Abstract
Background
The highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) continues to pose one of the greatest threats to the swine industry. M protein is the most conserved and important structural protein of PRRSV. However, information about the host cellular proteins that interact with M protein remains limited.
Methods
Host cellular proteins that interact with the M protein of HP-PRRSV were immunoprecipitated from MARC-145 cells infected with PRRSV HuN4-F112 using the M monoclonal antibody (mAb). The differentially expressed proteins were identified by LC-MS/MS. The screened proteins were used for bioinformatics analysis including Gene Ontology, the interaction network, and the enriched KEGG pathways. Some interested cellular proteins were validated to interact with M protein by CO-IP.
Results
The PRRSV HuN4-F112 infection group had 10 bands compared with the control group. The bands included 219 non-redundant cellular proteins that interact with M protein, which were identified by LC-MS/MS with high confidence. The gene ontology and Kyoto encyclopedia of genes and genomes (KEGG) pathway bioinformatic analyses indicated that the identified proteins could be assigned to several different subcellular locations and functional classes. Functional analysis of the interactome profile highlighted cellular pathways associated with protein translation, infectious disease, and signal transduction. Two interested cellular proteins—nuclear factor of activated T cells 45 kDa (NF45) and proliferating cell nuclear antigen (PCNA)—that could interact with M protein were validated by Co-IP and confocal analyses.
Conclusions
The interactome data between PRRSV M protein and cellular proteins were identified and contribute to the understanding of the roles of M protein in the replication and pathogenesis of PRRSV. The interactome of M protein will aid studies of virus/host interactions and provide means to decrease the threat of PRRSV to the swine industry in the future.
Similar content being viewed by others
Background
Porcine reproductive and respiratory syndrome virus (PRRSV) is the etiologic agent of porcine reproductive and respiratory syndrome (PRRS) [1–4], an economically devastating pandemic disease of swine. PRRS is typically characterized by severe reproductive failure in sows and respiratory disorders in pigs of all ages [5, 6]. The disease is now found in most pig-producing countries and affects the swine industry and food safety worldwide [7–9]. In particular, the emergence of highly pathogenic PRRSVs (HP-PRRSVs) in China and Vietnam in 2006 [10–14] and their rapid spread to several neighboring Asian countries [15] has raised concerns that a new pathogenic PRRSVs could spread throughout the world, posing a substantial threat to the global agricultural community [16–18].
PRRSV is an enveloped, single-stranded, positive-sense RNA virus belonging to the order Nidovirales, family Arteriviridae, and genus Arterivirus [3, 19]. The viral genome is approximately 15 kb in length and encodes at least 10 open reading frames (ORFs), comprising of ORF1a, ORF1b, ORF2a, ORF2b, ORFs3–7 and the recently discovered ORF5a [20, 21]. ORF1a and ORF1b encode viral replicase polyproteins, which are proteolytically processed by virally encoded proteinases into 14 mature nonstructural proteins and the newly discovered transframe fusion (TF) in the NSP2-coding region [22–24]. The rest of the ORFs of PRRSV encode eight structural proteins: GP2, E, GP3, GP4, GP5, M, N, and ORF5a [20, 25, 26]. The M protein, an 18 to 19 kDa class III membrane protein, is unglycosylated and the most conserved structural protein of arteriviruses and PRRSV [27, 28]. The M protein is a key target for PRRSV neutralization [29]. A bacillus Calmette-Guérin vaccine strain of Mycobacterium bovisbacille expressing the M protein successfully induced the development of M protein neutralizing antibodies in mice, further indicating that the M protein contains neutralizing epitopes [30]. Co-expression of GP5 and M protein as heterodimers significantly improves the potency of PRRSV DNA vaccination [31]. The M protein and GP5 are found as a disulfide -linked heterodimer in the virion, which is essential for the infectivity of arteriviruses [32, 33]. The M protein and the M/GP5 complex contribute to PRRSV attachment to a heparinlike receptor on pulmonary alveolar macrophages (PAMs) [34]. The M/GP5 complex was identified as a ligand for sialoadhesin, which is involved in the entry process of PRRSV in to PAMs [35, 36]. These findings reveal that the M protein is involved in not only PRRSV infection and immunity but also the entry process of the pathogen. However, the molecular mechanisms of its involvement in these functions have not been elucidated clearly.
We used the co-immunoprecipitation (Co-IP) technique coupled with LC-MS/MS and bioinformatics analysis to screen and analyze host cellular proteins interaction with PRRSV M protein. An interactome profile of M protein was generated to understand the mechanism of PRRSV infection and immunity.
