Abstract
Background
Giant cell-rich solitary fibrous tumour (GCR-SFT), previously referred to as giant cell angiofibroma, is an uncommon soft tissue tumour that classically occurs in the orbit but very rarely presents in deep organs. Here, we present a case of GCR-SFT occurring in the urinary bladder, which is one of the unusual histological subtypes of SFT.
Case presentation
A 56-year-old man was incidentally found to have a mass measuring 4.5 × 4.3 × 4.0 cm located in the left posterior wall of the bladder by computed tomography during a physical examination. The lesion was confirmed as GCR-SFT by pathological examination after laparoscopic radical surgery. Histopathologically, the tumour was a well-circumscribed, nonencapsulated lesion that was composed of bland spindle-ovoid tumour cells alternating with hypocellular and hypercellular areas, staghorn-like vasculatures and scattered large dark-stained multinucleate giant cells lining pseudovascular spaces. The spindle-ovoid cells and multinucleate giant cells showed strong and diffuse expression of CD34 and nuclear STAT6. In addition, the hallmark of the NAB2ex4-STAT6ex5 fusion gene was detected by RT‒PCR. The patient was classified as having a low risk of recurrence or metastasis according to the risk stratification criteria. The patient underwent regular follow-up for 34 months after surgery, and there was no evidence of local recurrence or metastasis.
Conclusion
This is the first reported case of GCR-SFT occurring in the urinary bladder with underlying NAB2ex4-STAT6ex5 fusion. Complete surgical excision of the tumour and long-term follow-up are recommended to ensure no local recurrence or metastasis.
Similar content being viewed by others
Introduction
Solitary fibrous tumour (SFT) is an uncommon mesenchymal neoplasm of fibroblastic origin that usually involves the pleura and was first described by Klemperer and Rabin in 1931 [1]. Subsequently, it has been found that it can occur in numerous extrathoracic anatomical regions, such as the orbit [2], salivary glands [3], respiratory tract [4], mediastinum [5], adrenal glands [6], pelvis [7], skin [8], liver [9], and retroperitoneum [10]. However, SFTs occurring in the urinary bladder have seldom been reported.
Histopathologically, SFT characterized by NAB2-STAT6 gene rearrangement mainly included “classic SFT” and “cellular SFT” previously recognized as haemangiopericytoma. Since giant cell angiofibroma (GCA), fat-forming haemangiopericytoma and the dedifferentiated type were essentially confirmed as SFT variants [11], the morphological spectrum of SFT had been greatly expanded, which posed great challenges to the diagnosis. However, the specific use of STAT6 immunohistochemistry and NAB2-STAT6 gene detection by RT‒PCR make it possible to accurately diagnose SFT [12,13,14,15].
GCR-SFT, as a rare variant of SFT, has occasionally been reported to occur in the head and neck region, back, retroperitoneum, hip and vulva, etc., according to the literature [3, 10, 16]. However, to the best of our knowledge, there have been no reports of GCR-SFT occurring in the urinary bladder. It is not yet known whether tumour with different morphological features have different clinical courses. Hence, we detailed the clinical presentation, imaging examination, pathological features, immunophenotypes, molecular features and prognosis of the rare case in this study.
Case presentation
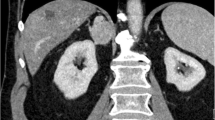
A 56-year-old man presented to Maoming People’s Hospital for routine physical examination with no discomfort symptoms. Pelvic CT with contrast displayed a heterogeneously enhancing oval solid mass measuring 4.5 × 4.3 × 4.0 cm in the left posterior wall of the bladder (Fig. 1). The laboratory examination results showed that the urine occult blood test and urine protein were positive, while the liver function, renal function test and cancer markers were within normal limits. The patient underwent laparoscopic radical tumour resection.
