Abstract
Purpose
Few studies have compared patient characteristics, clinical management, and outcome of patients with COVID-19 between the different epidemic waves. In this study, we describe patient characteristics, treatment, and outcome of patients admitted for COVID-19 in the Antwerp University Hospital over the first three epidemic waves of 2020–2021.
Methods
Retrospective observational study of COVID-19 patients in a Belgian tertiary referral hospital. All adult patients with COVID-19, hospitalized between February 29, 2020, and June 30, 2021, were included. Standardized routine medical data was collected from patient records. Risk factors were assessed with multivariable logistic regression.
Results
We included 722 patients, during the first (n = 179), second (n = 347) and third (n = 194) wave. We observed the lowest disease severity at admission during the first wave, and more elderly and comorbid patients during the second wave. Throughout the subsequent waves we observed an increasing use of corticosteroids and high-flow oxygen therapy. In spite of increasing number of complications throughout the subsequent waves, mortality decreased each wave (16.6%,15.6% 11.9% in 1st, 2nd and 3rd wave respectively). C-reactive protein above 150 mg/L was predictive for the need for intensive care unit admission (odds ratio (OR) 3.77, 95% confidence interval (CI) 2.32–6.15). A Charlson comorbidity index ≥ 5 (OR 5.68, 95% CI 2.54–12.70) and interhospital transfers (OR 3.78, 95% CI 2.05–6.98) were associated with a higher mortality.
Conclusions
We observed a reduction in mortality each wave, despite increasing comorbidity. Evolutions in patient management such as high-flow oxygen therapy on regular wards and corticosteroid use may explain this favorable evolution.
Similar content being viewed by others
Background
Since December 2019, the SARS-CoV-2 pandemic has spread across the world. In Belgium, a first epidemic wave unfolded during spring 2020, a second in autumn and winter of 2020–2021, and a third wave spring 2021, coinciding with the emergence of the Alpha variant, lineage B.1.1.7 [1, 2].
Few studies have compared patient characteristics, treatments, and outcomes of patients with COVID-19 between these different epidemic waves [3,4,5,6,7]. Those who did, observed slightly older patients during the second pandemic wave, and a higher mortality during the first wave [3, 5,6,7,8], while one study observed lower mortality during the first wave, but fewer hospitalizations [4]. Higher mortality was observed in older patients, men, and in patients with cardiovascular diseases, hypertension, obesity, diabetes, chronic pulmonary disease, advanced chronic kidney disease, pregnancy, active solid or hematological cancer, and other immunodeficiency [9,10,11]. Other apparent predictive factors include laboratory values, for example a high C-reactive protein (CRP) [12].
Treatment of hospitalized patients with COVID-19 evolved throughout the pandemic. However, the use of antimicrobial agents was consistently high, with approximately 60% of patients receiving antibiotics, despite the low frequency (≤ 5%) of documented early bacterial coinfection [13,14,15,16,17,18,19,20]. Whether the different epidemic waves (with their own specific epidemiological characteristics, viral variants, and treatment options) are associated with different patient outcomes is yet unclear.
In this study, we aim to address differences in patient characteristics, treatment, and outcome during different epidemic waves of COVID-19 in a Belgian tertiary hospital, and to identify and quantify the effect of different risk factors.
Methods
Study design and context
This is an observational study; the registry was started at the start of the pandemic using data from medical and laboratory records. It was conducted at a single tertiary referral hospital, the Antwerp University Hospital in Belgium. The hospital has 573 beds, of which 45 are intensive care unit (ICU) beds. Patients were hospitalized from the emergency department or immediately after transfer from another hospital. Patients were hospitalized on regular medical wards, including temporally dedicated COVID-19 wards, and transferred to the ICU, if necessary, after discussion with an ICU physician, as in routine non-COVID-19 medical care. Treating physicians received guidance in case management through the hospital’s COVID-19 guidance documents formulated by a multidisciplinary team and updated in accordance with Belgian and international guidelines. In particular during peak periods within the pandemic with high pressure on hospital and ICU beds, case management was discussed in a multidisciplinary group to ensure proper patient care and anticipate future ICU admissions. The ICU surge capacity for COVID-19 and non-COVID-19 intensive care unit patients was organized in the ICU and the post-anesthesia care unit, respectively.
