Abstract
Background
Deep rooting is one of the most promising plant traits for improving crop yield under water-limited conditions. Most root phenotyping methods are designed for laboratory-grown plants, typically measuring very young plants not grown in soil and not allowing full development of the root system.
Results
This study introduced the 15N tracer method to detect genotypic variations of deep rooting and N uptake, and to support the minirhizotron method. The method was tested in a new semifield phenotyping facility on two genotypes of winter wheat, seven genotypes of spring barley and four genotypes of ryegrass grown along a drought stress gradient in four individual experiments. The 15N labeled fertilizer was applied at increasing soil depths from 0.4 to 1.8 m or from 0.7 to 2.8 m through a subsurface tracer supply system, and sampling of aboveground biomass was conducted to measure the 15N uptake. The results confirm that the 15N labeling system could identify the approximate extension of the root system. The results of 15N labeling as well as root measurements made by minirhizotrons showed rather high variation. However, in the spring barley experiment, we did find correlations between root observations and 15N uptake from the deepest part of the root zone. The labeled crop rows mostly had significantly higher 15N enrichment than their neighbor rows.
Conclusion
We concluded that the 15N tracer method is promising as a future method for deep root phenotyping because the method will be used for phenotyping for deep root function rather than deep root growth. With some modifications to the injection principle and sampling process to reduce measurement variability, we suggest that the 15N tracer method may be a useful tool for deep root phenotyping. The results demonstrated that the minirhizotrons observed roots of the tested rows rather than their neighboring rows.
Similar content being viewed by others
Introduction
Drought and nutrient deficiency are the main constraints limiting plant growth and productivity [1, 2]. It is a frequent occurrence for plants that water and nutrient uptake fail to meet requirements for growth. The grain yield is especially sensitive to resource deficiency during grain filling, when vegetative growth is limited and the absorption of water and nutrients mainly supports grain development [3,4,5]. To cope with this situation, crop species have evolved both structural and physiological traits to improve resource acquisition and utilization. Deep rooting is one of the most effective traits, which enable plants to access deep soil resources and support yield formation [6,7,8,9]. To improve drought tolerance and subsoil exploration, deep rooting is a desirable trait for plant breeders.
The expression of root traits and performance is a complex process under field conditions, which is regulated by the morpho-physiological abilities of the genotype, growth environment and interactions between them [10,11,12]. For example, roots can grow deeper to explore more resources when soil water and N become limiting in upper soil layers [13, 14]. Cereal roots mainly grow before flowering and the deep root traits form before reproductive growth [4,5,6]. Under terminal drought stress, deep rooting genotypes have the advantage of accessing extra water and nutrients for yield formation compared to shallow rooting genotypes. For example, an additional 10.5 mm subsoil water uptake from the 1.35–1.85 m soil layer after the flowering stage led to a yield increase of 0.62 t/ha in wheat [7]. The structure and functioning of roots vary among the genotypes and growth environment, which makes field root phenotyping a challenging task.
Considerable efforts have focused on the phenotyping of roots in soilless media, which provides visualization of the root system and efficient analysis of root images [15,16,17]. Such laboratory studies are conducted on young plants with roots grown in small containers under controlled environments, where the plants fail to reflect actual field root traits or root traits during the reproductive stage [18, 19]. These studies overlook the influences of soil hardiness, pore size and distribution, moisture and fertility on root traits in the field [20]. Therefore, direct field root phenotyping is essential.
The methods used to measure roots in the field can be categorized as (i) traditional excavation methods, such as trenching and soil coring [21, 22], (ii) nondestructive minirhizotrons, ground-penetrating radar and electrical capacitance [23,24,25], and (iii) indirect root activity estimations, e.g., tracers [26]. However, most of these methods are not suitable for deep root phenotyping because the methods do not efficiently detect root traits or functions for a large number of genotypes.
In general, there are few methods for root phenotyping available to plant breeders [21, 27]. Some studies have used soil coring and minirhizotron methods to identify deep rooting among a few genotypes [22, 28, 29]. The soil coring method involves the collection of a large number of soil samples and root washing, which are labor-intensive and time-consuming [30]. The soil coring method has been improved in the core-break method to enable rapid assessment of deep root distribution [22]. In contrast, a minirhizotron is a nondestructive method for field root phenotyping in situ, which provides a dynamic visualization of root growth [31, 32]. Nevertheless, a large investment is needed to establish a minirhizotron facility for field root phenotyping. Aiming at developing efficient and low-cost methods, the 15N tracer method is introduced in this study and is compared to the minirhizotron method for deep root phenotyping in situ. The 15N tracer method relies on the measurement of deep root uptake ability through deep placement of 15N-labeled nitrate [33, 34]. Additionally, a linear correlation of the 15N uptake and root density at different soil depths has been reported [26]. It is a potential phenotyping method for estimates of deep rooting traits and activity.