Grossly, the tumour was a 5.0 × 4.0 × 4.0 cm in size with no obvious macroscopic haemorrhage or tumour necrosis on the cut surface. Histopathologically, the tumour was a well-circumscribed, nonencapsulated lesion that was composed of bland spindle-ovoid tumour cells alternating with hypocellular and hypercellular areas, staghorn-like vasculatures and scattered large dark-stained multinucleate giant cells lining pseudovascular spaces (Fig. 2A-B). There was no evidence of necrosis or mitotic activity (0/10HPF). Spindle-ovoid cells and multinucleate giant cells showed strong and diffuse expression of CD34 (Fig. 2C) and nuclear STAT6 (Fig. 2D), while being negative for S-100, Desmin, CD117, DOG-1, SMA, MDM2, P16, Pankeratin and CD68 (the antibody information is detailed in Table 1). The Ki-67 proliferation index of the tumour cells was 3%. In addition, RT‒PCR confirmed the presence of the NAB2ex4-STAT6ex5 fusion gene in the tumour (Fig. 2E). Based on morphology, immunohistochemistry and molecular detection results, it was diagnosed as a GCR-SFT of the urinary bladder.
Histopathological features of GCR-SFT. (A) The tumour was characterized by the presence of spindle- or ovoid-shaped cells among sparse collagen fibres (H&E, magnification×40). (B) Vessels were dilated, staghorn-like appearance with remarkable scattered multinucleate giant cells lining pseudovascular spaces (H&E, magnification×200). The tumour cells were strongly positive for CD34 (C) and nuclear STAT6 (D) (immunohistochemistry, magnification×200). (E) The NAB2ex4-STAT6ex5 fusion gene was revealed by RT‒PCR
Based on the 3-tiered model (age at diagnosis, tumour size, mitotic count), the patient was given a score of 2 points (age ≥ 55 years and tumour size = 5 cm, shown in Table 2) and was stratified into low risk of metastasis or recurrence according to the risk assessment criteria of the 2020 WHO classification of soft tissue and bone tumours [17]. The patient underwent regular follow-up for 34 months after surgery, and there was no evidence of local recurrence or metastasis.
Discussion and conclusions
GCR-SFT, formerly known as GCA, was first reported in the orbital region by Dei Tos et al. in 1995 [18]. Since then, GCR-SFT has been described in some extraorbital anatomical locations, including the mediastinum, back, retroperitoneum, hip, vulva, and inguinal region [5, 16, 19]. In the most comprehensive review of the English literature to date, approximately 38 reports of GCR-SFT involving 66 cases have been identified between 1995 and 2023 [13, 16, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. However, GCR-SFT, as a rare variant, has never been reported to occur in the urinary bladder.
The clinical characteristics of the 66 reported cases of GCR-SFT are detailed in Table 3. Patients (Male38: Female28) ranged in age from 18 to 84 years with a mean age of 48.2 years. GCR-SFT mostly involved the orbit (n = 19, 28.8%) followed by the eyelid (n = 9, 13.6%), conjunctiva (n = 4, 6.1%), buccal mucosa (n = 4, 6.1%) and other head and neck regions (n = 17, 25.8%) including the parotid, occipital scalp, vocal cord, retroauricular region, submandibular, nasolacrimal duct, neck, tongue, parietal region, external auditory, nasopharynx, cheekbone, and sublingual regions. However, rare sites outside the head and neck region were noted in only 13 cases (back, groin, mediastinum, retroperitoneum, vulva, hip, axillary, gallbladder).
The clinical symptoms and signs depend on tumour size and location. The vast majority of bladder SFTs exhibit well-defined and slow-growing masses, with symptoms related to local compression caused by tumour growth, including urinary tract irritation, haematuria, difficulty urinating and lower abdominal discomfort [51,52,53]. The maximum diameter of GCR-SFT ranges from 0.5 to 14 cm with a mean size of 3.25 cm. Of note, tumours that occurred in areas such as the orbit and the eyelid often had a smaller diameter than tumours that occurred in subcutaneous soft tissue and deep organs. Although larger tumor diameters are positively correlated with higher risk stratification according to the risk assessment criteria, Feasel et al. demonstrated that 26 cases of SFTs occurring in cutaneous/subcutaneous soft tissue showed no recurrence or metastasis, and 2 cases of histologically malignant SFT were included [8].