Study period and epidemic waves
Definition of the start and end dates of subsequent epidemic waves was based on national epidemiological data and Antwerp university hospital admission data (Fig. 1) [21]. The first wave was defined as February 29th, 2020, until April 30th, 2020; the second wave, May 1st, 2020 to January 31st, 2021, and the third wave, February 1st, 2021, to June 30, 2021. Nationwide vaccination was initiated December 28, 2020, initially focusing on elderly comorbid people and healthcare providers. By June 30, 2021, at the end of the third wave, a nationwide vaccine coverage of 62% for the first dose and 35% for the second dose was achieved [21].
Eligibility criteria
We included adult patients who were admitted for or developed symptomatic COVID-19 during their hospital stay at the Antwerp University Hospital between February 29, 2020, until June 30, 2021. Both laboratory and radiologically confirmed patients were considered, consistent with the case definition of the Belgian National Institute of Public Health, Sciensano [22]. Criteria for exclusion were hospitalization for a reason other than COVID-19 and, a hospital stay shorter than 24 h.
Data collection
Data collection was retrospective, but structured medical notes were used for COVID-19 patients during the pandemic. Pseudonymized demographic (age and sex) and clinical patient data were collected from the electronic patient file system and the laboratory data system. Clinical data included date of onset of symptoms and hospital admission, the presence of comorbidities, and CRP. Charlson comorbidity index (CCI) was calculated based on age and the presence of comorbidities [12]. The World Health Organization COVID-19 severity classification was determined at admission to our hospital, categorizing them into non-severe, severe, or critical disease [23]. Different treatments were recorded, including supportive therapies, antimicrobial therapies, and COVID-19 directed medical therapies; as well as the occurrence of complications during hospital stay. The collected outcome measures are ICU admission and hospital mortality.
Statistical analysis
Statistical analyses were performed using SPSS version 29. Descriptive statistics were used to compare patient characteristics, management, and outcome between the successive epidemic waves. Absolute and relative frequencies were used to analyze categorical and binary variables. Continuous variables (age, CCI, body mass index (BMI), CRP), were categorized and analyzed using absolute and relative frequencies. Differences of the categorical and binary variables were examined with Chi-square test. Length of stay, time to intensive care unit admission, time to death, duration of treatments, and timing of initiation of certain drugs are presented as median (in days) with corresponding interquartile range (IQR). Differences of the continuous variables were examined with Kruskal-Wallis test.
Risk factors for intensive care unit admission and in-hospital death were determined using a multivariable logistic regression, presented as odds ratios (OR) and 95% confidence intervals (CI). Preselected covariates were age, sex, and wave. Other covariates of interest were selected based on statistical significance (p < 0.1) in univariable analysis. As we are tertiary care hospital with a lot of tertiary referrals mostly directly to ICU, we described the ICU population in more detail.
Ethics
The study protocol was approved by the local institutional ethics committee in April 2020 (study number 3461). Informed consent was waived given the retrospective and observational non-interventional nature of this study, focusing on routine medical care.
Results
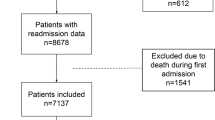
In total 722 of 813 adult patients who were admitted with COVID-19 to the Antwerp University Hospital during the observation period were included, 181, 347 and 194 during the first, second and third wave respectively. Excluded patients were not fulfilling the case definition (n = 75), hospitalized for less than 24 h (n = 2) or readmitted during the observation period (n = 14), of which 5 were readmitted for COVID-19.
Patient characteristics
Table 1 displays the baseline characteristics of patients presenting in the consecutive waves. Patients presenting during the second wave, were significantly older and had more comorbidity. Distribution of CRP upon hospital admission was similar over the successive waves. The timing of hospital admission after symptom onset (n = 641) did not change during the first, second and third wave (median 7 days (IQR 4–11), median 7 days (IQR 3–9), median 7 (IQR 4–10), respectively), also disease severity of patients primarily presenting at our hospital did not change over the successive waves.
