Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a progressive and fatal motor neuron disease. Due to the limited knowledge about potential biomarkers that help in early diagnosis and monitoring disease progression, today’s diagnoses are based on ruling out other diseases, neurography, and electromyography examination, which takes a time-consuming procedure.
Methods
PubMed, ScienceDirect, and Web of Science were explored to extract articles published from January 2015 to June 2023. In the searching strategy following keywords were included; amyotrophic lateral sclerosis, biomarkers, cerebrospinal fluid, serum, and plama.
Results
A total number of 6 studies describing fluid-based exosomal biomarkers were included in this study. Aggregated proteins including SOD1, TDP-43, pTDP-43, and FUS could be detected in the microvesicles (MVs). Moreover, TDP-43 and NFL extracted from plasma exosomes could be used as prognostic biomarkers. Also, downregulated miR-27a-3p detected through exoEasy Maxi and exoQuick Kit in the plasma could be measured as a diagnostic biomarker. Eventually, the upregulated level of CORO1A could be used to monitor disease progression.
Conclusion
Based on the results, each biomarker alone is insufficient to evaluate ALS. CNS-derived exosomes contain multiple ALS-related biomarkers (SOD1, TDP-43, pTDP-43, FUS, and miRNAs) that are detectable in cerebrospinal fluid and blood is a proper alternation. Exosome detecting kits listed as exoEasy, ExoQuick, Exo-spin, ME kit, ExoQuick Plus, and Exo-Flow, are helpful to reach this purpose.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a complex fatal neurodegenerative disease characterized by the progressive degeneration of upper motor neurons (UMNs) and lower motor neurons (LMNs) in different areas of the brain and spinal cord [1,2,3], eventuating in muscle paralysis, atrophy, weakness, respiratory failure, and lastly death within 3–5 years of disease onset [4]. According to study reports, 223,000 people worldwide were affected by ALS, and estimated that this number will increase by 69% in 2040, primarily due to the aging population [5]. As this fatal disease is expected to spread over time and affect more individuals, improving our understanding of ALS seems vital. ALS is described in two types: Familial ALS (fALS) and sporadic ALS (sALS). The latter, with no specific inheritance pattern, accounts for nearly 90% of all ALS cases, whereas fALS accounts for almost 5–10% of the ALS population [4].
When ALS symptoms initially appear in the limbs, leading to movement or walking difficulties, it is called limb-onset ALS. Another presentation of ALS, which is more progressive than limb-onset ALS [6], is classified as bulbar-onset ALS, when symptoms occur in the face or neck, leading to difficulties in swallowing or speech [6, 7]. Although fALS severity is higher than sALS, both types are clinically identical [5]. Clinical diagnosis of ALS is usually tricky due to its overlapping symptoms with other neurological disorders and the lack of a specific diagnostic test [8]. To date, due to ALS’s complex nature and limited knowledge of the underlying mechanisms that cause it, there is no treatment. Existing strategies are based only on disease management, survival enhancement, and symptom therapy [9]. Therefore, the discovery and development of strategies that can help in early diagnosis, demonstrate target engagement, monitor the course of the disease, and serve as an indicator of treatment efficacy seems vital for ALS patients [5, 10, 11]. To achieve these goals, biomarkers are a suitable option.
Currently, the ALS pathogenic mechanisms are still largely unknown [12], however, possible mechanisms may include mitochondrial dysfunction, the mutant SOD1 effects, and glutamate excitotoxicity [7, 12,13,14]. In addition, several mutant genes associated with ALS have been identified [5, 7]. For instance, in 1993, due to the discovery by a group of scientists supported by the National Institute of Neurological Disorders and Stroke, the mutant SOD1 gene was associated with some fALS patients [15]. Afterward, other additional genetic mutations have been in ALS like TDP-43, [16] which is primarily responsible for protein synthesis and RNA processing [16, 17]. These pathogenic proteins have been observed to accumulate in the plasma-derived and central nervous system (CNS)-derived extracellular vesicles and act as diagnostic biomarkers [18]. Attempts to discover fluid-based ALS biomarkers have been accomplished chiefly using CSF due to its close position to the neuroanatomical region affected by the disease. However, recent findings have also studied serum and plasma, and scientists’ attempt to conduct studies based on urine and saliva is emerging [10, 19]. For instance, cystatin C, detected in the CSF samples of ALS patients, also known as a cysteine protease inhibitor is conceptualized to participate in the procedures resulting in the formation of the Bunina bodies in the intraneuronal areas [10]. In addition to mutant gene products, bio-fluids contain neurofilament proteins, inflammatory mediators, and metabolic markers that provide information about ALS progression.
In this review, we aim to investigate relevant findings about ALS diagnostic fluid-based biomarkers by introducing the potential ALS-associated proteins or factors accumulated in the exosomes of blood and cerebrospinal fluid (CSF), whether as a result of gene mutations or other causes. Improving our knowledge of ALS diagnosis can get us closer to developing novel yet definitive ALS therapeutics in the future.
Materials and methods
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance [11, 12]. Moreover, the protocol of the study was submitted in the PROSPERO website.
