Abstract
We evaluated the inter-physician variability in the target contouring of the radiotherapy for anal squamous cell carcinoma (ASCC). Clinical target volume (CTV) of three patients diagnosed with ASCC was delineated by seven experienced radiation oncologists from multi-institution. These patients were staged as pT1N1a, cT2N0, and cT4N1a, respectively, according to 8th edition of the American Joint Committee on Cancer staging system. Expert agreement was quantified using an expectation maximization algorithm for Simultaneous Truth and Performance Level Estimation (STAPLE). The maximum distance from the boundaries of the STAPLE generated volume with confidence level of 80% to those of the contour of each CTV in 6 directions was compared. CTV of pelvis which includes primary tumor, perirectal tissue and internal/external iliac lymph node (LN) area (CTV-pelvis) and CTV of inguinal area (CTV-inguinal) were obtained from the seven radiation oncologists. One radiation oncologist did not contain inguinal LN area in the treatment target volume of patient 2 (cT2N0 stage). CTV-inguinal displayed moderate agreement for each patient (overall kappa 0.58, 0.54 and 0.6, respectively), whereas CTV-pelvis showed substantial agreement (overall kappa 0.66, 0.68 and 0.64, respectively). Largest variation among each contour was shown in the inferior margin of the CTV-inguinal. For CTV-pelvis, anterior and superior margin showed the biggest variation. Overall, moderate to substantial agreement was shown for CTV delineation. However, large variations in the anterior and cranial boarder of the CTV-pelvis and the caudal margin of the CTV-inguinal suggest that further studies are needed to establish a clearer target volume delineation guideline.
Similar content being viewed by others
Introduction
Anal squamous cell carcinoma (ASCC) is a rare type of cancer comprising only 0.3% of all malignancy1. Concurrent chemoradiation (CCRT) with 5-fluorouracil plus mitomycin-C has been the standard therapy for ASCC2. Radiotherapy volume encompasses primary tumor within mesorectum and elective nodal area including obturator, internal iliac, external iliac, presacral and/or inguinal lymph node (LN) area. Advances in radiation therapy technology have led IMRT to replace 3-dimensional (3D) conformal radiotherapy. Whereas large randomized trials utilized mainly 3D conformal radiotherapy3,4, recent clinical trials are testing the application of intensity modulated radiotherapy (IMRT) to reduce toxicity by minimizing radiation dose to normal organs without compromising target coverage5,6. Accurate and well-defined target volume definition is the essential element of IMRT to avoid missing the treatment target and to minimize the dose to surrounding normal tissue. Accurate target delineation is even more critical in the era of image guided radiotherapy (IGRT) using daily conebeam CT or ultrasonogram, because IGRT sided by incorrect target volumes only allows to precisely hit the wrong ones7. Moreover, it is also crucial to reduce toxicity profile of CCRT by specifically targeting organs at risk, such as bowel for gastro-intestinal (GI), genitals for genito-urinary (GU) and bone marrow for hematologic toxicity8,9,10.
There existed different contouring guidelines for IMRT of ASCC11,12,13. While these guidelines provide robust evidence and reproducibility in routine radiation therapy at the clinic, there are still ambiguous definition in the field margin of the clinical target volume (CTV) for elective LN irradiation. Moreover, there may be inter-physician variation resulting from differences in experience, and/or various institutional policy. To date, no studies have shown how varying CTV’s are among experienced radiation oncologists in the real-world clinical settings.
Therefore, in the current study, we evaluated the inter-physician variability of target contouring of the radiotherapy for ASCC.
Materials and methods
Three ASCC patients previously treated with radiotherapy were selected in this study. All patients were diagnosed as ASCC through pathologic examination. For staging work up, all patients underwent colonoscopy, abdomino-pelvic computed tomography (CT), magnetic resonance image (MRI) of pelvis, and positron emission tomography (PET)/CT. TNM staging was described according to the 8th edition of the American Joint Committee on Cancer staging system. Patient 1 was a 49-year-old woman diagnosed with stage pT1N1a ASCC. The patient underwent excisional biopsy. Pathologic examination revealed a 1.4 cm tumor and the resection margin was positive. There was a metastatic LN (1.2 cm in size) in the left inguinal chain which was identified in CT and MRI, and increased maximum standardized uptake value (SUVmax) of 5.0 was observed in PET/CT images. Patient 2 was a 77-year-old woman with stage cT2N0. The tumor size was 3.9 cm. No LN was identified in the CT, MR and PET/CT images. Patient 3 was a 72-year-old woman with stage cT4N1a. About 4.5 cm-sized tumor involved anus, perineum and posterior wall of vagina. There was a 1.2 cm-sized and pathologically confirmed LN in the right inguinal area, which showed increased SUVmax value of 3.07 in PET/CT images. Clinical information of the patients was described in Table 1.