Inpatient medical management
Overall, 83% received oxygen therapy, for a median duration of 3 days (IQR 2–7) (Table 2). An increasing use of noninvasive ventilation, mostly high-flow oxygen therapy, was observed over the subsequent waves. This increase was observed in the ICU after the first wave, and in patients who were never transferred to the ICU, respectively in 1%, 3%, and 9% of all patients in the first, second and third wave. During the first wave, hydroxychloroquine was used as an antiviral and immunomodulatory agent in 77% of patients; this practice was completely abandoned thereafter. Corticosteroids were occasionally used during the first wave, but were initiated later than in subsequent waves, 9 (4–14), 1 (0–3), and 1 (0–3) days (IQR) after hospital admission during the first, second and third wave respectively. Remdesivir was the only used antiviral agent in this stage of the pandemic, but its use was also limited (2.5% (18/722)) and abandoned after the second wave. We observed a decrease in antibiotic prescriptions at hospital admission (excluding patients transferred from another hospital) from 55% (94/171) during the first wave to less than 30% (44/153) in the third wave.
Outcome and complications
We observed an increase in complications over the different waves, including acute respiratory distress syndrome (ARDS), thrombo-embolic disease, ventilator associated pneumonia, and neurologic complications (Table 3). One hundred seven patients died because of COVID-19 or its complications. The overall in-hospital mortality decreased across the waves, although not statistically significant, mostly driven by a decreased mortality in non-ICU patients; 16%, 8%, and 5% during the first, second and third wave respectively (p = 0.007). Patients died after a median duration of 8 days after hospital admission (IQR 5–14) on a regular medical ward, and 20 days (IQR 14–38) for ICU patients. Median length of hospital stay was 9 days (IQR 5–19), but 27 days (IQR 16–47) in patients admitted to the intensive care unit.
Characteristics, management and outcome of patients admitted to the ICU
31% of all patients were admitted to the ICU (Table 4). Almost half of the patients were older than 65 years old with fewer people having a CCI < 2 (34% vs. 40% in all patients admitted to our hospital). More than one in three patients admitted to our ICU was transferred from another hospital. Intubation and invasive ventilation were necessary in 73%, with a median number of invasive ventilation days of 20 (IQR 13–39). 20% of patients received more than one course of corticosteroids, the second course being an additional treatment for ARDS. Besides respiratory and infectious complications, we observed neurological sequelae (non-stroke) in one third of ICU patients. Most of these were ICU acquired weakness (critical illness polyneuropathy). Length of ICU stay was shorter during the second wave (median 15 days, IQR 6–34), compared to the first wave (median 18 days, IQR 12–35) and third wave (median 23 days, IQR 11–51). Median length of stay was 49 days (IQR 31–70) in patients with neurological sequelae, compared to 18 days (IQR 12–32) in patients without neurological sequelae.
Risk factors for ICU admission and hospital mortality
Cases occurring during the second (OR 0.30, 95% CI 0.18–0.52) and third wave (OR 0.24, 95% CI 0.13–0.44) had a decreased risk of death, though no differences in risk for ICU admission were found (Table 5). In our population increasing age above 50 years old was found protective against mortality, on the other hand patients above 80 years old were less likely to be admitted to ICU. We found that patients with a CCI ≥ 5 were less likely to be admitted to the ICU but at a higher risk of dying in the hospital. Both for admission to ICU as well as hospital mortality having a BMI above 25 kg/m² was protective. High CRP at hospital admission was a risk factor for ICU admission (OR 3.59, 95% CI 2.26–5.72) in our cohort, but was not associated with death. Transfers from another hospital were associated with mortality during hospital stay. Comparable results were found after excluding nosocomial, and transferred patients (supplementary Table S1).