Eligibility criteria
In this study, retrospective, prospective, and cross-sectional studies published in peer-reviewed journals with the aim of investigating exosomal biomarkers in the ALS disease were included. Also, studies with less than 10 participants classified as case reports and case series were excluded. Moreover, there was a publication year restriction, in which only novel biomarkers described from articles published since 2015 were included. Finally, studies published as a conference proceedings were not eligible for this study. In this study, biomarkers as a prediction, diagnostic, or prognosis measured in the fluid samples, including serum/plasma and CSF, were included.
Search strategy and databases
Three databases (PubMed, ScienceDirect, and Web of Science) were explored to extract articles with publication years ranging from January 2015 to June 2023. In the searching strategy following keywords were considered; amyotrophic lateral sclerosis, biomarkers, cerebrospinal fluid, serum, and plasma. Eventually, the following search strings were utilized to find relevant articles from PubMed, ScienceDirect, and Web of Science databases.
-
• In the PubMed database, based on the purpose of the study and keywords following search string was built: ((amyotrophic lateral sclerosis [Title/Abstract] AND biomarker[Title/Abstract]) AND exosomes[Title/Abstract] AND (CSF[Title/Abstract] OR serum[Title/Abstract] OR plasma[Title/Abstract]) NOT (spinal cord injury)))
-
• In Web of Science and ScienceDirect databases articles were extracted based on the following search string: ((amyotrophic lateral sclerosis AND biomarker AND exosomes) AND (CSF OR serum OR plasma) NOT (spinal cord injury))
Study selection
Conference abstracts, case reports, and case series were omitted in advance of initial screening. Evaluating the title and abstract of the extracted articles were implemented through three reviewers (AA, SD, and DA). In the evaluation of the title and abstract, the presence of relevant keywords was considered. Subsequently, the methodology of the remaining articles was evaluated from multiple aspects, including statistical analysis, sample size, and the tool by which biomarkers were assessed. If the article’s full text were not free to access, a request would be made to obtain the full text. If the request was responded to without a positive outcome, the manuscript was excluded from further evaluation.
Data extraction
The required data from the inputs were extracted by one of the researchers (AA) into a table, in which assessed tools and the cutoff point for diagnostic accuracy were extracted. It is worth mentioning that only confirmed biomarkers detected in serum/plasma or CSF were reported in the tables. Other biomarkers were mentioned in the main text.
Quality assessment
The Critical appraisal (CA) test as a questionnaire assessment tool to evaluate the risk of bias in the selected studies was utilized with the judgment of two of the reviewers (AR and AA). Moreover, the included studies were assessed based on the criteria discussed in the Standards for Reporting of Diagnostic Accuracy (STARD) checklist. Based on STARD checklists included studies should contain a diagnostic accuracy test (including predictive values, specificity, sensitivity, or area under the curve), enrollment of patients with valid criteria (El Escorial criteria), and report the statistical results with their precision.
Results
In the initial searching, 378 inputs were obtained from three databases (PubMed, ScienceDirect, and Web of Science). After removing duplicated inputs and applying inclusion criteria, 6 original studies reported exosomal fluid-based biomarkers were included in this study (Fig. 1).
Extracellular vesicles (EVs)
Nearly all cells [20], including the nervous system cells, are observed to release 30 to 2000 nm diameter vesicles to the extracellular space [21]. Being found in physiological fluids, blood-derived EVs and CNS-derived EVs cross the blood-brain barrier (BBB) and are readily detectable in blood, CSF, and urine. Besides, these vesicles can be isolated from blood, urine, or CSF by several minimally invasive techniques. Since the secretion of macromolecules, including mRNAs, miRNAs, lipids, and proteins, is mediated by EVs [3, 21, 22] carrying specific biochemical signals [23], they provide us with precious information about cell status, especially during disease conditions. Two main EV types have been described as exosomes and microvesicles (MVs). MVs of ALS patients have been identified to be enriched by the accumulation of ALS-related proteins, namely SOD1, TDP-43, pTDP-43, and FUS [16], suggesting EV-mediated prion-like propagation of ALS disease [19], these mutant proteins retrieved in EVs are delivered across the brain cells and spread the disease (Table 1). In addition, the Chen et al. 2020 results investigating TDP-43, NFL, and pNFL in the exosomes derived from plasma samples reported an enhancement of TDP-43 and NFL in exosomes of the ALS group, over time highlighted its prognostic role [24]. Furthermore, exosomes are reported to transport ALS-specific downregulated miRNA biomarkers, including miR-27a-3p which could be detected in patients’ plasma through exoEasy Maxi and exoQuick Kit for exosome isolation and qRT-PCR method for miRNA detection [11, 25]. Also, the CORO1A protein could be detected in the exomes extracted from ALS patients’ plasma. It was suggested as a novel fluid biomarker highlighted by an upregulation that was highly correlated with disease progression [26] (Table 1).
Superoxide dismutase 1 (SOD1)
Superoxide dismutase 1 (SOD1) is an abundant cytosolic and mitochondrial enzyme responsible for the clearance of superoxide molecules by breaking down superoxide radicals. As mentioned above, SOD1 was the first ALS-related protein to be detected in extracellular vesicles (EVs). Misfolded disulfide-cross-linked SOD1 aggregates have been observed in EVs from spinal motor neurons and glial cells of familial ALS (fALS) and some proportion of sporadic (sALS) cases [15]. It is noteworthy that in some other fALS and sALS cases, instead of mutations in SOD1, wild-type SOD1 has been observed [27]. As Sibilla et al. propose, SOD1 can self-replicate in vitro and transfer aggregates in culture [28]. Further, the aggregates of mutant SOD1 in EVs are associated with the prion-like propagation of the ALS pathology in the CNS [16]. This term is applied to diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) that are caused by the deposition of several misfolded proteins. Moreover, by measuring SOD1 concentration in CSF we perceive an elevation in the values versus healthy controls [29].