After approval of institutional review board (IRB) of Dongnam Institute of Radiological and Medical Sciences(DIRAMS) (IRB no. D-1809-035-002), clinical information including medical history, colonoscopy, abdomino-pelvic CT, pelvic MRI and PET/CT images and pathologic report of these patients were sent to seven radiation oncologists practicing in different institutions. Participants’ written informed consent was waived by the IRB of DIRAMS since the data was provided as de-identified form. Our research was performed in accordance with relevant guidelines and regulations. The careers of these clinicians range from three to 13 years. They were asked to delineate CTV of pelvis (CTV-pelvis), which includes primary tumor, perirectal tissue, and presacral/obturator/internal/external iliac LN area. Delineation of CTV for inguinal LN area (CTV-inguinal) was decided to the clinician’s decision.
After acquisition of Dicom file of contours from each institution, we analyzed the target volume using MATLAB software (MathWorks, Natick, MA). For the quantification of the agreement in volume definition, we analyzed using two different method. Generalized conformity index (CIgen) can be simplified into an expression as:\({\mathrm{CI}}_{gen}=\frac{{\sum }_{pairs i,j}\left|{A}_{i}\cap {A}_{j}\right|}{{\sum }_{pairs i,j}\left|{A}_{i}\cup {A}_{j}\right|}\) , where Ai and Aj represent the volumes described by the i-th and j-th physicians, respectively. Here, CIgen < 0.5 is generally considered a weak correlation, while CIgen ≥ 0.7 is acceptable14. For the other analysis of the agreement among CTVs of different physicians, we applied the Simultaneous Truth and Performance Level Estimation (STAPLE) algorithm included in the Computational Environment for Radiotherapy Research (CERR) software. This is known as the expectation–maximization algorithm15; it estimates the true contour by implementing an optimization process through the spatial uniformity condition by weighting the performance level of each delineated contour. The performance level is the probability of how each contour is close to the virtual true contour16. In CERR, sensitivity, specificity, and agreement level measurements are expressly provided as an apparent agreement, a kappa-corrected agreement, and a STAPLE-estimated probability. The apparent agreement evaluates the probability of correspondence between observers for each voxel. The kappa-corrected agreement is the corrected consistency to exclude the possibility of coincidence15. In general, a kappa value of < 0.00 indicates poor agreement; 0.00–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.00, almost perfect agreement17. Based on the STAPLE analysis, we generated a contour for each CTV set using the 80% confidence level and used it as a reference (CTV-80) for comparison with each CTV.
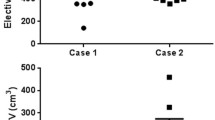
For the difference analysis, we calculated the maximum distance from the boundary of the CTV-80 to that of each CTV contour in six directions. This distance does not necessarily have to be on the same plane along the axis. Pirateplot18 of these values, showing descriptive statics (mean and median) and inferential statistics (95% confidence interval) was generated using R software version 3.6 (R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
Results
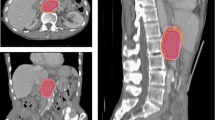
Each CTVs was obtained from the seven radiation oncologists. One radiation oncologist did not contain inguinal LN area in the treatment target volume of patient 2. Analysis of CTV-inguinal of patient 2 was conducted using six contours. Volume and level of agreement of the contours was described in Table 2. Mean and standard deviation of CTV-inguinal was 181.37 ± 65.33 cm3, 158.04 ± 65.37 cm3 and 91.69 ± 335.59 cm3 for each patients, and those of CTV-pelvis were 633.30 ± 158.98 cm3, 658.32 ± 140.16 cm3 and 609.24 ± 143.21 cm3, respectively. Overall kappa value ranged from 0.54 to 0.75. CTV-inguinal displayed moderated agreement for each patient, whereas CTV-pelvis showed substantial agreement. The CIgen value ranged from 0.45 to 0.55. CIgen of CTV-pelvis of three patients had values over 0.5, whereas CIgen of CTV-inguinal was below 0.5. Each CTV of seven clinicians and CTV-80 were delineated in Fig. 1. The differences of boarders between CTV-80 and each CTV along the 6 directions were described in Table 3 and Fig. 2. For CTV-inguinal, the largest variation among each contour was shown in the inferior margin. For CTV-pelvis, anterior and superior margin showed the biggest variation. (Fig. 2).