Methods
Cells, virus, and plasmid
The MARC-145 and 293 T cell lines were cultured in Dulbecco’s modified eagle medium (DMEM) (Gibco BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS) (Hyclone Laboratories, Inc., South Logan, UT) at 37 °C, with 5% CO2. The hybridoma cell line named 3 F7 secreting PRRSV M protein mAb was prepared by our lab [37]. The 3 F7 monoclonal antibody subclass was IgG1. The IFA titer of the 3 F7 culture supernatant was 1:512, and the Western blot titer of it was at least 1:512. The most important is that the binding of the 3 F7 to PRRSV could be blocked by the anti-serum to PRRSV in blocking ELISA. A HP-PRRS vaccine strain HuN4-F112 was obtained by culturing its parent strain, HP-PRRSV HuN4 [12, 13], with MARC-145 cells for 112 passages [38]. The 5th-passage HuN4 (HuN4-F5) used in animal’s challenge [38] was used in CO-IP together with HuN4-F112. The eukaryotic expression vector pCAGGS-Flag-HuN4-F112-M was maintained in our lab.
Purification of M protein mAb
BALB/c mice aged 12 weeks (from the Laboratory Animal Center of Harbin Veterinary Research Institute, CAAS) were primed with Freund’s incomplete adjuvant (Sigma, St. Louis, MO, USA) and administered an intraperitoneal injection of 1-3 × 106 hybridoma cell 3 days later. Ascitic fluids were collected using syringes when abdominal distension became marked. The mice were euthanized after three collections. The M protein mAb was purified by Protein G resin (GenScript, Nanjing, China) according to the manufacturer’s instructions.
Plasmid construction
A 4-week-old SPF landrace piglet was obtained from the Laboratory Animal Center of Harbin Veterinary Research Institute, CAAS. The piglet was euthanized, and its pulmonary alveolar macrophages were collected according to a previously described method [39]. The ORFs of NF45 and PCNA were amplified from the total RNA of PAMs by RT-PCR using the designed primers based on the sequences available from GenBank (XM_005663409.1, NF45; GQ913657.1, PCNA). The reverse transcriptions were performed using AMV reverse transcriptase (Takara, Dalian, China) in a reaction system with a total volume of 20 μL. The ORF6 gene of PRRSV was amplified by PCR using pCAGGS-Flag-HuN4-F112-M as the template. The pCMV-HA-NF45/PCNA, pCAGGS-Flag-NF45/PCNA and pCMV-HA-M plasmids were constructed by conventional techniques. All the primers used in this study are listed in Table 1.
Detecting the expression of M protein
MARC-145 cells in a 60-mm dish were infected with the HuN4-F112 at an MOI of 0.1. The cells were collected at different time points (12 to 84 h post-infection). The samples were subjected to Western blot with anti-M protein mAb. The assay was repeated in triplicate.
Co-Immunoprecipitation
HuN4-F112/HuN4-F5 infected MARC-145 cells and uninfected MARC-145 cells were lysed in NP-40 lysis buffer (Beyotime, Nanjing, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) (Beyotime, Nanjing, China) and 1% protease inhibitor cocktail (Sigma, St. Louis, MO, USA) by incubation at 4 °C on a shaker for 30 min, followed by centrifugation at 12,000 × g for 20 min. Clarified extracts were precleared with protein G beads for 1 h. A total of 1 mL of each supernatant at a final concentration of 5 mg/mL was precipitated with anti-M protein mAb 3 F7 in conjunction with protein G resin and incubated with gentle rocking overnight at 4 °C. The beads were washed five times with PBS and boiled with 1 × SDS loading buffer for 5 min, followed by SDS-PAGE and Coomassie brilliant blue staining or Western blot. 293 T cells were transfected with the constructed plasmids as described above to verify the interaction between M protein and host proteins. Cells co-transfected with empty vector pCMV-HA or pCAGGS-Flag served as controls. The assay was repeated in triplicate.
Coomassie blue staining and mass spectrometric identification of proteins
The immunoprecipitated proteins were separated by electrophoresis on 5% and 12% SDS-PAGE gels and the separation gel was stained using Coomassie brilliant blue for Mass Spectrometry. All distinct bands in the lane of HuN4-F112 infection group and the gel at parallel areas in the lane of the control group were excised and subjected to LC-MS/MS. Briefly, gel pieces were distained with 30% acetonitrile/100 mM NH4HCO3 and freeze-dried. The gel pieces were reduced with 100 mM of DTT (56 °C, 30 min), followed by alkylation with 200 mM iodoacetamide (in the dark, 25 °C, 20 min). The gels were incubated with 100 mM NH4HCO3 and shrunk with acetonitrile again, and incubated with trypsin (2.5-10 ng/μL) for 20 h at 37 °C. Peptides were extracted with 60% acetonitrile/0.1% TFA. Peptides were separated using a nano-flow HPLC (LTQ VELOS, Thermo Finnigan, San Jose, CA, USA).
Bioinformatics analysis
The functional annotation and classification of all the proteins were determined using Blast2GO program [40] against the non-redundant protein database (nr) at NCBI and the KEGG pathway database [41]. The protein-protein interact network was performed using Cytoscape software [42].
Western blot analysis
Protein samples were separated by 12% SDS-PAGE and then transferred onto PVDF membranes (Millipore, Bedford, MA, USA). The membranes were incubated with anti-HA mAb, anti-Flag mAb, and anti-M mAb, respectively. After the membranes were rinsed with PBST, each membrane was treated with DyLight 800-Goat Anti-Mouse IgG (H + L) as the secondary antibody. The proteins were visualized by scanning the membranes with a LI-COR Odyssey infrared image system (LI-COR Biosciences, Lincoln, NE, USA).