RNA-binding protein (FUS)
RNA-binding protein FUS is involved in DNA transcription, protein synthesis, RNA metabolism, stress granules formation resulting in neural death [30], and disarrangement of the splicing phenomenon [31]. Abnormal FUS protein aggregates have been observed in several neurodegenerative diseases, including ALS, FTLD, and polyglutamine disease [30], FUS aggregation in ALS appears at the early stages of the disease, contributing well to the disease pathogenesis [32] Compared to SOD1 or TDP-43 mutation carriers, FUS mutation carriers have shown a slightly younger age of disease onset and their survival time appeared to be shorter [33]. Similar to the TDP-43 protein, the mouse model expressing the human FUS protein has a short lifespan and exhibits an aggressive phenotype [34]. It is proposed that the FUS protein aggregations within stress granules might be associated with disease initiation [35]. However, the exact roles of FUS and stress granules in the pathogenesis of ALS and FTLD remain unclear [34]. Attempts to identify FUS-based biomarkers in plasma or CSF might offer developments in the therapy of inherited FUS-linked ALS [30].
TAR DNA-binding protein 43 (TDP-43)
Another protein retrieved in EVs is TAR DNA-binding protein 43 (TDP-43) and phosphorylated-TDP-43 (pTDP-43) [36]. The pathological hallmark underlying most ALS cases and half of the cases of frontotemporal lobar degeneration (FTLD) is the deposition of nuclear and cytoplasmic inclusions of (TDP-43), which regulates translational regulation, pre-mRNA splicing, and transcriptional repression [16, 37, 38]. Similar to SOD1, TDP-43 presents a prion-like structure, appearing misfolded in extracellular fluids (Fig. 2). Plasma-derived EVs in familial types of ALS have been observed to be enriched with mutant TDP-43 aggregates; however, the serum has not shown the same result [39]. Furthermore, TDP-43 accumulations have been found in the EV fraction of CSF from ALS and Frontotemporal dementia (FTD) [21]. Similar to TDP-43, many other mutant genes provide ALS degenerative conditions that may resemble FTD [16]; thus, biomarkers should be examined carefully (Fig. 3).
The ALS disease pathological signaling pathway. MFG-E8: milk fat globule-EGF factor 8, PGRN: Progranulin, MCP-1: macrophage chemoattractant protein-1, MIP-1β: macrophage inflammatory protein-1β, ROS: reactive oxygen species, SOD1: Superoxide dismutase 1, FUS: Fused in sarcoma, TDP-43: TAR DNA-binding protein 43, NFL: neurofilament light, NFH: neurofilament heavy, DPRs: dipeptide repeat proteins
The ALS disease biomarkers in the CSF, Blood, Exomes, Extracellular vesicles, and Stress granules. Although, still there is no available method to detect C9orf72, based on in vitro and animal research this biomarker has the potential to be measured in the blood and CSF. CSF: cerebrospinal fluid, MFG-E8: milk fat globule-EGF factor 8, PGRN: Progranulin, IL-6: interleukin-6, MCP-1: macrophage chemoattractant protein-1, MIP-1β: macrophage inflammatory protein-1β, SOD1: Superoxide dismutase 1, FUS: Fused in sarcoma, TDP-43: TAR DNA-binding protein 43, NFL: neurofilament light, miRNA: micro RNA
MicroRNA (miRNA)
As it was previously mentioned, miR-27a-3p can be detected in the exosomes of ALS patients. However, there are a wide range of miRNAs in which some proportion of them can be detected in exosome, while others are presented as circulating biomarkers in the serum/plasma or CSF [40,41,42,43]. miRNAs are small, non-coding RNA molecules playing roles as endogenous transcriptional regulators of gene expression. Being present in human physiological fluids, miRNAs are considered promising biomarkers for neurodegenerative diseases like PD, AD, HD, and ALS [44]. Detectable changes in many serum miRNA levels have been observed, of which miR-206 seems to be very promising as its higher expression level in ALS patients has been confirmed by many studies [45,46,47,48]. However, the miR-206 circulating level has been reported to decrease along the disease progression [45, 49]. Moreover, considering different stages of ALS, some miRNAs are involved in apoptosis and the neurodegenerative phase of the disease, including miR-338, miR-142, and miR-183, whereas miR-206, miR-133a, and miR-133b associate with the muscular atrophy [50]. (Table 1) The serum level of all these miRNAs increases during the disease progression, except for miR-183 [50]. Although these biomarkers seem to represent a valid fluid-based biomarker for ALS, further investigation might still be required, as these changes in miRNAs might appear in other neurodegenerative conditions, particularly ALS-mimic disorders, and thus not specific to ALS pathogenesis. Apart from miRNAs, different types of RNAs, including lncRNAs, and circRNAs, all detectable in blood, may also represent other novel biomarkers for ALS [47] as they have already been proposed for some neurodegenerative diseases and cancers [51]. For example, an elevated level of circRNA hsa_circ_0023919 in blood samples [43, 47], as well as CSF [39] of SALS patients, has been observed, suggesting the potential utility of other types of RNAs. Moreover, in a cohort study conducted by Banack et al., the plasma samples were obtained from ALS and normal participants to determine the level of miRNAs in the neural-enriched EVs. In this research, first, the plasma was acquired from participants, subsequently, EVs were derived from the plasma, and the level of miRNAs was determined by the real-time PCR. The result of the study concluded in five upregulated (miR-146a-5p, miR-199a-3p, miR-151a-3p, miR-151a-5p, and miR-199a-5p) and three downregulated (miR-4454, miR-10b-5p, and miR-29b-3p) miRNAs [52].