Pirateplot of differences of borders in 6 directions between each CTV and STAPLE generated CTV with confidence level of 80% (CTV-80). Positive values represent that border of each CTV is larger than CTV-80 in each direction. Plots were generated using R software version 3.6 (R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
Discussion
The result of the current study demonstrated variations among radiation oncologists in the CTV delineation of ASCC. Overall kappa and CIgen values demonstrated that CTV-inguinal had less agreement level than CTV-pelvis among the physicians.
There are three known contouring guidelines for IMRT of ASCC. These are Radiation Therapy Oncology Group (RTOG) consensus guideline11, Australasian Gastrointestinal Trials Group (AGITG) guideline13 and British National Guidance (BNG)12. In all these guidelines, the superior border of the CTV-pelvis is recommended as the bifurcation of the common iliac artery into the external and internal iliac arteries. However, in the current study, cranial border of the CTV-pelvis showed biggest variation (Fig. 2). The variation of the anterior and cranial border of the CTV-pelvis would be due to the concerning about the common iliac LN metastasis. Dapper et al. compared the three existing guidelines and suggested optimal CTVs considering the PET imaging-based LN distribution of 22 ASCC patients19. In three patients with extensive nodal involvement, there were LNs located superiorly to the recommended border of CTV-pelvis. However, common iliac LN relapse was uncommon in the ASCC. In the study reporting the pattern of relapse after definitive CCRT for ASCC using IMRT, common iliac LN relapse accounted for only 4.1 percent20. Moreover, Tomasoa et al. did not report any recurrence above the level of S321. Using the PET/CT evaluation for the LN involvement before treatment, bifurcation of the internal/internal iliac arteries would be sufficient as a cranial border for the CTV of pelvic LN.
Regarding the inguinal region, there was a discrepancy in the defining CTV. The anatomy in the inguinal region is very complex due to large differences between the individuals. Therefore, the three guidelines have different recommendations for inferior inguinal margins. The RTOG defines the caudal margin “2 cm caudal to the saphenous/femoral junction”, the BNG determines the “lesser trochanter” and the AGITG identifies “the lower edge of the ischial tuberosities” as a compromise between saphenous/femoral junction and sartorius/adductor longus junction22. Dapper et al. also pointed out that 10% to 29% of the inguinal LN was not covered by the CTV’s of RTOG, AGITG and BNG guidelines19. Twenty percent of the inguinal LN was located inferiorly to the RTOG inguinal CTV and only four LN’s were located below the lower edge of the ischial tuberosity, suggesting that inferior border of the inguinal LN should be anal verge or 2 cm caudal to anal verge if extensive disease or multiple LN’s19. Because of the large anatomical variation of the inguinal area, more clear definition of the target volume based on the obvious anatomical landmarks is needed for the consistent target contouring among physicians.
Regarding elective inguinal irradiation, one radiation oncologist did not include inguinal radiotherapy in patient 2 with cT2N0 stage ASCC in the current study. There is controversy concerning the necessity of inguinal LN irradiation in the treatment of early stage node-negative ASCC. While many studies support elective inguinal LN irradiation3,23,24,25, some reports suggest its omission for early stage disease26,27,28,29,30,31. Further research is needed regarding elective inguinal LN irradiation in the treatment of early stage node-negative ASCC.
Consensus contouring guideline is essential to reduce inter/intra-clinician variability in the target volume delineation32. However, visualization of contouring guideline in the representative case has a limitation that it does not reflect the patients’ anatomic variations and diverse clinical scenarios. Research on the usefulness of auxiliary contouring tools such as Anatom-e (Anatom-e Information Systems Ltd., Houston, Texas), a digital platform facilitating target delineation by providing atlas as well as guidelines and protocols should also be considered33.
Several limitations exist in our study. We did not provide information on the exact radiotherapy treatment modality, dose prescription and PTV margin. Different institutional protocols may have affected clinicians' CTV delineation. Moreover, we did not investigate which guideline each clinician referred to mainly. Despite these limitations, this study addresses clinicians’ different consideration in target delineation of ASCC, which will help to establish a clearer target delineation guideline in the future.