Confocal imaging
293 T cells were cotransfected with pCAGGS-Flag-NF45/PCNA (0.5 μg) and pCMV-HA-M (0.5 μg) in a 35-mm dish. After 48 h of incubation, transfected cells were fixed with 4% paraformaldehyde in PBS for 30 min and permeabilized with 0.1% Triton X-100 for 15 min. The cells were incubated with anti-HA mAb and anti-Flag pAb for 1 h. The cells were then incubated with goat anti-mouse IgG -FITC (F2012; Sigma) and goat anti-rabbit IgG-TRITC antibodies. Cells were stained with DAPI for 5 min and examined with a Leica SP2 confocal system (Leica Microsystems, Wetzlar, Hessen, Germany). The assay was repeated in triplicate.
Results
The expression of M protein upon PRRSV infection
MARC-145 cells were infected with the HuN4-F112 and collected at 12 h to 84 h post-infection to detect the expression of M protein arising from PRRSV infection. Samples were subjected to Western blot analysis with anti-M protein mAb. Expression of GAPDH served as an internal reference. The expression level of M protein increased during PRRSV infection and reached a peak between 48 and 60 h post-infection (Fig. 1a). We collected samples at 48–60 h post-infection for subsequent interactome analyses.
Identification of the cellular proteins that interact with PRRSV M protein by co-immunoprecipitation (Co-IP). a Cell lysates from HP-PRRSV strain HuN4-F112-infected MARC-145 cells at different time points were subjected to Western blot with anti-M protein mAb 3 F7 and anti-GAPDH mAb. b Cell lysates from HuN4-F112/HuN4-F5- or mock-infected MARC-145 cells were immunoprecipitated with anti-M protein mAb 3 F7. The three lanes (lane 1, lane 2, and lane 3) stand for HuN4-F5 infected group, HuN4-F112 infected group, and control group, respectively. The immunoprecipitated proteins were separated by 12% SDS-PAGE and visualized by Coomassie brilliant blue staining. The asterisks (*) show the differential protein bands between HuN4-F112- or mock-infected MARC-145 cells. c The experimental procedure was as the same as above, but the cell lysates were subjected to Western blot with anti-M protein mAb 3 F7
Identification of host cellular proteins that interact with PRRSV M protein
MARC-145 cells were infected with HuN4-F112/HuN4-F5 at an MOI of 0.1 to efficiently precipitate M protein and subsequently identify host proteins that interact with M protein. Infected cells were harvested at 48–60 h post-infection and immunoprecipitated with M protein mAb 3 F7. The immunoprecipitated proteins were resolved using 12% SDS-PAGE and visualized by Coomassie brilliant blue staining (Fig. 1b) and Western blot with 3 F7 (Fig. 1c). At least 10 additional bands of proteins specifically precipitated from HuN4-F112 infected cells compared with the control group (Fig. 1b). 3011 cellular proteins were identified by LC-MS/MS analysis of the 10 protein bands coming from the HuN4-F112 infection group. Of these, 219 proteins had a high confidence (Unique Peptide ≥ 2). A summary of the proteins that interact with M protein at 48–60 h following PRRSV infection are given in (Additional file 1: Table S1), with the UniquePepCount and CoverPercent of each protein.
Functional analyses of identified proteins
All of the identified proteins were assigned for bioinformatic analyses to gain functional insights into the interactome of M protein. Three main types of annotations, including biological processes, cellular components and molecular functions, were obtained from the gene ontology (GO) consortium website (Fig. 2a–c). Subclasses associated with cellular process (16.57%), metabolic process (14.32%), single-organism process (14.12%), biological regulation (9.56%), localization (8.34%) and cellular component organization or biogenesis (8.16%) were enriched in the biological process category (Fig. 2a). The most enriched subclasses in the cellular components included cell (29.21%), organelle (27%), macromolecular complex (17%), membrane (11.57%) and membrane-enclosed lumen (10.72%) (Fig. 2c). The enrichments based on molecular function were binding (49.96%), catalytic activity (23.9%) and structural molecule activity (12.8%) (Fig. 2b). A more detailed summary containing the GO annotation for individual proteins is provided in (Additional file 2: Table S2).
The annotation of proteins interacting with PRRSV M protein using Gene Ontology, the interaction network, and the enriched KEGG pathways. a Biological process. b Cellular components. c Molecular function. d Interaction network. e Classification of the enriched KEGG pathways of the cellular proteins interacting with PRRSV M protein
The interactions between the differentially expressed proteins and other proteins were determined by querying the IntAct data base according to differentially expressed proteins’ Gene Symbol. The interaction network of the cellular proteins interacting with M protein were drawn using the CytoScape software (Fig. 2d).