Non-exosomal fluid-based biomarkers
Levels of the NFH and NFL have been reported to be significantly elevated in the CSF of ALS than in healthy controls and patients without parenchymal CNS disease, indicating axonal impairment in ALS [8, 53, 54] With regards to blood, NFL has appeared in serum-derived EVs with a validity of 84–100% and specificity of 76–97%, whereas the data for NFH in serum has been 61–80% for validity and 72.1–83.7% for specificity, implying that serum NFH seems to be slightly a less potent biomarker [55]. Notably, the rise of NFL levels has been detected in several other CNS diseases, for example, in subarachnoid hemorrhage [56, 57], and thus is not specific to ALS. However, NFL and NFH are excellent biomarkers in differentiating ALS patients from healthy ones and ALS mimic diseases, based on the Sugimoto et al. 2020 study with a sensitivity and specificity of 100% [58] (Tables 1 and 2).
The C9orf72 repeat expansion GGGGCC (G4C2)n is the most common genetic cause of FTLD and 25–40% of all FALS cases [7, 10, 12, 15]. C9orf72 encodes five dipeptide repeat proteins (DPRs) observed in patients with ALS and FTLD [10], exhibiting neurotoxic effects. These proteins have been detected in CSF and blood and thus could be a valuable fluid-based biomarker tracking ALS pathology [59] (Fig. 2).
Due to recent proteomic findings, inflammatory factors were identified to be altered in the CSF of patients with ALS, reflecting the potential role of inflammatory mediators as biomarkers of ALS. For instance, elevated levels of interleukin-6 (IL-6) in astrocyte-derived exosomes of SALS patients have been identified, showing a positive correlation with the rate of disease progression [8, 10, 60]. Progranulin (PGRN), a secretory protein involved in inflammation and other cellular functions, is another mediator that changes in CSF of FTD, ALS, and AD patients. Multiple studies detected higher cerebrospinal fluid PGRN levels in ALS-diagnosed patients [61,62,63]. Moreover, milk fat globule-EGF factor 8 (MFG-E8), a secretory glycoprotein with anti-inflammatory properties [64], is another mediator involved in the pathogenesis of neurodegenerative diseases, including ALS. It has been found that MFG-E8 plays a role in distinguishing ALS from healthy controls, as ALS patients exhibit a higher CSF level of MFG-E8 [64, 65]. As some papers report, macrophage chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1β (MIP-1β) levels significantly increased in the CSF fluid of ALS patients compared to the healthy controls [54, 66, 67] (Table 2). In one cohort study, these cytokine levels were analyzed concerning ALS duration and severity, as well as its pathogenesis. Although these cytokines affected ALS pathogenesis, no correlation with the duration or severity of the disease was found [66] (Fig. 3). Besides, the enhanced level of CD14, secreted from hepatocytes, in the ALS group could be detected in the CSF and serum of the patients [68, 69].
Lastly, blood metabolic parameters can also be examined as potential biomarkers in diagnosing ALS. A metabolomics study of plasma approved by the Institutional Review Board at Massachusetts General Hospital identified a 32-member panel of metabolites that differentiated patients with ALS from healthy controls [70]. This biomarker panel included creatinine, creatine, glutamine, pyroglutamine, and urate. Other studies have demonstrated that uric acid level in ALS patients was significantly lower [8, 48, 62], similar to serum albumin level in those patients [8, 54]. Furthermore, creatinine level in serum was observed to be increased in the early stages of ALS [71] but decreased along the progression of the disease [72,73,74]. Nonetheless, additional investigations might be needed to confirm the utility and specificity of these biomarkers in the ALS diagnosis [10, 70] (Fig. 3).
Discussion
While many studies elaborate on the research aspect of the biomarkers and report all potential biomarkers, our study only includes the biomarkers and detecting kits that currently could be measured in rural laboratories without implementing high-tech instruments. Moreover, unlike similar articles, we reported commercial kits of the ALS detecting disease. Therefore, identifying and measuring ALS biomarkers is critical for accurate and rapid diagnosis at the early stages, helping physicians and caregivers handle the situation most effectively [75].