In conclusion, moderate to substantial agreement was shown for ASCC CTV target delineation. However, large variations in the upper margin of the CTV-pelvis and the lower margin of the inguinal LN area suggest that further studies are needed to establish a clearer target delineation guideline.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Glynne-Jones, R. et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother. Oncol. 111, 330–339. https://doi.org/10.1016/j.radonc.2014.04.013 (2014).
Gunderson, L. L. et al. Long-term update of US GI intergroup RTOG 98–11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J. Clin. Oncol. 30, 4344–4351. https://doi.org/10.1200/JCO.2012.43.8085 (2012).
James, R. D. et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2 x 2 factorial trial. Lancet Oncol. 14, 516–524. https://doi.org/10.1016/s1470-2045(13)70086-x (2013).
Kachnic, L. A. et al. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int. J. Radiat. Oncol. Biol. Phys. 86, 27–33. https://doi.org/10.1016/j.ijrobp.2012.09.023 (2013).
Registry, I. PLATO—Personalising Anal Cancer Radiotherapy Dose.
Arcadipane, F. et al. Image-guided IMRT with simultaneous integrated boost as per RTOG 0529 for the treatment of anal cancer. Asia-Pac. J. Clin. Oncol. 14, 217–223. https://doi.org/10.1111/ajco.12768 (2018).
Arians, N. et al. Intrafractional vaginal dilation in anal cancer patients undergoing pelvic radiotherapy (DILANA)—A prospective, randomized, 2-armed phase-II-trial. BMC Cancer 20, 52. https://doi.org/10.1186/s12885-020-6547-7 (2020).
Arcadipane, F. et al. Bone marrow-sparing IMRT in anal cancer patients undergoing concurrent chemo-radiation: Results of the first phase of a prospective phase II trial. Cancers https://doi.org/10.3390/cancers12113306 (2020).
Ng, M. et al. Intensity-modulated radiotherapy for anal cancer: Dose-volume relationship of acute gastrointestinal toxicity and disease outcomes. Clin. Oncol. (Royal College of Radiologists (Great Britain)) 30, 634–641, https://doi.org/10.1016/j.clon.2018.07.020 (2018).
Myerson, R. J. et al. Elective clinical target volumes for conformal therapy in anorectal cancer: A radiation therapy oncology group consensus panel contouring atlas. Int. J. Radiat. Oncol. Biol. Phys. 74, 824–830. https://doi.org/10.1016/j.ijrobp.2008.08.070 (2009).
Muirhead R, A. R., Gilbert DC, Harrison M, Glynne-Jones R, Sebag-Montefiore D, Hawkins MA. National Guidance for IMRT in Anal Cancer. (2016).
Ng, M. et al. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int. J. Radiat. Oncol. Biol. Phys. 83, 1455–1462. https://doi.org/10.1016/j.ijrobp.2011.12.058 (2012).
Gwynne, S. et al. Improving radiotherapy quality assurance in clinical trials: Assessment of target volume delineation of the pre-accrual benchmark case. Br. J. Radiol. 86, 20120398 (2013).
Allozi, R. et al. Tools for consensus analysis of experts’ contours for radiotherapy structure definitions. Radiother. Oncol. 97, 572–578 (2010).
Warfield, S. K., Zou, K. H. & Wells, W. M. Simultaneous truth and performance level estimation (STAPLE): An algorithm for the validation of image segmentation. IEEE Trans. Med. Imaging 23, 903–921. https://doi.org/10.1109/tmi.2004.828354 (2004).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174 (1977).
Phillips, N. Pirateplot. https://cran.r-project.org/web/packages/yarrr/vignettes/pirateplot.html. Accessed 22 Dec 2020.
Dapper, H. et al. Have we achieved adequate recommendations for target volume definitions in anal cancer? A PET imaging based patterns of failure analysis in the context of established contouring guidelines. BMC Cancer 19, 742. https://doi.org/10.1186/s12885-019-5970-0 (2019).
Shakir, R. et al. Patterns and predictors of relapse following radical chemoradiotherapy delivered using intensity modulated radiotherapy with a simultaneous integrated boost in anal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys.https://doi.org/10.1016/j.ijrobp.2019.10.016 (2019).