In this study, the 15N tracer method was tested in a new root phenotyping facility (RadiMax) to select deep rooting genotypes based on deep 15N uptake and to support the minirhizotron method by analyzing the neighboring effect. The 15N tracer uptake was further compared with the minirhizotron method for the identification of deep-rooted genotypes. The hypotheses of this study are that (1) deep 15N uptake will be correlated with the rooting depth of different crop species, (2) there will be genotypic differences in the deep 15N uptake related to deep root growth, and (3) deep roots of a crop row grow mainly below the crop row itself and do not use resources from their neighboring rows.
Materials and methods
Experimental design
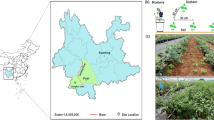
Four experiments were carried out in a new root phenotyping facility, RadiMax (Fig. 1), which was built on a farm of the University of Copenhagen in Taastrup (55° 40́ 90.35ʹ N, 12° 18́ 24.84ʺ E), Denmark, in 2015. The 15N tracer method was applied at increasing depths along the crop rows to crops of winter wheat and spring barley to test deep 15N uptake and to estimate root depth. The first experiment (Exp. 1) was conducted on two genotypes of winter wheat, ‘Hystar’ and ‘Tabasco’, in 2016. The second experiment (Exp. 2) was conducted on two genotypes of spring barley, ‘Evelina’ and ‘Laurikka’, in 2016. The third experiment (Exp. 3) was conducted on seven genotypes of spring barley in 2017. Except for ‘Kenia’ and ‘Evelina’, others are modern genotypes. The fourth experiment (Exp. 4) was conducted on four genotypes of perennial ryegrass (Lolium perenne L.) in 2017, including diploid genotypes ‘Esquire’ and ‘Mercitwo’, and tetraploid genotypes ‘Fabian’ and ‘Tetrastar’ (Table 1).
Experimental sites and methods used in the root phenotyping facility, RadiMax. a The four black squares show units 1, 2, 3 and 4 of the RadiMax facility. The blue squares in units 1, 2 and 3 show the experimental sites with 30 crop rows and the timelines of the four experiments. b The tracer pipes and minirhizotrons were set along the V-shaped bottom in each unit
The RadiMax facility includes movable rain-out shelters to cover the plants and subject them to drought stress, as described by Svane et al. [32]. A subsurface irrigation system was established in the facility to create drought stress gradients along the crop rows by irrigating at increasing depths from the edge to the middle of the facility (Fig. 2). Weather data was obtained from a meteorological station located less than 700 m from the experimental site. The monthly reference evapotranspiration and 10-day average air temperature are shown in Fig. 3. The daily precipitation is shown in Fig. 4.
Schematic of the experimental set-up. Each unit of the RadiMax facility had a V-shaped bottom. Minirhizotrons were overlain on the bulkheads at 0.25 m intervals, sloping from the edge towards the middle of the facility. A subsurface tracer supply system involved 15 tracer pipes, set next to every second minirhizotron. The crop rows above the tracer pipes were labeled crop rows, and those above minirhizotrons were neighbor crop rows. A subsurface irrigation system was established along the bulkheads, enabling irrigation at ten soil depths separately
Daily precipitation and hourly volumetric water content (VWC) at 0.5 m, 1.0 m and 1.5 m soil depth. Four experiments were done in two seasons with rainout shelters used to create terminal drought stress in each experiment individually. Rainout shelters were used to cover winter wheat from 20 May to 24 May, from 27 May to 28 May and from 12 June to 21 July in Exp. 1 (a), and to cover spring barley from 15 June to 2 August in Exp. 2 (b) in 2016. In 2017, rainout shelters were used to cover spring barley from 7 June to 2 August in Exp. 3 (c) and ryegrass from 15 June to 15 July in Exp. 4 (d) in 2017
Experimental set up
The facility consists of four units (Fig. 1), each 40 m long and 9.7 m wide. Each unit has a V-shaped bottom in the belowground. The vertical depth of the V-shaped bottom ranges from 1.1 to 3.0 m soil depth in units 1 and 2 and from 0.7 to 2.1 m soil depth in units 3 and 4. Ten bulkheads of 0.28 m height were inserted horizontally on each side slope of the V-shaped bottom. Drip pipes were placed along the bulkheads, which enables irrigation at 10 soil depths separately. The flux of each drip was 0.85 L h−1. The minirhizotrons (70 mm in outer diameter and 60 mm in inner diameter) were overlain on the bulkheads at 0.25 m intervals, sloping from the edge towards the middle of the facility [32]. In total, 15 tracer pipes from a subsurface tracer supply system were set next to every second minirhizotron at one end of each unit. A drainage pipe was located in the deepest part of the V-shaped bottom to drain excess soil water. The bottom of each unit was sealed by a plastic membrane. Within each unit, 150 crop rows planted at 0.25 m intervals can be grown above the minirhizotrons. Each experiment involved 30 crop rows, i.e., 15 neighbor crop rows and 15 labeled crop rows (Fig. 2), for further details, see [32].
