are now offered by international cancer societies and clinicians can have their volumes peer reviewed at their centre and through participation in clinical trials incorporating radiotherapy quality assurance.
Tumour control probability
IMRT is the treatment of choice for AC [31]. It enhances the therapeutic window by reducing dose to OAR and resultant acute toxicity [23]. Fewer treatment breaks for toxicity are required and it offers the prospect of safe dose escalation for large tumours. However, AC contouring is complex and considerable variation exists in target volume delineation [29, 31].
We have shown that minor contour variations generated from a common protocol do not translate into significant differences in TCP. By applying a template-based IMRT planning protocol, we isolated the impact of contour variation on TCP. Further TCP variability would be expected should centres develop their own treatment plans or employ different treatment doses.
Contours for this study were generated from a common contouring guideline [14]. Contouring guidelines lead to greater inter-observer agreement [32] and the provision of an anorectal contouring atlas has been shown to increase TCP and reduce normal tissue complication probability (NTCP) [29].
GTV contours were supplied in the TROG study which may have reduced CTV variation. Inaccurate GTV delineation occurred in 21% of plans submitted to RTOG 0529 [23]. Target volume delineation is more accurate with the use of co-registered Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) and is recommended for treatment planning [31]. PET is recommended to identify lymph nodes requiring inclusion in the GTV [33].
Sensitivity analysis
We investigated the influence of prescription dose and N0 (number of initial clonogens) on the TCP predicted by the linear quadratic model. Increasing N0 led to a reduction in TCP (Fig. 4a) and an increase in the TCP variation between plans (Fig. 4b). Decreasing dose led to a reduction in TCP (Fig. 4c) and increase in TCP variation between plans (Fig. 4d).
While the influence of dose and N0 on the absolute TCP is not surprising, the effect on TCP variation is quite interesting. It suggests contour variations become more critical as one moves down the dose response curve either by increasing tumour cell load or reducing dose. These are important insights for radiation dose de-escalation trials such as ACT 3 and 4 [34] and highlights the central role real-time radiotherapy quality assurance should play in these studies.
Limitations and future directions
In radiation therapy, TCP models have traditionally been limited to the relative comparison of treatment plans rather than their absolute predictive power. Indeed, the difference in tumour control estimated by the two models used in our study highlights the dangers of over interpreting TCP results or using them to inform radiotherapy dose selection.
That contours were completed in the context of a trial using a common protocol with a GTV already provided is a shortcoming of this study potentially limiting its applicability to the wider community. Our results are likely to underestimate contour variation and do not capture the effect of radiotherapy plan generation.
Our study aim was to isolate the impact of contour variation on TCP. While we appreciate there is manual bias in planning [35], it is out of the scope of this study. We tried to mitigate against this by using a single planner and single patient anatomy, deploying a class solution beam arrangement, objectives and priorities.
While a NTCP assessment was explored, this was abandoned due to a lack of reliable radiobiological parameters in the literature. The principle benefit of IMRT is reduced OAR dose, avoidance of toxicity induced treatment gaps and the prospect of safe dose escalation for larger tumours. To achieve this, tumour volumes must balance adequate coverage of at risk sites with toxicity risk.
The ACT 3–5 [34] studies will provide valuable insights into the radiation dose response curve for AC. The incorporation of prognostic factors other than stage such as human papilloma virus status could help further refine this relationship [36]. Treatment adaptation represents another promising approach and may ultimately provide the best means of individualising treatment [37].
Conclusions
In the context of a clinical study, with the dose and parameters used, minor contour variations generated from a common guideline did not significantly impact TCP. With increasing tumour cell load or reducing RT dose, contour variations may be more critical.
Abbreviations
- 3DCRT:
-
Three-dimensional conformal radiation therapy
- AC:
-
Anal cancer
- AGITG:
-
Australian Gastrointestinal Trials Group
- ANROTAT:
-
Assessment of New Radiation Oncology Technology and Treatments
- CERR:
-
Computational Environment for Radiotherapy Research
- CI:
-
Concordance Index
- CRT:
-
Chemo-radiotherapy
- CT:
-
Computed tomography
- CTV:
-
Clinical target volume
- CTV45:
-
Elective clinical target volume to receive 45Gy
- CTV54n:
-
Nodal clinical target volume to receive 54Gy
- CTV54p:
-
Primary clinical target volume to receive 54Gy
- DSC:
-
Dice similarity coefficient,
- DVH:
-
Dose Volume Histogram
- GTV:
-
Gross tumour volumes,
- IMRT:
-
Intensity modulated radiotherapy
- LN:
-
Lymph node
- MRI:
-
Magnetic Resonance Imaging
- OAR:
-
Organs at risk
- PET:
-
Positron Emission Tomography
- PTV:
-
Planning Target Volume
- SCC:
-
Squamous cell carcinoma
- SD:
-
Standard Deviation
- TCP:
-
Tumour Control Probability
- TROG:
-
Trans-Tasman Radiation Oncology Group
References
Steele SR, Varma MG, Melton GB, Ross HM, Rafferty JF, Buie WD. Practice parameters for anal squamous neoplasms. Dis Colon rectum. 2012;55:735–49.