The problem in finding potential biomarker arise in the specificity aspect, in which almost all of the reported biomarkers are common between two or more diseases. Implementing specific instruments, including neuroimaging and electromyography (EMG) in differentiated diagnostics of ALS mainly has a doubtful result, which leads the physician to roll out other diseases rather than directly justify ALS. Researchers mainly perform neuroimaging techniques listed as T1 weighted, diffusion tensor imaging (DTI), and magnetic resonance spectroscopy (MRS) to diagnose ALS. In the T1 weighted images, multiple areas are claimed to be changed as a result of ALS disease bulbar onset. Among them, hypothalamus atrophy is more prominent compared to the control group [76]. Nevertheless, the issue of specificity arises due to the same reduction observed in the autism spectrum [77]. The thickness of the temporal muscle is reported to be altered in the ALS group. Besides its novelty, the temporalis muscle is susceptible to several diseases such as glioblastoma [78]. To evaluate the DTI method, Agosta et al. systematic review could be referred to, in which five studies reported decreased FA parameter as a biomarker in the corpus callosum (CC) [79]. Likewise, in AD at the mild stage, the FA value decreased significantly in the CC [80]. Finally, the value of the diagnosis of the MRS was assessed in a systematic review of six studies, in which it could be observed a decrease in the N-Acetyl-aspartate (NAA) concentration. Accordingly, the NAA/creatine (NAA/Cr) ratio and the NAA/choline (NAA/Cho) ratio were diminished [81]. Nevertheless, the decreased ratio of NAA to creatine is observed in multiple sclerosis (MS) and it construes as the fatigue sign of the disease, whereas this ratio in MS is mostly affected by creatine rather than NAA [82]. Another utmost important instrument in ALS detection is known as EMG and nerve conduction study (NCS). The overall evaluation through NCS is concluded by measuring F-wave and compound motor action potential (CMAP). F-waves and CMAP alone do have not a potential diagnostic value in differentiating ALS from other neuromuscular diseases [83]. Alternatively, a scale calculated from the CMAP value of the ulnar and median nerve (Abductor pollicis brevis (APB) CMAP divided by Abductor digiti minimi (ADM) CMAP) named split hand has a high specificity and sensitivity in ALS detection with 81% and 78%, respectively [84]. Among the multiple limitations in performing the mentioned instruments, two of them are more significant in clinical approaches, including case-by-case difference explanations of the data results along with vague cut-off points. ALS-specific biomarkers in the EVs could be a potential candidate for overcoming with mentioned challenges since the coincidence of positive results of a complex set of ALS biomarkers in the EVs has the least chance to be observed in other diseases. Nevertheless, one of the current study limitation was lack of reporting sensitivity and specificity in the included articles reported exosomal biomarkers, in which conducting a comparison between the sensitivity and specificity of the exosomal and non-exosomal biomarkers was not possible.
Conclusion
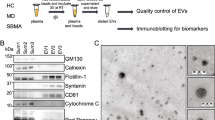
In conclusion, due to the heterogeneity of the ALS disease manifestation and onset, finding a single specific fluid-based biomarker for ALS is far-reaching. Alternatively, a combination of biomarkers should be measured. Biomarkers are the indicators of the different cellular pathways involved in disease onset and progression which help to develop effective therapeutics in the future as well as monitor the disease progression. In this regard, diagnosis based on plasma and CSF EVs can provide helpful information about abnormal protein segregations and changes in miRNA levels in the physiological fluids of the human body. To reach this purpose, six commercial kits are listed: ExoQuick, ExoQuick Plus, Exo-spin, ExoEasy, Exo-Flow, and ME kits are beneficial in extracting exosomes from the body fluids [22]. Exosome markers including surface markers (CXCL5, S100A9, CXCL12, CD63, CD9, and TSG101), can be detected by western blot and Luminex assay to justify the accuracy of the exome extracting procedure [22].
Data availability
Not applicable.
References
Ding Y, Botchway BOA, Zhang Y, Jin T, Liu X. The combination of autologous mesenchymal stem cell-derived exosomes and neurotrophic factors as an intervention for amyotrophic lateral sclerosis. Ann Anat. 2022;242:151921.
Hongo Y, Matsui T, Nakata T, Furukawa H, Ono T, Kaida K, et al. Morphological characterization of cholinergic partition cells: a transmitter-specific tracing study by Cre/lox-dependent viral gene expression. Ann Anat. 2022;240:151857.
Wilkins HM, Dimachkie MM, Agbas A. Blood-based Biomarkers for Amyotrophic lateral sclerosis. In: Araki T, editor. Amyotrophic lateral sclerosis. Brisbane (AU): Exon Publications Copyright: The Authors.;; 2021.
Morimoto S, Takahashi S, Fukushima K, Saya H, Suzuki N, Aoki M, et al. Ropinirole hydrochloride remedy for amyotrophic lateral sclerosis – protocol for a randomized, double-blind, placebo-controlled, single-center, and open-label continuation phase I/IIa clinical trial (ROPALS trial). Regenerative Therapy. 2019;11:143–66.
Vejux A, Namsi A, Nury T, Moreau T, Lizard G. Biomarkers of amyotrophic lateral sclerosis: current status and interest of oxysterols and phytosterols. Frontiers Media S.A.; 2018.
Walhout R, Verstraete E, van den Heuvel MP, Veldink JH, van den Berg LH. Patterns of symptom development in patients with motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degeneration. 2018;19(1–2):21–8.
Wales S, Kiernan Dsc C, Cheah Mbiostat AM, Burrell Mbbs BC, Zoing Bnurs J, Kiernan MC. Seminar Amyotrophic lateral sclerosis. Lancet. 2011;377:942–55.