Semifield experiment
Each unit was filled with topsoil (0–0.4 m) and subsoil (below 0.4 m), which were taken from a local farm at 0–0.3 m soil depth and a field at 0.5–2.0 m soil depth, respectively. The soil is classified as a sandy loam. The soil texture, pH and soil fertility are shown in Table 2.
In all the units, the soil was plowed to a depth of 0.2 m and was harrowed before seeding. In Exp. 1, two winter wheat genotypes were sown in unit 1 on 1 October 2015. Crops were fertilized with 70 kg N ha−1 on 15 March and again on 11 May 2016. In Exp. 2, two spring barley genotypes were sown in unit 3 on April 12, 2016. N fertilizer was supplied at a rate of 70 kg ha−1 on the same day as sowing. In Exp. 3, seven spring barley genotypes were sown in unit 1 on 28 March and in unit 2 on April 4 in 2017. N fertilizer was added at the rate of 100 kg ha−1 on the sowing day. The sowing densities of wheat and barley were 350 seeds m−2 and 300 seeds m−2, respectively. In Exp. 4, four ryegrass genotypes were sown in unit 3 on September 27 in 2016. Fertilizers containing 40, 120, 50 and 94.5 kg N ha−1 were supplied on 27 September 2016, 16 March 2017, 16 May 2017 and 02 June 2017, respectively. The sowing density of ryegrass was 800 seeds m−2. Within each of the four experiments, the genotypes were sown in four replicates in a randomized plot design.
The subsurface irrigation system was manually controlled to irrigate at increasing depth along the V-shaped bottom. The soil moisture was measured by time domain transmission (TDT) sensors (Acclima, Inc., USA), which were installed at 0.5 m intervals along the V-shaped bottom with 0.25 m distance and in the west, middle and east of each unit at 0.5 m, 1 m and 1.5 m soil depths. The volumetric soil water content along the soil depth gradient is shown in Fig. 4. The irrigation and rain-out shelter were combined to create drought stress gradients along the crop rows. The subsurface water supply at increasing depths from the edge towards the middle of the facility makes it easy for plants growing towards the edge to reach the subsoil water, while plants along the planting rows towards the middle of the facility needed to grow deeper to access the water supply. The subsurface irrigation system was placed c. 0.28 m below the minirhizotrons. Weed and disease control were performed by herbicide and fungicide spraying as recommended according to local conditions.
15N tracer method
The 15N-labeled fertilizer was supplied to the labeled crop rows through the tracer supply system (Table 1). The 15N tracer was assumed to be evenly distributed along the labeled rows, following the depth gradient of the V-shaped bottom. The 15N uptake was measured by aboveground plant matter of the labeled rows (Fig. 2) and neighbor rows.
Spikes were sampled to measure 15N uptake at maturity in Exp. 1, Exp. 2, and Exp. 3 (Table 1). Samples were collected at four different intervals of each crop row on the labeled side of each unit (Table 3). The samples were oven-dried for 3 days at 75 °C. The dry matter was ground and placed into tin capsules for 15N analysis at the UC Davis Stable Isotope Facility, USA. The total N and 15N concentrations were obtained. The procedure for calculating of 15N enrichment was performed according to Walley et al. [35].
Minirhizotron method
Minirhizotrons provided an interface between a transparent tube and the soil, and a semiautomated minirhizotron camera was used to take images at intervals along the minirhizotrons, i.e., at increasing soil depths. Root images were recorded on 18 May in Exp. 1 and 3 June in Exp. 2 in 2016 and on 21 June in Exp. 3 and 23 May in Exp. 4 in 2017. Root depth was estimated based on these images, as the deepest soil depth where roots were visible on the images. Root intensity was measured by attaching transparent sheets with grid lines on the images and counting the number of roots crossing the grid lines [27].