Nelson RA, Levine AM, Bernstein L, Smith DD, Lai LL. Changing patterns of Anal Canal carcinoma in the United States. J Clin Oncol. 2013;31:1569–75.
Jemal A, Simard EP, Dorell C, Noone A-M, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, Cronin KA, Watson M, Schiffman M, Henley SJ, Schymura MJ, Anderson RN, Yankey D, Edwards BK. Annual report to the nation on the status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. JNCI Journal of the National Cancer Institute. 2013;105:175–201.
National Comprehensive Cancer Network: Anal Carcinoma (Version 2.2017) https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf. 2017.
Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, Arnold D, ESMO, ESSO, ESTRO: Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother oncol. 2014;11:330–39.
Martenson JA, Lipsitz SR, Lefkopoulou M, Engstrom PF, Dayal YY, Cobau CD, Oken MM, Haller DG. Results of combined modality therapy for patients with anal cancer (E7283). An eastern cooperative oncology group study. Cancer. 1995;76:1731–6.
Peiffert D, Seitz JF, Rougier P, Francois E, Cvitkovic F, Mirabel X, Nasca S, Ducreux M, Hannoun-Levi JM, Lusinchi A, Debrigode E, Conroy T, Pignon JP, Gerard JP. Preliminary results of a phase II study of high-dose radiation therapy and neoadjuvant plus concomitant 5-fluorouracil with CDDP chemotherapy for patients with anal canal cancer: a French cooperative study. Ann Oncol. 1997;8:575–81.
Gerard JP, Ayzac L, Hun D, Romestaing P, Coquard R, Ardiet JM, Mornex F. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother Oncol. 1998;46:249–56.
Bentzen AG, Guren MG, Vonen B, Wanderås EH, Frykholm G, Wilsgaard T, Dahl O, Balteskard L. Radiotherapy and oncology. Radiother Oncol. 2013;108:55–60.
Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran H, Willett CG. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33.
Peters LJ, O'Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, Bourhis J, Yuen K, Fisher R, Rischin D. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck Cancer: results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001.
Chen AM, Chin R, Beron P, Yoshizaki T, Mikaeilian AG, Cao M. Inadequate target volume delineation and local-regional recurrence after intensity-modulated radiotherapy for human papillomavirus-positive oropharynx cancer. Radiother Oncol. 2017;123:412–8.
Amini A, Jones BL, Ghosh D, Schefter TE, Goodman KA. Impact of facility volume on outcomes in patients with squamous cell carcinoma of the anal canal: analysis of the National Cancer Data Base. Cancer. 2017;123:228–36.
Trans Tasman Radiation Oncology Group: The assessment of new radiation oncology technologies and treatments (ANROTAT): study protocol B (IMRT Anal Canal) final report. 2012.
Ng M, Leong T, Chander S, Chu J, Kneebone A, Carroll S, Wiltshire K, Ngan S, Kachnic L. Australasian gastrointestinal trials group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Radiat Oncol Biol. 2012;83:1455–62.
Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–21.
Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30:979–85.
Holloway LC, Miller J-A, Kumar S, Whelan BM, Vinod SK. Comp plan: a computer program to generate dose and radiobiological metrics from dose-volume histogram files. Med Dosim. 2012;37:305–9.
Muirhead R, Partridge M, Hawkins MA. A tumor control probability model for anal squamous cell carcinoma. Radiother Oncol. 2015;116:192–6.
Gay HA, Barthold HJ, O'Meara E, Bosch WR, Naqa El I, Al-Lozi R, Rosenthal SA, Lawton C, Lee WR, Sandler H, Zietman A, Myerson R, Dawson LA, Willett C, Kachnic LA, Jhingran A, Portelance L, Ryu J, Small W, Gaffney D, Viswanathan AN, Michalski JM. Pelvic normal tissue contouring guidelines for radiation therapy: a radiation therapy oncology group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–62.
Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Haken Ten RK, Miften M. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101–7.
Dominello MM, Nalichowski A, Paximadis P, Kaufman I, McSpadden E, Joiner M, Miller S, Konski A. Limitations of the bowel bag contouring technique in the definitive treatment of cervical cancer. Practical Radiation Oncology. 2014;4:e15–20.
Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, Willins JD, Ryan DP, Hong TS. Dose-painted intensity-modulated radiation therapy for anal Cancer: a multi-institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys. 2012;82:153–8.
Brooks C, Hansen VN, Riddell A, Harris VA, Tait DM. Proposed genitalia contouring guidelines in anal cancer intensity-modulated radiotherapy. Br J Radiol. 2015;88:20150032–7.
Brown E, Cray A, Haworth A, Chander S, Lin R, Subramanian B, Ng M. Dose planning objectives in anal canal cancer IMRT: the TROG ANROTAT experience. J Med Radiat Sci. 2015;62:99–107.
Hornby CJ, Ackerly T, See A, Geso M. Exploring the effect of marked normal structure volume on normal tissue complication probability. Med Dosim. 2003;28:223–7.