Aydemir D, Ulusu NN. Importance of the serum biochemical parameters as potential biomarkers for rapid diagnosis and evaluating preclinical stage of ALS. Med Hypotheses. 2020;141.
Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, De Carvalho M, et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12(3):310–22.
Vu LT, Bowser R. Fluid-Based Biomarkers for Amyotrophic Lateral Sclerosis. Springer New York LLC; 2017. p. 119 – 34.
Barbo M, Ravnik-Glavač M. Extracellular vesicles as potential biomarkers in amyotrophic lateral sclerosis. Genes. 2023;14(2):325.
Ewis L, Owland PR, Hneider EAS. Medical Progress 1688 ·. 2001.
Bonafede R, Mariotti R. ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Frontiers Research Foundation; 2017.
Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. 2006. p. 787–95.
Institute of Neurological Disorders N. Amyotrophic Lateral Sclerosis fact sheet. 2021.
Opris I, Bonetto V, Basso M, Ferrara D, Pasetto L. Role of Extracellular vesicles in amyotrophic lateral sclerosis. Front Neurosci. 2018;12:574.
Silverman JM, Christy D, Shyu CC, Moon KM, Fernando S, Gidden Z, et al. CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)G93A ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem. 2019;294(10):3744–59.
Elsner C, Ergün S, Wagner NJAA-AA. Biogenesis and release of endothelial extracellular vesicles: morphological aspects. 2022:152006.
Floeter MK, Gendron TF. Biomarkers for amyotrophic lateral sclerosis and Frontotemporal Dementia Associated with Hexanucleotide Expansion mutations in C9orf72. Frontiers Media S.A.; 2018.
Kreimer S, Ivanov AR. Rapid isolation of Extracellular vesicles from blood plasma with size-exclusion chromatography followed by Mass Spectrometry-based proteomic profiling. Methods in molecular biology. (Clifton NJ). 2017;1660:295–302.
Feneberg E, Steinacker P, Lehnert S, Schneider A, Walther P, Thal DR, et al. Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5–6):351–6.
Macías M, Rebmann V, Mateos B, Varo N, Perez-Gracia JL, Alegre E, et al. Comparison of six commercial serum exosome isolation methods suitable for clinical laboratories. Effect in cytokine analysis. Clin Chem Lab Med. 2019;57(10):1539–45.
Hornung S, Dutta S, Bitan G. CNS-Derived blood exosomes as a Promising source of biomarkers: opportunities and challenges. Front Mol Neurosci. 2020;13:38.
Chen PC, Wu D, Hu CJ, Chen HY, Hsieh YC, Huang CC. Exosomal TAR DNA-binding protein-43 and neurofilaments in plasma of amyotrophic lateral sclerosis patients: a longitudinal follow-up study. J Neurol Sci. 2020;418:117070.
Chen QY, Wen T, Wu P, Jia R, Zhang R, Dang J. Exosomal Proteins and miRNAs as mediators of amyotrophic lateral sclerosis. Front Cell Dev Biol. 2021;9:718803.
Zhou Q, He L, Hu J, Gao Y, Shen D, Ni Y et al. Increased expression of coronin-1a in amyotrophic lateral sclerosis: a potential diagnostic biomarker and therapeutic target. Front Med. 2022:1–13.
Rotunno MS, Bosco DA. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. 2013.
Sibilla C, Bertolotti A. Prion properties of SOD1 in amyotrophic lateral sclerosis and potential therapy. Cold Spring Harb Perspect Biol. 2017;9(10).
Winer L, Srinivasan D, Chun S, Lacomis D, Jaffa M, Fagan A, et al. SOD1 in cerebral spinal fluid as a pharmacodynamic marker for antisense oligonucleotide therapy. JAMA Neurol. 2013;70(2):201–7.
Deng H, Gao K, Jankovic J. The role of FUS gene variants in neurodegenerative diseases. Nature Publishing Group; 2014. pp. 337–48.
Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. 2011. p. 616–30.
Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, MacKenzie IRA. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol. 2009;118(5):605–16.
Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: What do we really know? 2011. p. 603 – 15.
Mitchell JC, McGoldrick P, Vance C, Hortobagyi T, Sreedharan J, Rogelj B, et al. Overexpression of human wild-type FUS causes progressive motor neuron degeneration in an age- and dose-dependent fashion. Acta Neuropathol. 2013;125(2):273–88.
Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in als and FTD: disrupted RNA and protein homeostasis. 2013. p. 416–38.
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–3.
Lee JD, Woodruff TM. TDP-43 puts the STING in ALS. Trends Neurosci. 2021;44(2):81–2.
Beyer L, Günther R, Koch JC, Klebe S, Hagenacker T, Lingor P, et al. TDP-43 as structure-based biomarker in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2021;8(1):271–7.
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141–57.
De Felice B, Guida M, Guida M, Coppola C, De Mieri G, Cotrufo R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene. 2012;508(1):35–40.
Freischmidt A, Müller K, Ludolph AC, Weishaupt JH. Systemic dysregulation of TDP-43 binding microRNAs in amyotrophic lateral sclerosis. 2013.
Toivonen JM, Manzano R, Oliván S, Zaragoza P, García-Redondo A, Osta R. MicroRNA-206: a potential circulating biomarker candidate for amyotrophic lateral sclerosis. PLoS ONE. 2014;9(2).