Analysis of the infection network based on the KEGG revealed an enrichment of 219 pathways (Additional file 3: Table S3). The more prominent pathways were involved in ribosome (83 proteins), phagosome (31 proteins), protein processing in endoplasmic reticulum (26 proteins), pathogenic Escherichia coli infection (25 proteins), spliceosome (24 proteins) and glycolysis/gluconeogenesis (22 proteins) (Fig. 2e and Additional file 3: Table S3).
Validation of the proteins that interact with the M protein of PRRSV by CO-IP
The total RNA of PAMs was extracted and the ORFs of two interested protein (NF45 and PCNA) were amplified by RT-PCR. PCR products were cloned into the pCMV-HA/pCAGGS-Flag vectors and confirmed by sequencing. A pCMV-HA-HuN4-F112-M vector was constructed in the same way. After the 293 T cells were transfected with pCAGGS-Flag-M and pCMV-HA-NF45/PCNA or with pCMV-HA-M and pCAGGS-Flag-NF45/PCNA, Co-IP was performed with ANTI-FLAG® M2 Affinity Gel (Sigma, St. Louis, MO, USA). The immune complexes were resolved by 12% SDS-PAGE and probed for the presence of NF45/PCNA or M protein using anti-HA mAb and anti-M protein mAb. Both NF45 and PCNA were readily detected in the presence of M protein, but not in the presence of empty vector (Fig. 3a). M protein was only detected in the presence of NF45 or PCNA (Fig. 3b). These results confirmed that PRRSV M protein was able to interact with the overexpressed proteins NF45 and PCNA.
Confirmation of the interaction of PRRSV M protein with NF45 and PCNA by CO-IP. The interaction of M protein and exogenous NF45 and PCNA. 293 T cells were co-transfected with 5 μg of the indicated plasmids in 60-mm dishes. Cell lysates were prepared at 36–48 h after transfection and the proteins were immunoprecipitated with anti-Flag mAb. Proteins in cell lysates (input) and immunoprecipitated samples were detected with the antibodies against Flag, HA, and M by Western blot. The asterisk (*) indicates IgG (Flag mAb) heavy chains. a NF45 and PCNA were immunoprecipitated by M protein. b M protein was immunoprecipitated by NF45 and PCNA
Confocal analyses of M protein and NF45/PCNA
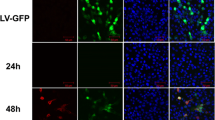
To examine the colocalization of M protein with NF45 or PCNA, 293 T cells were co-transfected with plasmids expressing HA-M and Flag-NF45/PCNA proteins and the subcellular localization of M protein and NF45 or PCNA were examined by confocal microscopy (Fig. 4). Both HA-M protein and Flag-NF45/PCNA were distributed throughout the cytoplasm, and M protein colocalized with NF45 or PCNA. This finding confirms that M protein interacts with exogenous NF45 or PCNA in 293 T cells.
Colocalization of M protein with NF45 and PCNA. 293 T cells were cotransfected with HA-M and Flag-NF45/PCNA. Cells were fixed at 48 h and subjected to indirect immunofluorescence to detect HA-M (green) and Flag-NF45/PCNA (red) with mouse anti-HA and rabbit anti-Flag antibodies. The position of the nucleus is indicated by DAPI (blue) staining in the merged image
Discussion
PRRSV causes persistent infection and immunological tolerance in pigs [43], but the specific molecular mechanisms of these effects have not been absolutely elucidated. The virus proteins carry out some functions that depend on interaction with the host cellular proteins, so it is necessary to explore the mechanisms associated with viral pathogenesis and host anti-virus response using a protein interactions approach.
The M protein encoded by ORF6 is an unglycosylated membrane protein of 18–19 kDa [27, 43]. The M protein is important in virus assembly and budding [44] and is linked to GP5 as heterodimers via a disulfide bond at the N-terminal ectodomains [27, 45]. The M/GP5 complex could combine sialoadhesin, which is involved in the entry process of PRRSV in to PAM [35, 36]. Investigating the interactome profile of M protein with the host cellular proteins is very valuable because PRRSV M protein has important functions associated with viral entry and replication. In the present study, the HP-PRRSV vaccine strain HuN4-F112 was used to further investigate the direct and indirect interaction of cellular proteins with M protein in PRRSV-infected MARC-145 cells. This viral strain was chosen because the vaccine strain HuN4-F112 was more adaptive to the MARC-145 cells than other HP-PRRSVs including HuN4-F5, which was useful to screen more host cellular proteins. This method can present the native protein conformation during virus replication and explore the cellular proteins that interact directly or indirectly with M protein in the presence of other viral proteins. These interactions are easily missed using the classical method of Co-IP of a single protein with host cells [46, 47].