Statistical analysis
The estimated root depth, 15N uptake and 15N enrichment of different genotypes in the four experiments were analyzed using R statistical software [36]. A normal distribution test was performed with the Shapiro–Wilk method (P ≤ 0.05) prior to statistical testing for the equality of means by F-tests in analysis of variance (ANOVA). Tukey’s Honestly Significant Difference (HSD) (P ≤ 0.05) was used for post hoc analysis among genotypes. A linear mixed-effects model was used to take the block effects into account in Exp. 3 [37].
Results
Crop performance
The grain crops grew well in the facility and produced generally good yields, i.e., on average 7.9, 4.8 and 6.6 Mg DM of grain per hectare for winter wheat in Exp. 1 and spring barley in Exp. 2 and 3, respectively (Table 3). Genotype differences in yield were limited, but in Exp. 3, the old cultivar ‘Kenia’ showed a lower yield than the other genotypes. In Exp. 1 and 2 in 2016, there was a tendency towards higher yields and N uptake in the shallow part of the facility, with better water supply than the deep part. In Exp. 3 in 2017, the trend was opposite, with higher yields and N uptake in the deep part of the facility.
Root growth and 15N uptake
Root depth development varied among the three species studied (Table 4), with ryegrass reaching 1.1 to 1.2 m depth, spring barley reaching 1.2 to 1.5 m and winter wheat reaching c. 1.65 m.
The results showed significant 15N uptake of wheat, barley and ryegrass in all four experiments, with a general decrease with soil depth. There was much higher 15N uptake from the two shallow soil depths in Exp. 1, 3 and 4 compared to the crop receiving 15N at larger depths. Within these three experiments, the maximum depth of 15N uptake corresponded to the measured rooting depth of the crop at the time of 15N injection. In the three experiments, limited uptake was estimated from the depth below the estimated average rooting depth, but no uptake was found from the deepest soil layer. In Exp. 2, there was significant 15N uptake at the four soil depths (Table 4), and the uptake depth thus clearly exceeded the rooting depth at the time of 15N injection.
In three of the experiments, there were no significant differences in root depth measured by the minirhizotrons among the genotypes, except in Exp. 3, where Tocada (1.48 m) was found to have significantly deeper roots than Laurikka (1.21 m) (Table 4).
When correlating 15N uptake with root intensity in Exp. 3, in the two upper layers 0.87–1.30 m and 1.30–1.74 m, a significant correlation was shown in the deeper layer (1.30–1.74 m; R = 0.90, P = 0.006), while no correlation was observed in the upper layer (0.87–1.30 m; R = 0.09, P = 0.844) (Fig. 5).
15N enrichment of labeled and neighbor rows
To test the validity of the measurements in the facility we compared 15N uptake in the labeled crop lines to uptake in their neighbor rows, 0.25 m and 0.50 m away (Fig. 6). Significant uptake in neighbor rows may show either roots spreading across rows or movement of labeled N away from the injection point towards the neighbor row.
15N enrichment of the labeled and neighboring crop rows at four soil depths. The 15N enrichment measurements were performed on grain at harvest (a winter wheat in Exp. 1, b spring barley in Exp. 2, and c spring barley in Exp. 3) or on green tillers 25 days after labeling (d ryegrass in Exp. 4). The neighbor crop rows were 0.25 m or 0.50 m from the labeled crop rows. Different lowercase letters indicate significant differences among crop rows at P < 0.05. The furthest neighbor crop rows were only replicated once, and their statistical significance was ignored
In all experiments, the 15N enrichment in the labeled row was higher than in neighbor rows 0.25 m away. The higher enrichment in the labeled row was observed from all injection depths where significant enrichment was observed, and the enrichment was generally 3 to 5 times higher in the labeled row than in the neighbor rows (Fig. 6). The effect was significant in most cases. In deep layers in Exp. 1, 3 and 4, where very little enrichment was observed, no difference between labeled and neighbor rows was observed. In Exp. 2, enrichment was observed at all four soil depths, and from all layers, the labeled genotypes showed significantly higher 15N enrichment than the neighbor rows (Fig. 6b). Very little 15N enrichment was observed in any of the samples collected 0.50 m away from the test rows in any of the experiments (Fig. 6).