Tomasoa, N. B., Meulendijks, D., Nijkamp, J., Cats, A. & Dewit, L. Clinical outcome in patients treated with simultaneous integrated boost - intensity modulated radiation therapy (SIB-IMRT) with and without concurrent chemotherapy for squamous cell carcinoma of the anal canal. Acta Oncol. (Stockholm, Sweden) 55, 760–766. https://doi.org/10.3109/0284186x.2015.1124141 (2016).
Portaluri, M. et al. Clinical and anatomical guidelines in pelvic cancer contouring for radiotherapy treatment planning. Cancer Radiother. 8, 222–229. https://doi.org/10.1016/j.canrad.2004.02.003 (2004).
Matthews, J. H. et al. T1–2 anal carcinoma requires elective inguinal radiation treatment—The results of Trans Tasman Radiation Oncology Group study TROG 99.02. Radiother. Oncol. 98, 93–98, https://doi.org/10.1016/j.radonc.2010.10.005 (2011).
Ortholan, C. et al. Anal canal cancer: Management of inguinal nodes and benefit of prophylactic inguinal irradiation (CORS-03 Study). Int. J. Radiat. Oncol. Biol. Phys. 82, 1988–1995. https://doi.org/10.1016/j.ijrobp.2011.02.010 (2012).
Thompson, S. R. et al. Radiotherapy for anal squamous cell carcinoma: Must the upper pelvic nodes and the inguinal nodes be treated?. ANZ J. Surg. 88, 870–875. https://doi.org/10.1111/ans.14398 (2018).
Hatfield, P., Cooper, R. & Sebag-Montefiore, D. Involved-field, low-dose chemoradiotherapy for early-stage anal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 70, 419–424. https://doi.org/10.1016/j.ijrobp.2007.06.072 (2008).
Gerard, J.-P. et al. Management of inguinal lymph node metastases in patients with carcinoma of the anal canal. Cancer 92, 77–84. https://doi.org/10.1002/1097-0142(20010701)92:1%3c77::aid-cncr1294%3e3.0.co;2-p (2001).
Crowley, C., Winship, A. Z., Hawkins, M. A., Morris, S. L. & Leslie, M. D. Size does matter: Can we reduce the radiotherapy field size for selected cases of anal canal cancer undergoing chemoradiation?. Clin. Oncol. 21, 376–379. https://doi.org/10.1016/j.clon.2009.01.015 (2009).
Zilli, T. et al. Elective inguinal node irradiation in early-stage T2N0 anal cancer: Prognostic impact on locoregional control. Int. J. Radiat. Oncol. Biol. Phys. 87, 60–66, https://doi.org/10.1016/j.ijrobp.2013.03.008 (2013).
Kim, H. et al. Can we omit prophylactic inguinal nodal irradiation in anal cancer patients?. Radiat. Oncol. J. 33, 83 (2015).
Ortholan, C. et al. Anal canal carcinoma: Early-stage tumors < or =10 mm (T1 or Tis): Therapeutic options and original pattern of local failure after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 62, 479–485. https://doi.org/10.1016/j.ijrobp.2004.09.060 (2005).
Fuller, C. D. et al. Prospective randomized double-blind pilot study of site-specific consensus atlas implementation for rectal cancer target volume delineation in the cooperative group setting. Int. J. Radiat. Oncol. Biol. Phys. 79, 481–489. https://doi.org/10.1016/j.ijrobp.2009.11.012 (2011).
Franco, P. et al. Variability of clinical target volume delineation for rectal cancer patients planned for neoadjuvant radiotherapy with the aid of the platform Anatom-e. Clin. Transl. Radiat. Oncol. 11, 33–39. https://doi.org/10.1016/j.ctro.2018.06.002 (2018).
Author information
Authors and Affiliations
Contributions
K.K. and H.J.P. organized and supervised this study together, as co-corresponding authors. K.S.K. and K.H.C. contributed equally to this study by analyzing data and writing the main manuscript, as co-first authors. All the authors including T.K., H.K.K., J.H.C. and A.R.C. did the target contouring used in this analyses.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, K.S., Cheong, KH., Kim, K. et al. Interobserver variability in clinical target volume delineation in anal squamous cell carcinoma. Sci Rep 11, 2785 (2021). https://doi.org/10.1038/s41598-021-82541-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82541-5
- Springer Nature Limited
This article is cited by
-
Contouring aid tools in radiotherapy. Smoothing: the false friend
Clinical and Translational Oncology (2024)