Histopathologically, bland spindle-ovoid tumour cells alternating with hypocellular and hypercellular areas, staghorn-like vessels and prominent dark-stained multinucleate giant cells lining pseudovascular spaces are important diagnostic clues for GCR-SFT. For a long time in the past, a combination of CD34, BCL2 and CD99 has been widely used for the diagnosis of SFT. These markers often had good sensitivity and expression intensity in the vast majority of cases. Unfortunately, these markers are not specific and are frequently positively expressed in many spindle cell tumours closely mimicking SFT histologically [54, 55]. STAT6 has emerged as a useful tool for the diagnosis of SFT, and its sensitivity and specificity reached to 90–100% and 95–100%, respectively [13,14,15]. Based on the diagnostic needs, the use of CD34 and STAT6, which are better than other markers, is strongly recommended. However, subsequent research found that STAT6 can also be expressed in some other soft tissue neoplasms such as dedifferentiated liposarcoma (12%), desmoid fibromatosis (8%), and neurofibroma (8%) [56].
Given the unique or atypical morphology of cases, NAB2-STAT6 fusion gene detection was performed to ensure accurate diagnosis. SFTs characteristically harbour inv12(q13q13)-derived NAB2-STAT6 fusions with variable breakpoints resulting in diverse fusion variants. According to the literature, only 5 cases of GCR-SFT underwent NAB2-STAT6 fusion gene detection, and fusion variants included NAB2ex6-STAT6ex17 (3 cases), NAB2ex3-STAT6ex18 (1 case) and NAB2ex3-STAT6ex19 (1 case) [13, 50]. In this study, we first confirmed the presence of the NAB2ex4-STAT6ex5 fusion gene in GCR-SFT of the urinary bladder. Nonetheless, the significance of such fusion variants in the GCR-SFT remains unclear. To date, no association has been found between a certain type of mutation variant and poor prognosis in SFT [13].
The differential diagnosis of GCR-SFT includes a number of soft tissue tumours, especially the so-called fibrohistiocytic tumours and fibroblastic/myofibroblastic tumours, such as deep benign fibrous histiocytoma and giant cell fibroblastoma. Deep benign fibrous histiocytoma, occurs mainly in the deep soft tissue or subcutaneous tissue and mostly presents as isolated, slow-growing nodules with branching haemangiopericytoma-like vessels. Furthermore, giant cell fibroblastoma is an intermediate soft tissue tumour that histologically overlaps with dermatofibrosarcoma protuberans.Giant cell fibroblastomas frequently occur in the subcutis and primarily affects children, although some adult cases have been reported. It consists of elongated wavy arrangements of spindle cells distributed in a mucinous-like or collagenous stroma, and multinucleate giant cells often lining larger lacunar or sinusoidal pseudovascular spaces that are irregularly distributed. However, STAT6 nuclear expression is absent in these tumours. A small number of other mesenchymal tumours express STAT6 including dedifferentiated liposarcoma, undifferentiated pleomorphic sarcoma, and nodular fasciitis [57]. However, their morphology is quite different from that of GCR-SFT.
In addition, the morphology of GCR-SFT may overlap with other giant cell-rich malignant bladder lesions including osteoclast-type giant cell-rich carcinoma and leiomyosarcoma with osteoclast-like multinucleated giant cells. Histologically, osteoclast-type giant cell-rich carcinoma showed biphasic morphology with polygonal to epithelioid to spindle mononuclear cells and scattered multinucleated osteoclast-like giant cells. Leiomyosarcoma with osteoclast-like multinucleated giant cells is composed of spindle cells and has the presence of numerous multinucleated, osteoclast-like giant cells. Immunohistochemically, in addition to expressing markers of their own intrinsic origin respectively, giant cells in both tumours expressed CD68 positively. However, the giant cells of GCR-SFT were negative for CD68.