Discussion
The subsurface tracer supply system
The subsurface tracer supply system is an innovative facility designed to apply tracers to the underground for deep root phenotyping. In this study, it enabled the supply of 15N-labeled fertilizer to the deep roots of wheat, barley and ryegrass along an increasing soil depth from 0.4 to 1.8 m or from 0.7 m to 2.8 belowground. By measuring aboveground 15N uptake at intervals along the depth gradient, depth activity was estimated. Additionally, the amount of 15N uptake can be related to the deep root density measured by the minirhizotrons. 15N as well as root growth and observation by the minirhizotrons is affected by a number of factors, such as heterogeneous soil compaction and moisture, and minirhizotron measurements rely on the appearance of visible roots on the tube surface and new image analysis strategies to separate new roots and dead roots or roots of other plants [38]. The semifield facility was designed to reduce these problems compared to direct field studies, where soil variability is often high. The 15N tracer method is able to detect the deep roots in situ. While the sloping subsurface tracer supply system is ideal to support studies of the tracer method in the RadiMax facility, a full-scale phenotyping approach would probably need to be based on a specific target injection depth, thereby labeling all rows within the unit could be conducted in future experiments.
Relationship between 15N uptake and deep root growth of crop species
In this study, it was confirmed that 15N injection using driplines in the RadiMax facility and measuring its uptake could be used to study differences in deep rooting among crop species and to reveal differences among genotypes where expected differences are smaller. The 15N uptake of all crops was high when it was injected at shallow soil depths, where minirhizotrons showed significant root growth. For all crops, 15N enrichment was low from larger soil depths where observations did not show root growth at the time of 15N supply. A good agreement between the 15N uptake and root depth of crops under field conditions has previously been reported by Kristensen and Thorup-Kristensen [26]. 15N was taken up when placed within the soil layers where root growth was measured using the minirhizotron method, but no 15N uptake was found when the 15N was placed below the measured rooting depth [26].
Relationship between 15N uptake and deep root growth of crop genotypes
In one of the four experiments, Exp. 3, where significant differences in deep rooting were observed among the genotypes, genotypic differences in 15N uptake and its relationship to root observations could be studied. Few significant genotype effects were found, but we did find a correlation between 15N tracer uptake and minirhizotron root observations in the deepest part of the root zone. For spring barley, significant differences were indicated in the shallowest soil depth interval studied, but as root density in that location was high for all genotypes, this may not be important for N uptake in this layer or indicates the ability of deep rooting. Other traits might be important for the effective utilization of soil N in this interval [25, 39]. Therefore, it is difficult to draw a conclusion on which method is superior because both of them had merits and limitations. Furthermore, the shallow and deep soil layers had different soil moisture contents, which can influence 15N uptake. In addition, 15N uptake also depends on the transpiration rate and N demand of the shoot [26]. Although a few significant genotypic differences were observed, and a good correlation between deep root length and deep 15N uptake in spring barley was observed, showing the potential of the method, it is also clear that the considerable variability of the measurements is a limitation of the method as it is. Reducing experimental variability will be important for the efficient use of the method for larger scale phenotyping.
Deep rooting traits are closely related to root uptake ability because deep-rooting genotypes can explore deep-stored resources and increase drought tolerance [6, 8, 40]. The deep rooting genotypes of spring barley generally had higher grain N content and yield than some of the shallow rooting genotypes in this study. Under terminal drought stress, water and nutrient use from subsoil are critical to reduce yield loss [41]. In this study, ‘Tocada’ with the deepest rooting had the highest 15N uptake at a larger soil depth.
A large number of studies have found that deep and branching root traits are cheap and effective way to improve root uptake ability and to reduce drought stress under water limited conditions [4, 6, 8, 42]. However, there is a lack of methods for phenotyping root traits and especially deep root traits in situ [21]. Therefore, many studies attempt to perform phenotyping for deep rooting on very young plants without well-developed root systems and grown in artificial growing media, which often fail to meet the expectations when grown in the field [18, 19, 43]. In contrast, the 15N tracer method is a direct way to phenotype deep rooting in situ. It is a further advantage that the tracer method measures the nitrogen uptake ability of deep roots, rather than just root growth, and potentially this can be applied to other tracers for water and other nutrients, using, e.g., 2H2O-labeled water or 32P-labeled fertilizer.
15N enrichment of neighbor crop rows
We hypothesized that roots of a crop row grow mainly below the row itself, and do not use resources from below neighbor rows. This was confirmed by the significantly higher 15N enrichment from the labeled crop rows than the neighbor rows at the root reached depth. Some 15N uptake did occur in the neighbor rows, but as the tracer itself will spread in the soil, to some degree towards the neighbor row, this indicates rather limited mingling with roots from neighbor lines, and that roots detected in a minirhizotron belong mainly to the row above it. The horizontal spread of roots can vary strongly among crops [21, 44, 45], but the horizontal spread of root systems of wheat, barley and ryegrass has rarely been measured due to technological bottlenecks. The 15N tracer method was useful for distinguishing the deep roots among crop rows in this study, and in previous studies it has even been used to map root distributions in intercropping systems [46].