Discussion
This retrospective observational study of COVID-19 patients in a Belgian university hospital provides us with an overview and comparison of patient characteristics and management during the first three consecutive epidemic waves. We found demographic differences between the waves, patients being older and with more comorbidities in the second wave. In ICU an increased number of transfers for tertiary care was observed across the waves, as well as more organ failure and increased ECMO use, reflecting an increasing selection of the most critically ill patients in a tertiary care hospital such as ours.
Our hospitalized patient population with SARS-Cov2 infection is comparable to other Belgian hospitals, with male predominance and 80% patients older than 50 years old [23, 24]. The proportion of patients admitted to ICU is slightly higher (31% vs. 25%), reflecting the tertiary referral function and the relative high proportion of ICU-beds in our hospital (8% of all beds), and is also higher than in multinational studies such as ISARIC (15.3% ICU admission) [25]. In spite of the hospital’s tertiary function, COVID-19 related mortality was lower than the Belgian in-hospital average during the first wave (17% vs. 21%) [23], and multinational data for first and second waves (21%) [25]. Moreover, although the third wave patients were probably infected with the more virulent Alpha variant [1, 2], outcome was better than in the preceding epidemic waves. It remains difficult to compare intensive care unit outcome data as criteria for intensive care unit admission differ significantly between centers and countries, and the influence of the pandemic pressure on ICU [26].
During the third wave the nationwide vaccination campaign started, prioritizing elderly and comorbid people, and reaching a vaccine uptake of two thirds for a first dose and one third of Belgian citizens received a second dose by the end of the third wave [24]. Vaccination with at least one dose was only reported in 10% of patients admitted during the third wave, while we observed a clear decrease in admission of patients older than 65 years with comorbidities.
Between the first and second waves, we noted a significant change in case management, including the abolishment of hydroxychloroquine use and widespread use of corticosteroids early in the disease course. Furthermore, we saw an increased use of non-invasive ventilation (mainly high-flow nasal oxygen therapy) after the first wave. This practice was also adopted on regular wards: before ICU transfer, step down from ICU and for patients who were considered poor candidates for intensive care unit admission. Another evolution in case management was the decrease in antibiotic prescriptions at hospital admission, which was the result of growing knowledge on the low incidence of early bacterial co- or superinfection and ongoing antimicrobial stewardship [14].
An intriguing observation in our cohort is that age is inversely associated with death. This is opposite to the findings in other studies [9,10,11, 23, 25]. We cannot completely explain this finding, probably several factors are contributing. The small sample size, and the fact that the analysis was carried out in a hospital with tertiary referral function might be an explanation for this finding, with younger critical COVID patients undergoing ECMO treatment as well as admitting patients treated in our tertiary care center (complex hematology and oncology, solid-organ transplant patients). As seen in Table 5, not only very old patients, but also patients with CCI of at least 5 were less likely admitted to ICU. This can probably be explained by the fact that these patients had more advanced care planning decisions with limitations in therapy such as mechanical ventilation during the multidisciplinary meetings. Their poorer prognosis in COVID 19, was noted in several studies reviewed in a meta-analysis in 2020 showing an increase in mortality by 16% with each in CCI by one point [27]. This might have led to a particular case mix where analysis of risk factors is different from analysis in more general population.
The strengths of our study include the clinical and treatment information, available for all individuals admitted for COVID-19 since the start of the pandemic until the end of the third epidemic wave. Furthermore, it involves real world data from a single center, allowing to compare influence of different demographics, virus variants and case management between the respective waves. Therefore, our data offer a detailed insight in the evolution of the patient population and the clinical impact of COVID-19 during the first pandemic year. Despite the retrospective design, data collection was standardized and of high quality (few missing data) during the entire study period. Our results are however specific for our regional setting, and not necessarily generalizable to other (similar) socio-geographical regions [28, 29]. Healthcare and policy may differ, as well as circulating infectious strains, uptake of vaccinations and infection control measurements, and preparedness. Therefore, we do strongly advocate for widespread data collection and analyses, to facilitate data-driven improvements in healthcare, and to be better prepared for future emergencies.