In this study, 219 host cellular proteins that interact with M protein were identified in HuN4-F112-infected cells with high confidence by Co-IP and LC-MS/MS. We used bioinformatic analysis to comprehensively evaluate and characterize the identified proteins to further explore the biological significance of the interaction between M protein and host cellular proteins. The results implicate a large number of host cellular proteins that were related to the ribosome, protein processing in the endoplasmic reticulum, spliceosome, phagosome, pathogenic Escherichia coli infection, and glycolysis pathways. Of these, the first three pathways were related to protein translation and it is reasonable to find these translation pathways were enriched during the PRRSV infection. The translation process is initiated after virus entry and release of the viral genome into the cytoplasm. The PRRSV first translates its two replicase proteins coded by ORF1a and ORF1b, by employing the host translation system, to yield the polyprotein precursors pp1a and pp1ab [22, 48, 49]. Our data suggest that M protein could interact with proteins related to protein translation. The GO annotations of the host cellular proteins that interacted with M protein indicated they were located on the membrane and had binding and catalytic activities, and we inferred that M protein could combine with membrane proteins. Previous studies showed that heparin interacted with the virus and reduced infection of PAM by up to 92% or 88% for the American and European types of PRRSV, respectively [34]. Heparinase treatment of PAM resulted in a significant reduction of the infection. The structural M protein and the M/GP5 complex were verified to contribute to PRRSV attachment on a heparinlike receptor on PAM using heparin-affinity chromatography and SDS-PAGE [34]. These results further suggested that M protein could combine with membrane proteins. The interaction network of differentially expressed proteins is shown in Fig. 2d, which identified over 20 proteins with six small dispersed protein networks. The networks of proteins interacting with PRRSV M protein were poorly understood until now. There are three proteins located in the center, eukaryotic elongation factor 1A (eEF1A), ADP-ribosylation factor (ARF) 6, and the cellular chaperone HSP90AB1. The eEF1A is one of the most abundant protein synthesis factors, and constitutes 1% to 4% of the total soluble proteins in actively dividing cells [50, 51]. eEF1A takes part in viral transcription, translation and assembly as a cofactor for many viruses, including tombusvirus (TBSV) [52] and human immunodeficiency type 1 (HIV-1) [53]. Moreover, eEF1A interacts with the NS5A protein and inhibits the growth of classical swine fever virus (CSFV) [54]. ARFs are 21-kDa GTP-binding proteins that belong to a group of ras-related small GTPases that regulate various events associated with membrane trafficking. The ARFs constitute a family of gene products composed of six ARF proteins and nine ARF-like proteins. The ARFs are divided into three classes based on size and amino acid identity: ARFs 1, 2, 3 and ARFs 4, 5 constitute classes I and II, respectively, with ARF6 belonging to class III. In fact, ARF6 is the only member of the Ras-related ARF family of small GTPases that affects cell-surface dynamics, thereby regulating plasma membrane/endosome trafficking and cortical actin reorganization [55]. HIV-1 requires ARF6-mediated membrane dynamics to efficiently enter and infect T lymphocytes [56]. HSP90AB1 is an abundant, highly conserved cellular chaperone that functions as a key component of a multiprotein chaperone complex. These complex includes Cdc37 and several other proteins that regulate folding, maturation, stabilization, and renaturation of a select group of target proteins [57, 58]. A previous study has demonstrated that hepatitis B virus polymerase suppresses NF-κB signaling by inhibiting the activity of IKKs via interaction with HSP90AB1 [59]. All of these findings show that these three protein participated in virus replication and innate immunity, which may also play a role in PRRSV life cycle via interaction with M protein.
A proportion of proteins were shown to be associated with the infectious disease (Fig. 3 and Additional file 3: Table S3). These results implicate that like others pathogens, PRRSV may exploit similar host cellular components and share a common or similar pathogenesis. Thus the research on other pathogens could be useful in the study of PRRSV pathogenesis.
We selected two novel proteins from the 219 cellular proteins that interact with M protein, namely NF45 and PCNA, and the interactions between PRRSV M protein and porcine NF45 or PCNA were further confirmed by Co-IP (Fig. 4). NF45 is a versatile nuclear protein that associates with various factors in multifunctional complexes involved in mitosis, microRNA biogenesis [60], interleukin 2 (IL-2) production [61], IRES-dependent translational control [62], and cellular inhibitor of apoptosis protein 1 (cIAP1)-mediated antiapoptosis [63]. Recent observations suggest that NF45 and its heterodimer NF90 are significantly involved in the replication process of several different RNA viruses. Both NF45 and NF90 were indicated to be part of viral replication machineries and suggested to part of the regulation of viral translation and RNA replication for two Flaviviridae family members, bovine viral diarrhea virus (BVDV) and hepatitis C virus (HCV) [64–66]. NF45 interacts with viral proteins of infectious bursal disease virus and inhibits viral replication [67]. We identified another protein-proliferating cell nuclear antigen (PCNA) among the proteins identified from the interactome profile of M protein (Fig. 4). PCNA is a member of the sliding clamp family of DNA-replication accessory proteins. Their functions are critical to processes such as cell cycle control, chromatin remodeling, gene expression, apoptosis, and DNA repair [68–71]. PCNA is a homo episomal trimer in most organisms, with three subunits that adopt a doughnut-shaped structure in a head-to-tail arrangement. This toroidal structure is highly conserved in protozoa, humans, yeasts and plants [72–75]. Ubiquitylation of PCNA participates directly in the meiotic process and the diversification of the Ig locus through class-switch recombination and somatic hypermutation [76]. PCNA was identified as an H5N1 PA-host interacting protein in chicken cells [77]. All seven viral replication proteins of herpes simplex virus were enriched on the viral genome, along with cellular PCNA [78]. PCNA was recruited by LANA to the Kaposi’s Sarcoma-associated herpesvirus genome via Bub1 to initiate viral replication during the cell division S phase [79]. We inferred that PCNA was involved in the replication of many viral genomes. Both NF45 and PCNA are found predominantly in the nucleus, and they may interact with M protein in the cytoplasm after nuclear export.