Conclusion
This study tested deep 15N placement through drip irrigation lines for deep root phenotyping of wheat, barley and ryegrass in four individual experiments in a new root phenotyping facility, RadiMax. The results showed significant 15N uptake of all crops from upper soil layers, but uptake from deeper layers was related to the rooting depth of the crops. Few significant genotypic variations in rooting depth and 15N uptake were detected, but a significant correlation between deep root length and deep 15N uptake was found among seven spring barley genotypes. The results show that the method has potential for use as a phenotyping method and is of interest because it measures actual root uptake activity rather than root growth, but they also indicate that improvements will be needed to reduce measurement variability. There were significant differences in 15N enrichment between the labeled crop rows and the neighbor rows, showing that roots grow mainly below the crop row itself, rather than mingling with roots of neighbor rows when the row spacing was 0.25 m.
Availability of data and materials
The datasets in this study are available from the corresponding author on reasonable request.
Abbreviations
- N:
-
nitrogen
- TDT:
-
time domain transmission
References
Lynch JP. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot. 2013;112(2):347–57. https://doi.org/10.1093/aob/mcs293.
Samarah N, Alqudah A, Amayreh J, McAndrews G. The effect of late-terminal drought stress on yield components of four barley cultivars. J Agron Crop Sci. 2009;195(6):427–41. https://doi.org/10.1111/j.1439-037X.2009.00387.x.
Gooding MJ, Ellis RH, Shewry PR, Schofield JD. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J Cereal Sci. 2003;37(3):295–309. https://doi.org/10.1006/jcrs.2002.0501.
Krishnamurthy L, Kashiwagi J, Upadhyaya HD, Gowda CLL, Gaur PM, Singh S, Purushothaman R, Varshney RK. Partitioning coefficient-A trait that contributes to drought tolerance in chickpea. Field Crop Res. 2013;149:354–65. https://doi.org/10.1016/j.fcr.2013.05.022.
Prasad P, Pisipati S, Momčilović I, Ristic Z. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J Agron Crop Sci. 2011;197(6):430–41. https://doi.org/10.1111/j.1439-037X.2011.00477.x.
Kashiwagi J, Krishnamurthy L, Crouch JH, Serraj R. Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crop Res. 2006;95(2):171–81. https://doi.org/10.1016/j.fcr.2005.02.012.
Kirkegaard J, Lilley J, Howe G, Graham J. Impact of subsoil water use on wheat yield. Aust J Agric Res. 2007;58(4):303–15. https://doi.org/10.1071/AR06285.
Ramamoorthy P, Lakshmanan K, Upadhyaya HD, Vadez V, Varshney RK. Root traits confer grain yield advantages under terminal drought in chickpea (Cicer arietinum L.). Field Crop Res. 2017;201:146–61. https://doi.org/10.1016/j.fcr.2016.11.004.
Wasson AP, Richards R, Chatrath R, Misra S, Prasad SS, Rebetzke G, Kirkegaard J, Christopher J, Watt M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot. 2012;63(9):3485–98. https://doi.org/10.1093/jxb/ers111.
Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WBT, Wojciechowski T, Young IM. Root phenomics of crops: opportunities and challenges. Funct Plant Biol. 2009;36(11):922–9. https://doi.org/10.1071/FP09150.
Kamoshita A, Babu RC, Boopathi NM, Fukai S. Phenotypic and genotypic analysis of drought-resistance traits for development of rice cultivars adapted to rainfed environments. Field Crop Res. 2008;109(1):1–23. https://doi.org/10.1016/j.fcr.2008.06.010.
Malamy J. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005;28(1):67–77. https://doi.org/10.1111/j.1365-3040.2005.01306.x.
López-Bucio J, Cruz-Ramı́rez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003;6(3):280–287. https://doi.org/10.1016/S1369-5266(03)00035-9.
Merrill SD, Tanaka DL, Hanson JD. Root length growth of eight crop species in Haplustoll soils. Soil Sci Soc Am J. 2002;66(3):913–23. https://doi.org/10.2136/sssaj2002.0913.
Bengough A, Gordon D, Al-Menaie H, Ellis R, Allan D, Keith R, Thomas W, Forster B. Gel observation chamber for rapid screening of root traits in cereal seedlings. Plant Soil. 2004;262(1/2):63–70. https://doi.org/10.1023/B:PLSO.0000037029.82618.27.