Koeck J, Lohr F, Buergy D, Büsing K, Trunk MJ, Wenz F, Mai S. Genital invasion or perigenital spread may pose a risk of marginal misses for intensity modulated radiotherapy (IMRT) in anal cancer. Radiat Oncol. 2016:1–8.
Koeck J, Lohr F, Buergy D, Büsing K, Trunk MJ, Wenz F, Mai S. Genital invasion or perigenital spread may pose a risk of marginal misses for Intensity Modulated Radiotherapy (IMRT) in anal cancer. Radiat Oncol. 2016;11:53.
Dell’Acqua V, Kobiela J, Kraja F, Leonardi MC, Surgo A, Zerella MA, Arculeo S, Fodor C, Ricotti R, Zampino MG, Ravenda S, Spinoglio G, Biffi R, Bazani A, Luraschi R, Vigorito S, Spychalski P, Orecchia R, Glynne-Jones R, Jereczek-Fossa BA. Genital marginal failures after intensity-modulated radiation therapy (IMRT) in squamous cell anal cancer: no higher risk with IMRT when compared to 3DCRT. Medical Oncology. 2018;35:59.
Vinod SK, Min M, Jameson MG, Holloway LC. A review of interventions to reduce inter-observer variability in volume delineation in radiation oncology. Journal of Medical Imaging and Radiation Oncology. 2016;60:393–406.
Glynne-Jones R, Tan D, Hughes R, Hoskin P. Squamous-cell carcinoma of the anus: progress in radiotherapy treatment. Nat Rev Clin Oncol. 2016;13:447–59.
Fuller CD, Nijkamp J, Duppen JC, Rasch CRN, Thomas CR, Wang SJ, Okunieff P, Jones WE, Baseman D, Patel S, Demandante CGN, Harris AM, Smith BD, Katz AW, McGann C, Harper JL, Chang DT, Smalley S, Marshall DT, Goodman KA, Papanikolaou N, Kachnic LA, Radiation Oncology Committee of the Southwest Oncology Group. Prospective randomized double-blind pilot study of site-specific consensus atlas implementation for rectal cancer target volume delineation in the cooperative group setting. Int J Radiat Oncol Biol Phys. 2011;79:481–9.
Jones M, Hruby G, Solomon M, Rutherford N, Martin J. The role of FDG-PET in the initial staging and response assessment of anal Cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22:3574–81.
PLATO: Personalising anal cancer radiotherapy dose http://www.isrctn.com/ISRCTN88455282?q=&filters=conditionCategory:Cancer&sort=&offset=6&totalResults=1847&page=1&pageSize=20&searchType=basic-search.
Batumalai V, Jameson MG, Forstner DF, Vial P, Holloway LC. How important is dosimetrist experience for intensity modulated radiation therapy? A comparative analysis of a head and neck case. Practical Radiation Oncology. 2013;3:e99–e106.
Gilbert DC, Williams A, Allan K, Stokoe J, Jackson T, Linsdall S, Bailey CM, Summers J. p16INK4A, p53, EGFR expression and KRAS mutation status in squamous cell cancers of the anus: correlation with outcomes following chemo-radiotherapy. Radiother Oncol. 2013;109:146–51.
Jones M, Hruby G, Stanwell P, Gallagher S, Wong K, Arm J, Martin J. Multiparametric MRI as an outcome predictor for anal canal cancer managed with chemoradiotherapy. BMC Cancer. 2015;15:281.
Acknowledgements
The authors acknowledge the Trans-Tasman Radiation Oncology group (TROG).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
KF conceived of the study and generated the IMRT plans with PE. JM provided expert opinion on anal cancer management. MJ and LH performed the geometric and tumour control probability analysis. MPJ wrote the protocol, consolidated the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Consent was obtained from the Trans-Tasman Radiation Oncology group (TROG) to use the Assessment of New Radiation Oncology Technology and Treatments (ANROTAT) data.
Competing interests
The authors declare that they have no competing interests.
Appendix
Appendix
Volume | Constraint |
|---|---|
PTV 45 | D95 ≥ 40.5Gy |
D98 ≥ 36Gy | |
D98 ≥ 36Gy | |
PTV 54 | D95 ≥ 48.6 |
D98 ≥ 43.2Gy | |
D2% ≤ 62.1Gy | |
Small bowel | V30Gy ≤ 200 cm3 |
V35Gy ≤ 150 cm3 | |
V45Gy ≤ 20 cm3 | |
V50Gy = 0 cm3 | |
Femoral heads | V30Gy ≤ 50% |
V40Gy ≤ 35% | |
V44Gy ≤ 5% | |
Iliac crest | V30Gy ≤ 50% |
V40Gy ≤ 35% | |
V50Gy ≤ 5% | |
External genitalia | V20Gy ≤ 50% |
V30Gy ≤ 35% | |
V40Gy ≤ 5% | |
Bladder | V35Gy ≤ 50% |
V40Gy ≤ 35% | |
V50Gy ≤ 5% |
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jones, M.P., Martin, J., Foo, K. et al. The impact of contour variation on tumour control probability in anal cancer. Radiat Oncol 13, 97 (2018). https://doi.org/10.1186/s13014-018-1033-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-018-1033-y