The interaction between virus and a host cell is not only the process that the virus replicates and cells releases progeny virus using host cell and viral proteins after breaking through multi-level barriers, but also the process that host cell resists virus invasion or self-sacrifice to clear the virus. These interactions ultimately results in changes in protein expression patterns, which influence normal physiology function of host cells and ultimately determines the processes and results of viral infection. The M protein has important biological functions during PRRSV infection and immunity. Our findings about the proteins that interact with the M protein provide scientific clues for understanding virus molecular pathogenesis and control.
These findings not only generate new sight on the cellular defense mechanism against PRRSV infections, but also provide a new view on PRRSV participating in cell cycle control.
Conclusions
In the present study, 219 host cellular proteins that interact with the M protein in PRRSV-infected cells were identified with high confidence using a HP-PRRSV vaccine by a Co-IP and LC/MS-MS coupled method. The identified proteins were assigned to different subcellular locations and functional classes according to the GO annotation and enriched KEGG pathway analysis. An interactome profile of M protein with the host cellular proteins was drawn to gain a functional insight into the host-virus proteins interaction.
Abbreviations
- DTT:
-
Dithiothreitol
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- LC-MS/MS:
-
Liquid Chromatography-Mass Spectrometry/Mass Spectrometry
- MOI:
-
Multiplicity of infection
- NF45:
-
Nuclear factor of activated T cells 45 kDa
- PCNA:
-
Proliferating cell nuclear antigen
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TFA:
-
Trifluoroacetic acid.
References
Wensvoort G, Terpstra C, Pol JM, ter Laak EA, Bloemraad M, de Kluyver EP, Kragten C, van Buiten L, den Besten A, Wagenaar F, et al. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Quart. 1991;13(3):121–30.
Terpstra C, Wensvoort G, Pol JM. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad virus: Koch’s postulates fulfilled. Vet Quart. 1991;13(3):131–6.
Benfield DA, Nelson E, Collins JE, Harris L, Goyal SM, Robison D, Christianson WT, Morrison RB, Gorcyca D, Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J Vet Diagn Invest. 1992;4(2):127–33.
Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 1992;4(2):117–26.
Russell P, Atkinson K, Krishnan L. Recurrent reproductive failure due to severe placental villitis of unknown etiology. J Reprod Med. 1980;24(2):93–8.
Albina E. Porcine reproductive and respiratory syndrome: ten years of experience (1986–1996) with this undesirable viral infection. Vet Res. 1997;28(4):305–52.
Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997;55(1–4):309–16.
Blaha T. The “colorful” epidemiology of PRRS. Vet Res. 2000;31(1):77–83.
Lunney JK, Benfield DA, Rowland RR. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Vet Res. 2010;154(1–2):1–6.
Li Y, Wang X, Bo K, Wang X, Tang B, Yang B, Jiang W, Jiang P. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the Mid-Eastern region of China. Vet J. 2007;174(3):577–84.
Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, et al. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One. 2007;2(6), e526.
Tong GZ, Zhou YJ, Hao XF, Tian ZJ, An TQ, Qiu HJ. Highly pathogenic porcine reproductive and respiratory syndrome. China Emerg Infect Dis. 2007;13(9):1434–6.
Zhou YJ, Hao XF, Tian ZJ, Tong GZ, Yoo D, An TQ, Zhou T, Li GX, Qiu HJ, Wei TC, et al. Highly virulent porcine reproductive and respiratory syndrome virus emerged in China. Transbound Emerg Dis. 2008;55(3–4):152–64.
An TQ, Tian ZJ, Xiao Y, Li R, Peng JM, Wei TC, Zhang Y, Zhou YJ, Tong GZ. Origin of highly pathogenic porcine reproductive and respiratory syndrome virus. China Emerg Infect Dis. 2010;16(2):365–7.
An TQ, Tian ZJ, Leng CL, Peng JM, Tong GZ. Highly pathogenic porcine reproductive and respiratory syndrome virus. Asia Emerg Infect Dis. 2011;17(9):1782–4.
Lunney JK, Chen H. Genetic control of host resistance to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Vet Res. 2010;154(1–2):161–9.
Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Vet Res. 2010;154(1–2):18–30.
Zhou L, Yang H. Porcine reproductive and respiratory syndrome in China. Vet Res. 2010;154(1–2):31–7.
Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142(3):629–33.
Johnson CR, Griggs TF, Gnanandarajah J, Murtaugh MP. Novel structural protein in porcine reproductive and respiratory syndrome virus encoded by an alternative ORF5 present in all arteriviruses. J Gen Virol. 2011;92(Pt 5):1107–16.