Conclusions
Despite increasing disease severity and more comorbid patients, mortality decreased each subsequent wave. Evolutions in patient management such as high-flow oxygen therapy on regular wards and corticosteroid use may explain this finding.
Data availability
Upon reasonable request and after required approval from Ethics Committee.
Abbreviations
- CRP:
-
C-reactive protein
- ICU:
-
intensive care unit
- CCI:
-
Charlson comorbidity index
- BMI:
-
body mass index
- OR:
-
odds ratio
- CI:
-
confidence interval
- IQR:
-
interquartile range
- ECMO:
-
extracorporeal membrane oxygenation
- ARDS:
-
acute respiratory distress syndrome
References
Malik YA. Covid-19 variants: impact on transmissibility and virulence. Malays J Pathol. 2022;44(3):387–96.
Vassallo M, Manni S, Klotz C, Fabre R, Pini P, Blanchouin E et al. Patients admitted for variant alpha COVID-19 have poorer outcomes than those infected with the Old strain. J Clin Med. 2021;10(16).
Blanca D, Nicolosi S, Bandera A, Blasi F, Mantero M, Hu C, et al. Comparison between the first and second COVID-19 waves in Internal Medicine wards in Milan, Italy: a retrospective observational study. Intern Emerg Med. 2022;17(8):2219–28.
Bociąga-Jasik M, Wojciechowska W, Terlecki M, Wizner B, Rajzer M, Garlicki A et al. Comparison between COVID–19 outcomes in the first 3 waves of the pandemic: a reference hospital report. Pol Arch Intern Med. 2022;132(10).
Buttenschøn HN, Lynggaard V, Sandbøl SG, Glassou EN, Haagerup A. Comparison of the clinical presentation across two waves of COVID-19: a retrospective cohort study. BMC Infect Dis. 2022;22(1):423.
Meschiari M, Cozzi-Lepri A, Tonelli R, Bacca E, Menozzi M, Franceschini E, et al. First and second waves among hospitalised patients with COVID-19 with severe pneumonia: a comparison of 28-day mortality over the 1-year pandemic in a tertiary university hospital in Italy. BMJ Open. 2022;12(1):e054069.
Oladunjoye O, Gallagher M, Wasser T, Oladunjoye A, Paladugu S, Donato A. Mortality due to COVID-19 infection: a comparison of first and second waves. J Community Hosp Intern Med Perspect. 2021;11(6):747–52.
Slim MA, Appelman B, Peters-Sengers H, Dongelmans DA, de Keizer NF, Schade RP, et al. Real-world evidence of the effects of Novel treatments for COVID-19 on mortality: a nationwide comparative cohort study of hospitalized patients in the First, Second, Third, and fourth waves in the Netherlands. Open Forum Infect Dis. 2022;9(12):ofac632.
Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS ONE. 2021;16(3):e0247461.
Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449–58.
Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92(10):1875–83.
Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26(3):107–8.
Alshaikh FS, Godman B, Sindi ON, Seaton RA, Kurdi A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: a systematic review and meta-analysis. PLoS ONE. 2022;17(8):e0272375.
Calderon M, Gysin G, Gujjar A, McMaster A, King L, Comandé D, et al. Bacterial co-infection and antibiotic stewardship in patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):14.
Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–8.
Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–9.
Karami Z, Knoop BT, Dofferhoff ASM, Blaauw MJT, Janssen NA, van Apeldoorn M, et al. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in the Netherlands. Infect Dis (Lond). 2021;53(2):102–10.
Lardaro T, Wang AZ, Bucca A, Croft A, Glober N, Holt DB, et al. Characteristics of COVID-19 patients with bacterial coinfection admitted to the hospital from the emergency department in a large regional healthcare system. J Med Virol. 2021;93(5):2883–9.
Moolla MS, Reddy K, Fwemba I, Nyasulu PS, Taljaard JJ, Parker A, et al. Bacterial infection, antibiotic use and COVID-19: lessons from the intensive care unit. S Afr Med J. 2021;111(6):575–81.