Dolinar A, Koritnik B, Glavač D, Ravnik-Glavač M. Circular RNAs as potential blood biomarkers in amyotrophic lateral sclerosis. Mol Neurobiol. 2019;56(12):8052–62.
Rajgor D. Macro roles for microRNAs in neurodegenerative diseases. Noncoding RNA Res. 2018;3(3):154–9.
de Andrade HM, de Albuquerque M, Avansini SH, de Dogini SRC, Nucci DB. MicroRNAs-424 and 206 are potential prognostic markers in spinal onset amyotrophic lateral sclerosis. J Neurol Sci. 2016;368:19–24.
Waller R, Goodall EF, Milo M, Cooper-Knock J, Da Costa M, Hobson E, et al. Serum miRNAs miR-206, 143-3p and 374b-5p as potential biomarkers for amyotrophic lateral sclerosis (ALS). Neurobiol Aging. 2017;55:123–31.
Ravnik-Glavač M, Glavač D. Circulating RNAs as potential biomarkers in amyotrophic lateral sclerosis. MDPI AG; 2020.
Freischmidt A, Müller K, Zondler L, Weydt P, Mayer B, von Arnim CAF, et al. Serum microRNAs in sporadic amyotrophic lateral sclerosis. Neurobiol Aging. 2015;36(9):2660. e15-.e20.
Dobrowolny G, Martone J, Lepore E, Casola I, Petrucci A, Inghilleri M, et al. A longitudinal study defined circulating microRNAs as reliable biomarkers for disease prognosis and progression in ALS human patients. Cell Death Discov. 2021;7(1):4.
Ricci C, Marzocchi C, Battistini S. MicroRNAs as biomarkers in amyotrophic lateral sclerosis. Cells. 2018;7(11).
Nuzziello N, Liguori M. The MicroRNA centrism in the orchestration of neuroinflammation in neurodegenerative diseases. MDPI; 2019.
Banack SA, Dunlop RA, Cox PA. An miRNA fingerprint using neural-enriched extracellular vesicles from blood plasma: towards a biomarker for amyotrophic lateral sclerosis/motor neuron disease. Open Biol. 2020;10(6):200116.
Xu Z, Henderson RD, David M, McCombe PA. Neurofilaments as biomarkers for amyotrophic lateral sclerosis: a systematic review and meta-analysis. Public Library of Science; 2016.
Sun J, Carrero JJ, Zagai U, Evans M, Ingre C, Pawitan Y, et al. Blood biomarkers and prognosis of amyotrophic lateral sclerosis. Eur J Neurol. 2020;27(11):2125–33.
Halbgebauer S, Steinacker P, Verde F, Weishaupt J, Oeckl P, von Arnim C, et al. Comparison of CSF and serum neurofilament light and heavy chain as differential diagnostic biomarkers for ALS. J Neurol Neurosurg Psychiatry. 2020;93(1):68–74.
Pijnenburg YAL, Verwey NA, van der Flier WM, Scheltens P, Teunissen CE. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimer’s Dementia: Diagnosis Assess Disease Monit. 2015;1(4):505–12.
Siedler DG, Chuah MI, Kirkcaldie MTK, Vickers JC, King AE. Diffuse axonal injury in brain trauma: insights from alterations in neurofilaments. Frontiers Media S.A.; 2014.
Sugimoto K, Han Y, Song Y, Gao Y. Correlational analysis of ALS Progression and serum NfL measured by Simoa Assay in Chinese patients. Front Neurol. 2020;11:579094.
Westergard T, Jensen BK, Wen X, et al. Cell-to-cell transmission of dipeptide repeat proteins linked to C9orf72-ALS/FTD. CellRep. 2016;17:645–52.
Chen Y, Xia K, Chen L, Fan D. Increased Interleukin-6 levels in the astrocyte-derived exosomes of sporadic amyotrophic lateral sclerosis patients. Front Neurosci. 2019;13:574.
Wilke C, Gillardon F, Deuschle C, Dubois E, Hobert MA, vom Müller J, et al. Serum levels of Progranulin do not reflect cerebrospinal fluid levels in neurodegenerative disease. Curr Alzheimer Res. 2016;13(6):654–62.
Feneberg E, Steinacker P, Volk AE, Weishaupt JH, Wollmer MA, Boxer A, et al. Progranulin as a candidate biomarker for therapeutic trial in patients with ALS and FTLD. J Neural Transm. 2016;123(3):289–96.
Schreiber S, Debska-Vielhaber G, Abdulla S, Machts J, Schreiber F, Kropf S, et al. Peripheral nerve atrophy together with higher cerebrospinal fluid progranulin indicate axonal damage in amyotrophic lateral sclerosis. Muscle Nerve. 2018;57(2):273–8.
Cheyuo C, Aziz M, Wang P. Neurogenesis in neurodegenerative diseases: role of mfg-e8. Frontiers Media S.A.; 2019.
Yang B, Wu Y, Wang Y, Yang H, Du B, Di W, et al. Cerebrospinal fluid MFG-E8 as a promising biomarker of amyotrophic lateral sclerosis. Neurol Sci. 2020;41(10):2915–20.