Firth AE, Zevenhoven-Dobbe JC, Wills NM, Go YY, Balasuriya UB, Atkins JF, Snijder EJ, Posthuma CC. Discovery of a small arterivirus gene that overlaps the GP5 coding sequence and is important for virus production. J Gen Virol. 2011;92(Pt 5):1097–106.
Music N, Gagnon CA. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Anim Health Res Rev. 2010;11(2):135–63.
Fang Y, Snijder EJ. The PRRSV replicase: exploring the multifunctionality of an intriguing set of nonstructural proteins. Vet Res. 2010;154(1–2):61–76.
Fang Y, Treffers EE, Li Y, Tas A, Sun Z, van der Meer Y, de Ru AH, van Veelen PA, Atkins JF, Snijder EJ, et al. Efficient −2 frameshifting by mammalian ribosomes to synthesize an additional arterivirus protein. Proc Natl Acad Sci U S A. 2012;109(43):E2920–8.
Meulenberg JJ, Petersen-den Besten A, De Kluyver EP, Moormann RJ, Schaaper WM, Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995;206(1):155–63.
Bautista EM, Meulenberg JJ, Choi CS, Molitor TW. Structural polypeptides of the American (VR-2332) strain of porcine reproductive and respiratory syndrome virus. Arch Virol. 1996;141(7):1357–65.
Mardassi H, Mounir S, Dea S. Molecular analysis of the ORFs 3 to 7 of porcine reproductive and respiratory syndrome virus, Quebec reference strain. Arch Virol. 1995;140(8):1405–18.
Mardassi H, Mounir S, Dea S. Structural gene analysis of a Quebec reference strain or porcine reproductive and respiratory syndrome virus (PRRSV). Adv Exp Med Biol. 1995;380:277–81.
Yang L, Frey ML, Yoon KJ, Zimmerman JJ, Platt KB. Categorization of North American porcine reproductive and respiratory syndrome viruses: epitopic profiles of the N, M, GP5 and GP3 proteins and susceptibility to neutralization. Arch Virol. 2000;145(8):1599–619.
Bastos RG, Dellagostin OA, Barletta RG, Doster AR, Nelson E, Osorio FA. Construction and immunogenicity of recombinant Mycobacterium bovis BCG expressing GP5 and M protein of porcine reproductive respiratory syndrome virus. Vaccine. 2002;21(1–2):21–9.
Jiang Y, Xiao S, Fang L, Yu X, Song Y, Niu C, Chen H. DNA vaccines co-expressing GP5 and M proteins of porcine reproductive and respiratory syndrome virus (PRRSV) display enhanced immunogenicity. Vaccine. 2006;24(15):2869–79.
Faaberg KS, Even C, Palmer GA, Plagemann PG. Disulfide bonds between two envelope proteins of lactate dehydrogenase-elevating virus are essential for viral infectivity. J Virol. 1995;69(1):613–7.
Snijder EJ, Dobbe JC, Spaan WJ. Heterodimerization of the two major envelope proteins is essential for arterivirus infectivity. J Virol. 2003;77(1):97–104.
Delputte PL, Vanderheijden N, Nauwynck HJ, Pensaert MB. Involvement of the matrix protein in attachment of porcine reproductive and respiratory syndrome virus to a heparinlike receptor on porcine alveolar macrophages. J Virol. 2002;76(9):4312–20.
Vanderheijden N, Delputte PL, Favoreel HW, Vandekerckhove J, Van Damme J, van Woensel PA, Nauwynck HJ. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J Virol. 2003;77(15):8207–15.
Van Breedam W, Van Gorp H, Zhang JQ, Crocker PR, Delputte PL, Nauwynck HJ. The M/GP(5) glycoprotein complex of porcine reproductive and respiratory syndrome virus binds the sialoadhesin receptor in a sialic acid-dependent manner. PLoS Pathog. 2010;6(1), e1000730.
Wang Q, Chen J, Peng J, An T, Leng C, Sun Y, Guo X, Ge X, Tian Z, Yang H. Characterisation of novel linear antigen epitopes on North American-type porcine reproductive and respiratory syndrome virus M protein. Arch Virol. 2014;159(11):3021–8.
Tian ZJ, An TQ, Zhou YJ, Peng JM, Hu SP, Wei TC, Jiang YF, Xiao Y, Tong GZ. An attenuated live vaccine based on highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) protects piglets against HP-PRRS. Vet Microbiol. 2009;138(1–2):34–40.
Zhang H, Guo X, Ge X, Chen Y, Sun Q, Yang H. Changes in the cellular proteins of pulmonary alveolar macrophage infected with porcine reproductive and respiratory syndrome virus by proteomics analysis. J Proteome Res. 2009;8(6):3091–7.
Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Snijder EJ, Meulenberg JJ. The molecular biology of arteriviruses. J Gen Virol. 1998;79(Pt 5):961–79.
Wieringa R, de Vries AA, van der Meulen J, Godeke GJ, Onderwater JJ, van Tol H, Koerten HK, Mommaas AM, Snijder EJ, Rottier PJ. Structural protein requirements in equine arteritis virus assembly. J Virol. 2004;78(23):13019–27.