Gioia T, Galinski A, Lenz H, Müller C, Lentz J, Heinz K, Briese C, Putz A, Fiorani F, Watt M. GrowScreen-PaGe, a non-invasive, high-throughput phenotyping system based on germination paper to quantify crop phenotypic diversity and plasticity of root traits under varying nutrient supply. Funct Plant Biol. 2017;44(1):76–93. https://doi.org/10.1071/FP16128.
Mathieu L, Lobet G, Tocquin P, Périlleux C. “Rhizoponics”: a novel hydroponic rhizotron for root system analyses on mature Arabidopsis thaliana plants. Plant Methods. 2015;11(1):3–11. https://doi.org/10.1186/s13007-015-0046-x.
Watt M, Moosavi S, Cunningham S, Kirkegaard J, Rebetzke G, Richards R. A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann Bot. 2013;112(2):447–55. https://doi.org/10.1093/aob/mct122.
Zhu J, Ingram PA, Benfey PN, Elich T. From lab to field, new approaches to phenotyping root system architecture. Curr Opin Plant Biol. 2011;14(3):310–7. https://doi.org/10.1016/j.pbi.2011.03.020.
Passioura J. Soil conditions and plant growth. Plant Cell Environ. 2002;25(2):311–8. https://doi.org/10.1046/j.0016-8025.2001.00802.x.
Chen X, Ding Q, Błaszkiewicz Z, Sun J, Sun Q, He R, Li Y. Phenotyping for the dynamics of field wheat root system architecture. Sci Rep. 2017;7(1):37649–60. https://doi.org/10.1038/srep37649.
Wasson AP, Rebetzke GJ, Kirkegaard JA, Christopher J, Richards RA, Watt M. Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. J Exp Bot. 2014;65(21):6231–49. https://doi.org/10.1093/jxb/eru250.
Amato M, Bitella G, Rossi R, Gómez JA, Lovelli S, Gomes JJF. Multi-electrode 3D resistivity imaging of alfalfa root zone. Eur J Agron. 2009;31(4):213–22. https://doi.org/10.1016/j.eja.2009.08.005.
Dalton F. In-situ root extent measurements by electrical capacitance methods. Plant Soil. 1995;173(1):157–65. https://doi.org/10.1007/BF00155527.
Rasmussen IS, Dresbøll DB, Thorup-Kristensen K. Winter wheat cultivars and nitrogen (N) fertilization-effects on root growth, N uptake efficiency and N use efficiency. Eur J Agron. 2015;68:38–49. https://doi.org/10.1016/j.eja.2015.04.003.
Kristensen HL, Thorup-Kristensen K. Root growth and nitrate uptake of three different catch crops in deep soil layers. Soil Sci Soc Am J. 2004;68(2):529–37. https://doi.org/10.2136/sssaj2004.5290.
Chen S, van der Graaff E, Ytting NK, Thorup-Kristensen K. Evaluation of deep root phenotyping techniques in tube rhizotrons. Acta Agric Scand B. 2018;69(1):62–74. https://doi.org/10.1080/09064710.2018.1500635.
Ohashi AYP, Pires RCDM, Ribeiro RV, Silva ALBDO. Root growth and distribution in sugarcane cultivars fertigated by a subsurface drip system. Bragantia. 2015; 74(2):1–9. https://doi.org/10.1590/1678-4499.0295.
White RG, Kirkegaard JA. The distribution and abundance of wheat roots in a dense, structured subsoil-implications for water uptake. Plant Cell Environ. 2010;33(2):133–48. https://doi.org/10.1111/j.1365-3040.2009.02059.x.
Samson BK, Sinclair TR. Soil core and minirhizotron comparison for the determination of root length density. Plant Soil. 1994;161(2):225–32. https://doi.org/10.1007/BF00046393.
Herrera JM, Stamp P, Liedgens M. Dynamics of root development of spring wheat genotypes varying in nitrogen use efficiency. In: Buck HT, Nisi JE, Salomón N. (eds) Wheat production in stressed environments developments in plant breeding. Springer, Dordrecht. 2007; 12: 197–201.https://doi.org/10.1007/1-4020-5497-1_25
Svane SF, Jensen CS, Thorup-Kristensen K. Construction of a large-scale semi-field facility to study genotypic differences in deep root growth and resources acquisition. Plant Methods. 2019;15(1):26. https://doi.org/10.1186/s13007-019-0409-9.