Although GCR-SFT exhibits benign histological morphology and slow growth process, incomplete tumour resection may lead to recurrence after many years [29, 58]. Henceforth, complete surgical excision should be performed immediately after detection when eligible for surgery to ensure a positive outcome and minimize the chance of malignant transformation or metastasis [52]. For the management of SFT, extensive and healthy surgical margin resection is currently considered the gold standard.
The clinical course of SFTs can be predicted by establishing a risk stratification model for low, intermediate and high metastatic risk that takes into account age at diagnosis, tumour size, mitotic count, and necrosis [59, 60]. Our case was scored 2 points and classified as low-risk progression. The patient underwent a follow-up of 34 months after complete resection of the tumour and did not experience any local recurrence or metastasis. However, in light of the specific location of the tumour, long-term follow-up is still needed.
In summary, this is the first reported case of GCR-SFT occurring in the urinary bladder with underlying NAB2ex4-STAT6ex5 fusion. Complete surgical excision of the tumour and long-term follow-up are recommended to ensure no local recurrence.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- SFT:
-
Solitary fibrous tumour
- GCA:
-
Giant cell angiofibroma
- STAT6:
-
Signal transducer and activator of transcription 6
- RT‒PCR:
-
Reverse transcription-polymerase chain reaction
- HPF:
-
High power field
References
Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med. 1992;22(1):1–31.
Richardt-Luhn J, McLean A, Mayer TE, Kirchhof K. [Solitary fibrous tumor of the orbit: a case report]. Laryngorhinootologie. 2023;102(5):371–2.
Velez Torres JM, Duarte EM, Diaz-Perez JA, Leibowitz J, Weed DT, Thomas G, Sargi Z, Civantos FJ, Arnold DJ, Gomez-Fernandez C, et al. Mesenchymal neoplasms of salivary glands: a clinicopathologic study of 68 cases. Head Neck Pathol. 2022;16(2):353–65.
Wang YX, Zhong Y, Fan SS, Zhu YS, Peng XR, Zhang X. Solitary fibrous tumors of the lung: a clinicopathological analysis of 52 cases. Curr Oncol. 2023;30(2):1784–93.
Witkin GB, Rosai J. Solitary fibrous tumor of the mediastinum. A report of 14 cases. Am J Surg Pathol. 1989;13(7):547–57.
Jha S, Mohanty SK, Sampat NY, Naik S, Baisakh MR, Pattnaik N, Lobo A, Rauta S, Sharma S, Munjal G, et al. Solitary fibrous tumor of the adrenal gland. Am J Clin Pathol. 2022;158(4):546–54.
Tsushimi T, Yagi T, Tomozawa N, Ohnishi H. Retroperitoneal solitary fibrous tumor of the pelvis with pollakiuria: a case report. BMC Res Notes. 2012;5:593.
Feasel P, Al-Ibraheemi A, Fritchie K, Zreik RT, Wang WL, Demicco E, Saeb-Lima M, Goldblum JR, Rubin BP, McKenney JK, et al. Superficial solitary fibrous tumor: a Series of 26 cases. Am J Surg Pathol. 2018;42(6):778–85.
Debs T, Kassir R, Amor IB, Martini F, Iannelli A, Gugenheim J. Solitary fibrous tumor of the liver: report of two cases and review of the literature. Int J Surg. 2014;12(12):1291–4.
Yuan X, Liu Y, Wang X, Chen Y, Zhang L, Wei J. Clinicopathological analysis of retroperitoneal solitary fibrous tumours: a study of 31 cases. Histol Histopathol. 2022;37(1):43–50.