Martínez HR, Escamilla-Ocañas CE, Camara-Lemarroy CR, González-Garza MT, Moreno-Cuevas J, Sarreón MAG. Increased cerebrospinal fluid levels of cytokines monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1 (MIP-1) in patients with amyotrophic lateral sclerosis. 2020.
Tanaka M, Kikuchi H, Ishizu T, Minohara M, Osoegawa M, Motomura K, et al. Intrathecal upregulation of granulocyte colony stimulating factor and its neuroprotective actions on motor neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006;65(8):816–25.
Beers DR, Zhao W, Neal DW, Thonhoff JR, Thome AD, Faridar A, et al. Elevated acute phase proteins reflect peripheral inflammation and disease severity in patients with amyotrophic lateral sclerosis. Sci Rep. 2020;10(1):15295.
Staats KA, Borchelt DR, Tansey MG, Wymer J. Blood-based biomarkers of inflammation in amyotrophic lateral sclerosis. Mol Neurodegener. 2022;17(1):11.
Lawton KA, Brown MV, Alexander D, Li Z, Wulff JE, Lawson R, et al. Plasma metabolomic biomarker panel to distinguish patients with amyotrophic lateral sclerosis from disease mimics. Amyotroph Lateral Scler Frontotemporal Degeneration. 2014;15(5–6):362–70.
Ito D, Hashizume A, Hijikata Y, Yamada S, Iguchi Y, Iida M, et al. Elevated serum creatine kinase in the early stage of sporadic amyotrophic lateral sclerosis. J Neurol. 2019;266(12):2952–61.
Chiò A, Calvo A, Bovio G, Canosa A, Bertuzzo D, Galmozzi F, et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA Neurol. 2014;71(9):1134–42.
Chen X, Guo X, Huang R, Zheng Z, Chen Y, Shang HF. An exploratory study of serum creatinine levels in patients with amyotrophic lateral sclerosis. Neurol Sci. 2014;35(10):1591–7.
Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. 2012;51(12):1501–8.
Tarawneh R, Biomarkers. Our path towards a cure for Alzheimer Disease. SAGE Publications Ltd; 2020.
Kocar TD, Müller H-P, Ludolph AC, Kassubek J. Feature selection from magnetic resonance imaging data in ALS: a systematic review. Therapeutic Adv Chronic Disease. 2021;12:20406223211051002.
Wolfe FH, Auzias G, Deruelle C, Chaminade T. Focal atrophy of the hypothalamus associated with third ventricle enlargement in autism spectrum disorder. NeuroReport. 2015;26(17):1017–22.
Furtner J, Genbrugge E, Gorlia T, Bendszus M, Nowosielski M, Golfinopoulos V, et al. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: translational imaging analysis of the EORTC 26101 trial. Neuro Oncol. 2019;21(12):1587–94.
Agosta F, Chiò A, Cosottini M, De Stefano N, Falini A, Mascalchi M, et al. The present and the future of neuroimaging in amyotrophic lateral sclerosis. Am J Neuroradiol. 2010;31(10):1769–77.
Di Paola M, Di Iulio F, Cherubini A, Blundo C, Casini A, Sancesario G, et al. When, where, and how the corpus callosum changes in MCI and AD: a multimodal MRI study. Neurology. 2010;74(14):1136–42.
Chiò A, Pagani M, Agosta F, Calvo A, Cistaro A, Filippi M. Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes. Lancet Neurol. 2014;13(12):1228–40.
Arm J, Ribbons K, Lechner-Scott J, Ramadan S. Evaluation of MS related central fatigue using MR neuroimaging methods: scoping review. J Neurol Sci. 2019;400:52–71.
Stålberg E, van Dijk H, Falck B, Kimura J, Neuwirth C, Pitt M, et al. Standards for quantification of EMG and neurography. Clin Neurophysiol. 2019;130(9):1688–729.
Hu N, Wang J, Liu M. Split hand in amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Clin Neurosci. 2021;90:293–301.
Xu Q, Zhao Y, Zhou X, Luan J, Cui Y, Han J. Comparison of the extraction and determination of serum exosome and miRNA in serum and the detection of miR-27a-3p in serum exosome of ALS patients. Intractable Rare Dis Res. 2018;7(1):13–8.
Yelick J, Men Y, Jin S, Seo S, Espejo-Porras F, Yang Y. Elevated exosomal secretion of mir-124-3p from spinal neurons positively associates with disease severity in ALS. Exp Neurol. 2020;333:113414.
Otake K, Kamiguchi H, Hirozane Y. Identification of biomarkers for amyotrophic lateral sclerosis by comprehensive analysis of exosomal mRNAs in human cerebrospinal fluid. BMC Med Genom. 2019;12:1–11.
Acknowledgements
The authors appreciate the Iran University of Medical Sciences (IUMS).
Funding
The authors did not receive any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, and data collection, were performed by A.A. The first draft of the manuscript was written by A.R and EA. CG. EA. CG, S.D, D.A, and L.D participated in the literature review, data gathering, and drafted the manuscript. All authors read, modified, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Consent to publish
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Darabi, S., Ariaei, A., Rustamzadeh, A. et al. Cerebrospinal fluid and blood exosomes as biomarkers for amyotrophic lateral sclerosis; a systematic review. Diagn Pathol 19, 47 (2024). https://doi.org/10.1186/s13000-024-01473-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-024-01473-6