Gass W, Peterson G, Hauck R, Olson R. Recovery of residual nitrogen by corn (Zea mays L.) from various soil depths as measured by 15N tracer techniques. Soil Sci Soc Am J. 1971;35(2):290–4. https://doi.org/10.2136/sssaj1971.03615995003500020032x.
Huang Y, Rickerl D, Kephart K. Recovery of deep-point injected soil nitrogen-15 by switchgrass, alfalfa, ineffective alfalfa, and corn. J Environ Qual. 1996;25(6):1394–400. https://doi.org/10.2134/jeq1996.00472425002500060033x.
Walley F, Fu G, van Groenigen J-W, van Kessel C. Short-range spatial variability of nitrogen fixation by field-grown chickpea. Soil Sci Soc Am J. 2001;65(6):1717–22. https://doi.org/10.2136/sssaj2001.1717.
Team RC. R: a language and environment for statistical computing. Vienna, Austria. 2019. https://cran.r-project.org/doc/manuals/fullrefman.pdf.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2014;67(1): 1–48. https://doi.org/10.18637/jss.v067.i01.
Svane SF, Dam EB, Carstensen JM. Thorup-Kristensen K. A multispectral camera system for automated minirhizotron image analysis. Plant Soil. 2019; 441: 657–672. https://doi.org/10.1007/s11104-019-04132-8.
Bingham I, Karley A, White P, Thomas W, Russell J. Analysis of improvements in nitrogen use efficiency associated with 75 years of spring barley breeding. Eur J Agron. 2012;42:49–58. https://doi.org/10.1016/j.eja.2011.10.003.
Kramer PJ, Boyer JS. Water relations of plants and soils. Soil Sci. 1996;161(4):257–60. https://doi.org/10.1097/00010694-199604000-00007.
Kashiwagi J, Krishnamurthy L, Purushothaman R, Upadhyaya HD, Gaur PM, Gowda CLL, Ito O, Varshney RK. Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.). Field Crop Res. 2015;170:47–544. https://doi.org/10.1016/j.fcr.2014.10.003.
Comas L, Becker S, Cruz VMV, Byrne PF, Dierig DA. Root traits contributing to plant productivity under drought. Front Plant Sci. 2013;4:442–58. https://doi.org/10.3389/fpls.2013.00442.
Gorim L, Rabani E, Barlow B, De Silva D, Vandenberg A. Are artificial media valid for root analysis? A case study comparing root traits of five lentil genotypes in artificial media versus soil. J Soil Sci Plant Health. 2018; 2(1): 1. https://www.scitechnol.com/peer-review/are-artificial-media-valid-for-root-analysis-a-case-study-comparing-root-traits-of-five-lentil-genotypes-in-artificial-media-versu-41NP.php?article_id=7136.
Liedgens M, Richner W. Minirhizotron observations of the spatial distribution of the maize root system. Agron J. 2001;93:1097–104. https://doi.org/10.2134/agronj2001.9351097x.
Thorup-Kristensen K. Root growth and nitrogen uptake of carrot, early cabbage, onion and lettuce following a range of green manures. Soil Use Manage. 2006;22:29–38. https://doi.org/10.1111/j.1475-2743.2005.00012.x.
Rowe EC, van Noordwijk M, Suprayogo D, Hairiah K, Giller KE, Cadisch G. Root distributions partially explain 15N uptake patterns in Gliricidia and Peltophorum hedgerow intercropping systems. Plant Soil. 2001;235(2):167–79. https://doi.org/10.1023/A:1011961409353.
Acknowledgements
The construction of this facility requires many peoples’ input. We would like to acknowledge their contributions and valuable skills. The project was funded by the Innovation Fund Denmark (Grant No. 46-2014-1), Crop Innovation Denmark, Promilleafgiftsfonden, Plan Danmark and Godfred Birkedal Hartmanns Familiefond (Grant No. RadiMax). We thank the China Scholarship Council for the financial support of Si Chen for her PhD research.
Author information
Authors and Affiliations
Contributions
KT-K obtained the grant, conceived the idea for the use of 15N tracers for root phenotyping. SFS and KT-K built the facility and designed the experiments. SC and SFS ran the experiments, collected plant samples and analysed the data. SC drafted the manuscript. KT-K and SFS revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
All authors agreed to publish this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, S., Svane, S.F. & Thorup-Kristensen, K. Testing deep placement of an 15N tracer as a method for in situ deep root phenotyping of wheat, barley and ryegrass. Plant Methods 15, 148 (2019). https://doi.org/10.1186/s13007-019-0533-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13007-019-0533-6