Kazazian K, Demicco EG, de Perrot M, Strauss D, Swallow CJ. Toward Better Understanding and Management of Solitary Fibrous Tumor. Surg Oncol Clin N Am. 2022;31(3):459–83.
Chuang IC, Liao KC, Huang HY, Kao YC, Li CF, Huang SC, Tsai JW, Chen KC, Lan J, Lin PC. NAB2-STAT6 gene fusion and STAT6 immunoexpression in extrathoracic solitary fibrous tumors: the association between fusion variants and locations. Pathol Int. 2016;66(5):288–96.
Kao YC, Lin PC, Yen SL, Huang SC, Tsai JW, Li CF, Tai HC, Lan J, Chuang IC, Yu SC, et al. Clinicopathological and genetic heterogeneity of the head and neck solitary fibrous tumours: a comparative histological, immunohistochemical and molecular study of 36 cases. Histopathology. 2016;68(4):492–501.
Cheah AL, Billings SD, Goldblum JR, Carver P, Tanas MZ, Rubin BP. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology. 2014;46(5):389–95.
Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, Asamura H, Kushima R. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38(4):552–9.
Guillou L, Gebhard S, Coindre JM. Orbital and extraorbital giant cell angiofibroma: a giant cell-rich variant of solitary fibrous tumor? Clinicopathologic and immunohistochemical analysis of a series in favor of a unifying concept. Am J Surg Pathol. 2000;24(7):971–9.
Anderson WJ, Doyle LA. Updates from the 2020 World Health Organization Classification of Soft Tissue and Bone Tumours. Histopathology. 2021;78(5):644–57.
Dei Tos AP, Seregard S, Calonje E, Chan JK, Fletcher CD. Giant cell angiofibroma. A distinctive orbital tumor in adults. Am J Surg Pathol. 1995;19(11):1286–93.
Sigel JE, Fisher C, Vogt D, Goldblum JR. Giant cell angiofibroma of the inguinal region. Ann Diagn Pathol. 2000;4(4):240–4.
Ganesan R, Hammond CJ, van der Walt JD. Giant cell angiofibroma of the orbit. Histopathology. 1997;30(1):93–6.
Fukunaga M, Ushigome S. Giant cell angiofibroma of the mediastinum. Histopathology. 1998;32(2):187–9.
Hayashi N, Borodic G, Karesh JW, Tolentino MJ, Remulla HD, Van Wesep RA, Grossniklaus HE, Jakobiec FA, Green WR. Giant cell angiofibroma of the orbit and eyelid. Ophthalmology. 1999;106(6):1223–9.
Rousseau A, Perez-Ordonez B, Jordan RC. Giant cell angiofibroma of the oral cavity: report of a new location for a rare tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(5):581–5.
Sonobe H, Iwata J, Komatsu T, Fukushima A, Hayashi N, Moriki T, Shimizu K, Ohtsuki Y. A giant cell angiofibroma involving 6q. Cancer Genet Cytogenet. 2000;116(1):47–9.
Kintarak S, Natiella J, Aguirre A, Brooks J. Giant cell angiofibroma of the buccal mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(6):707–13.
Wiebe BM, Gottlieb JO, Holck S. Extraorbital giant cell angiofibroma. APMIS. 1999;107(7):695–8.
Yazici B, Setzen G, Meyer DR, Williams EF, McKenna BJ. Giant cell angiofibroma of the nasolacrimal duct. Ophthalmic Plast Reconstr Surg. 2001;17(3):202–6.
Thomas R, Banerjee SS, Eyden BP, Shanks JH, Bisset DL, Hunt R, Byers RJ, Oogarah P, Harris M. A study of four cases of extra-orbital giant cell angiofibroma with documentation of some unusual features. Histopathology. 2001;39(4):390–6.
Keyserling H, Peterson K, Camacho D, Castillo M. Giant cell angiofibroma of the orbit. AJNR Am J Neuroradiol. 2004;25(7):1266–8.