Vaughn VM, Gandhi TN, Petty LA, Patel PK, Prescott HC, Malani AN, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with Coronavirus Disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72(10):e533–41.
Belgium, COVID-19 Epidemiological Situation.: Sciensano; 2021 https://epistat.wiv-isp.be/covid/covid-19.html.
COVID-19 - Gevalsdefinitie en testing: Sciensano. 2021 https://covid-19.sciensano.be/nl/covid-19-gevalsdefinitie-en-testing.
Faes C, Abrams S, Van Beckhoven D, Meyfroidt G, Vlieghe E, Hens N. Time between Symptom Onset, Hospitalisation and Recovery or Death: statistical analysis of Belgian COVID-19 patients. Int J Environ Res Public Health. 2020;17(20).
De Motqr L, Dockx Y, Vandromme M, De Pauw R, Serrien B, Van Goethem JC N.K. Blot. COVID-19 Clinical hospital surveillance report. Brussels, Belgium: Sciensano, diseases EaphEoi; 2023 August, 2023.
Kartsonaki C, Baillie JK, Barrio NG, Baruch J, Beane A, Blumberg L et al. Characteristics and outcomes of an international cohort of 600 000 hospitalized patients with COVID-19. Int J Epidemiol. 2023.
Taccone FS, Van Goethem N, De Pauw R, Wittebole X, Blot K, Van Oyen H, et al. The role of organizational characteristics on the outcome of COVID-19 patients admitted to the ICU in Belgium. Lancet Reg Health Eur. 2021;2:100019.
Tuty Kuswardhani RA, Henrina J, Pranata R, Anthonius Lim M, Lawrensia S, et al. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020 Nov-Dec;14(6):2103–9. https://doi.org/10.1016/j.dsx.2020.10.022. Epub 2020 Oct 28. PMID: 33161221; PMCID: PMC7598371.
Amado LA, Coelho WLDCNP, Alves ADR, Carneiro VCS, Moreira ODC, et al. Clinical Profile and Risk factors for severe COVID-19 in hospitalized patients from Rio De Janeiro, Brazil: comparison between the first and second pandemic waves. J Clin Med. 2023;12(7):2568. https://doi.org/10.3390/jcm12072568. PMID: 37048652; PMCID: PMC10094970.
Sadeghi F, Halaji M, Shirafkan H, Pournajaf A, Ghorbani H, et al. Characteristics, outcome, duration of hospitalization, and cycle threshold of patients with COVID-19 referred to four hospitals in Babol City: a multicenter retrospective observational study on the fourth, fifth, and sixth waves. BMC Infect Dis. 2024;24(1):55. https://doi.org/10.1186/s12879-023-08939-w. PMID: 38184533; PMCID: PMC10771668.
Acknowledgements
Thanks to Christophe Burms for support in setting up the electronic case report form. Thanks to the medical students for their support in the data collection.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
SVI was the principal investigator of the study. ADP and SVI were responsible for the data collection. Data cleaning was done by ADP. Analysis and interpretation were done by ADP with support by SVI, NB and EV. Drafting of the article was done by ADP, NB, EV, and SVI. All authors revised and contributed to the intellectual content of the article. All authors approved the final version of the article, including the authorship list.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Antwerp University Hospital (study number 3461).
Consent to participate and and consent for publication
Informed consent was waived by the Ethics Committee given the retrospective and observational non-interventional nature of this study, focusing on routine medical care.
Competing interests
No funding was received to assist with the preparation of this manuscript. EV is president of the national scientific advisory boards for covid-19 at Ministry of Health (GEES, GEMS, SSC). CT received support from ViiV for attenting EACS 2023. PJ is president of the Belgian Society of Intensive Care Medicine, unpaid.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Associations between different potential risk factors and outcome after exclusion of nosocomial infected patients and transferred patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
De Paepe, A., Vlieghe, E., Brusselaers, N. et al. COVID-19 in three waves in a tertiary referral hospital in Belgium: a comparison of patient characteristics, management, and outcome. Virol J 21, 119 (2024). https://doi.org/10.1186/s12985-024-02360-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02360-8