Song A, Syed N, Kirby PA, Carter KD. Giant cell angiofibroma of the ocular adnexae. Arch Ophthalmol. 2005;123(10):1438–43.
Qian YW, Malliah R, Lee HJ, Das K, Mirani N, Hameed M. A t(12;17) in an extraorbital giant cell angiofibroma. Cancer Genet Cytogenet. 2006;165(2):157–60.
Dudde F, Barbarewicz F, Telschow T, Knyphausen AZ, Henkel KO. Giant cell Angiofibroma in the Buccal Mucosa - A rising entity? Anticancer Res. 2023;43(4):1869–71.
Farmer JP, Lamba M, McDonald H, Commons AS. Orbital giant cell angiofibroma: immuno-histochemistry and differential diagnosis. Can J Ophthalmol. 2006;41(2):216–20.
Lazure T, Dimet S, Ndiaye N, Bourdin G, Ladouch-Badre A. Giant cell-rich solitary fibrous tumour of the gallbladder. First case report. Histopathology. 2007;50(6):805–7.
de Andrade CR, Lopes MA, de Almeida OP, León JE, Mistro F, Kignel S. Giant cell angiofibroma of the oral cavity: a case report and review of the literature. Med Oral Patol Oral Cir Bucal. 2008;13(9):E540–543.
Ereño C, López JI, Pérez J, Grande J, Bilbao FJ. Orbital giant cell angiofibroma. Apmis. 2006;114(9):663–5.
Piperi E, Rohrer MD, Pambuccian SE, Koutlas IG. Vascular solitary fibrous tumor with floret cells or giant cell angiofibroma? A lingual example highlighting the overlapping characteristics of these entities and positive immunoreaction for estrogen and progesterone receptors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):685–90.
Shu HR, Yang QT, Lai YY, Mo JG, Mao WH, Song JX, Zheng GJ. Giant cell angiofibroma in the vocal cord. Chin Med J (Engl). 2010;123(23):3479–81.
González-Pérez LM, Sánchez-Gallego F, Haro-Luna JJ, Infante-Cossío P. Giant cell angiofibroma of parapharyngeal space: a report of a new location for a rare tumour. Int J Oral Maxillofac Surg. 2010;39(10):1024–7.
Surace D, Blandamura S, Bernardini FP, Galan A, Lo Giudice G. Unusual presentation of giant cell angiofibroma of the eyelids. Eur J Ophthalmol. 2010;20(6):1073–5.
Pala EE, Beyhan R, Bayol U, Cumurcu S, Kucuk U. Giant cell angiofibroma in unusual localization: a case report. Case Rep Pathol. 2012;2012:408575.
Lynch MC, Chung CG, Specht CS, Wilkinson M, Clarke LE. Giant cell angiofibroma or localized periorbital lymphedema? J Cutan Pathol. 2013;40(12):1059–62.
Arifin MZ, Tjahjono FP, Faried A, Gill AS, Cahyadi A, Hernowo BS. Giant cell angiofibroma of the scalp: a benign rare neoplasm with bone destruction. Surg Neurol Int. 2013;4:131.
He Y, Zhang C, Liu G, Tian Z, Wang L, Kalfarentzos E. Giant cell angiofibroma misdiagnosed as a vascular malformation and treated with absolute alcohol for one year: a case report and review of the literature. World J Surg Oncol. 2014;12:117.
Yuzawa S, Tanikawa S, Kunibe I, Nishihara H, Nagashima K, Tanaka S. A case of giant cell-rich solitary fibrous tumor in the external auditory canal. Pathol Int. 2016;66(12):701–5.
Ekin MA, Ugurlu SK, Cakalagaoglu F. Unusual intraconal localization of orbital giant cell angiofibroma. Indian J Ophthalmol. 2018;66(1):160–3.
Dong SS, Wang N, Yang CP, Zhang GC, Liang WH, Zhao J, Qi Y. Giant Cell-Rich Solitary Fibrous Tumor in the nasopharynx: Case Report and Literature Review. Onco Targets Ther. 2020;13:6819–26.
Mudhar HS, Pavel M, Chung AKK, Jackson TE. Two cases of STAT6-Positive, primary Conjunctival Giant Cell-Rich Solitary Fibrous Tumour (Giant Cell Angiofibroma) with some unusual histological features. Ocul Oncol Pathol. 2020;6(5):328–32.
Dizdar SK, Salepci E, Hascicek SO, Turgut S. Giant cell Angiofibroma in Sublingual Area: a Case Report and Review of Literature. Sisli Etfal Hastan Tip Bul. 2021;55(1):134–7.
Alsaadi KA, Alwohaib M, Pinto K, Ali RH. Giant cell-rich solitary fibrous tumour of the lacrimal gland with prominent angiomatoid cystic changes and an underlying NAB2ex3-STAT6ex18 fusion. BMJ Case Rep 2022, 15(2).
Vargas JF, Gandhi D, Bajaj D, Serhal M, Erazo IS, Singh J. Solitary fibrous tumor of the urinary bladder: an unusual case report with literature review. Radiol Case Rep. 2021;16(12):3898–902.
Otta RJ, Acosta MA, Gordillo C. A rare case of solitary fibrous tumour of the bladder. Can Urol Assoc J. 2014;8(7–8):E552–553.
Spairani C, Squillaci S, Pitino A, Ferrari M, Montefiore F, Rossi C, Fusco W, Bigatti GL. A case of concomitant occurrence of solitary fibrous tumor and urothelial high-grade invasive carcinoma of the urinary bladder. Int J Surg Pathol. 2014;22(3):252–9.
Hirakawa N, Naka T, Yamamoto I, Fukuda T, Tsuneyoshi M. Overexpression of bcl-2 protein in synovial sarcoma: a comparative study of other soft tissue spindle cell sarcomas and an additional analysis by fluorescence in situ hybridization. Hum Pathol. 1996;27(10):1060–5.
Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathology: Official J United States Can Acad Pathol Inc. 2014;27(3):390–5.
Demicco EG, Harms PW, Patel RM, Smith SC, Ingram D, Torres K, Carskadon SL, Camelo-Piragua S, McHugh JB, Siddiqui J, et al. Extensive survey of STAT6 expression in a large series of mesenchymal tumors. Am J Clin Pathol. 2015;143(5):672–82.
Doyle LA, Tao D, Marino-Enriquez A. STAT6 is amplified in a subset of dedifferentiated liposarcoma. Mod Pathology: Official J United States Can Acad Pathol Inc. 2014;27(9):1231–7.
Demirci H, Shields CL, Eagle RC Jr., Shields JA. Giant cell angiofibroma, a variant of solitary fibrous tumor, of the orbit in a 16-year-old girl. Ophthalmic Plast Reconstr Surg. 2009;25(5):402–4.
Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, Lazar AJ, Wang WL. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25(9):1298–306.
Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang WL. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30(10):1433–42.
Acknowledgements
This study was supported by High-level Hospital Construction Research Project of Maoming People’s Hospital.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Zhou Su and Xiaolu Yuan wrote the paper. Xiaolu Yuan and Jianguo Wei carried out the histological, immunohistochemical studies and molecular examination of the tumor specimens. Xiaoiu Yuan collected the clinical, imaging and laboratory data of the patient. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The present study was approved by the Ethics Committee of Maoming People’s Hospital. The authors are accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent of the patient or Legal guardian to participate in this study has been obtained.
Consent for publication
Written consent for publication was obtained from the patient.
Conflict of interest
The authors have no conflicts of interest to declare.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Su, Z., Wei, J. & Yuan, X. Giant cell-rich solitary fibrous tumour of the urinary bladder: case report of an unusual histological variant and literature review. Diagn Pathol 19, 20 (2024). https://doi.org/10.1186/s13000-024-01442-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-024-01442-z