Mardassi H, Massie B, Dea S. Intracellular synthesis, processing, and transport of proteins encoded by ORFs 5 to 7 of porcine reproductive and respiratory syndrome virus. Virology. 1996;221(1):98–112.
Beura LK, Dinh PX, Osorio FA, Pattnaik AK. Cellular poly(c) binding proteins 1 and 2 interact with porcine reproductive and respiratory syndrome virus nonstructural protein 1beta and support viral replication. J Virol. 2011;85(24):12939–49.
Jourdan SS, Osorio F, Hiscox JA. An interactome map of the nucleocapsid protein from a highly pathogenic North American porcine reproductive and respiratory syndrome virus strain generated using SILAC-based quantitative proteomics. Proteomics. 2012;12(7):1015–23.
Gorbalenya AE, Enjuanes L, Ziebuhr J, Snijder EJ. Nidovirales: evolving the largest RNA virus genome. Vet Res. 2006;117(1):17–37.
Snijder EJ, Kikkert M, Fang Y. Arterivirus molecular biology and pathogenesis. J Gen Virol. 2013;94(Pt 10):2141–63.
Browning KS, Humphreys J, Hobbs W, Smith GB, Ravel JM. Determination of the amounts of the protein synthesis initiation and elongation factors in wheat germ. J Biol Chem. 1990;265(29):17967–73.
Condeelis J. Elongation factor 1 alpha, translation and the cytoskeleton. Trends Biochem Sci. 1995;20(5):169–70.
Li Z, Pogany J, Tupman S, Esposito AM, Kinzy TG, Nagy PD. Translation elongation factor 1A facilitates the assembly of the tombusvirus replicase and stimulates minus-strand synthesis. PLoS Pathog. 2010;6(5), e1001175.
Warren K, Wei T, Li D, Qin F, Warrilow D, Lin MH, Sivakumaran H, Apolloni A, Abbott CM, Jones A, Anderson JL, Harrich D. Eukaryotic elongation factor 1 complex subunits are critical HIV-1 reverse transcription cofactors. Proc Natl Acad Sci U S A. 2012;109(24):9587–92.
Li S, Feng S, Wang JH, He WR, Qin HY, Dong H, Li LF, Yu SX, Li Y, Qiu HJ. eEF1A Interacts with the NS5A Protein and Inhibits the Growth of Classical Swine Fever Virus. Viruses. 2015;7(8):4563–81.
Donaldson JG, Honda A. Localization and function of Arf family GTPases. Biochem Soc Trans. 2005;33(Pt 4):639–42.
Garcia-Exposito L, Barroso-Gonzalez J, Puigdomenech I, Machado JD, Blanco J, Valenzuela-Fernandez A. HIV-1 requires Arf6-mediated membrane dynamics to efficiently enter and infect T lymphocytes. Mol Biol Cell. 2011;22(8):1148–66.
Neckers L, Ivy SP. Heat shock protein 90. Curr Opin Oncol. 2003;15(6):419–24.
Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood). 2003;228(2):111–33.
Liu D, Wu A, Cui L, Hao R, Wang Y, He J, Guo D. Hepatitis B Virus Polymerase Suppresses NF-kB Signaling by Inhibiting the Activity of IKKs via Interaction with Hsp90b. PLoS One. 2014;9(3), e91658.
Sakamoto S, Aoki K, Higuchi T, Todaka H, Morisawa K, Tamaki N, Hatano E, Fukushima A, Taniguchi T, Agata Y. The NF90-NF45 complex functions as a negative regulator in the microRNA processing pathway. Mol Cell Biol. 2009;29(13):3754–69.
Zhao G, Shi L, Qiu D, Hu H, Kao PN. NF45/ILF2 tissue expression, promoter analysis, and interleukin-2 transactivating function. Exp Cell Res. 2005;305(2):312–23.
Merrill MK, Gromeier M. The double-stranded RNA binding protein 76:NF45 heterodimer inhibits translation initiation at the rhinovirus type 2 internal ribosome entry site. J Virol. 2006;80(14):6936–42.
Graber TE, Baird SD, Kao PN, Mathews MB, Holcik M. NF45 functions as an IRES trans-acting factor that is required for translation of cIAP1 during the unfolded protein response. Cell Death Differ. 2010;17(4):719–29.
Isken O, Baroth M, Grassmann CW, Weinlich S, Ostareck DH, Ostareck-Lederer A, Behrens SE. Nuclear factors are involved in hepatitis C virus RNA replication. RNA. 2007;13(10):1675–92.
Isken O, Grassmann CW, Sarisky RT, Kann M, Zhang S, Grosse F, Kao PN, Behrens SE. Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. EMBO J. 2003;22(21):5655–65.
Isken O, Grassmann CW, Yu H, Behrens SE. Complex signals in the genomic 3′ nontranslated region of bovine viral diarrhea virus coordinate translation and replication of the viral RNA. RNA. 2004;10(10):1637–52.
Stricker RL, Behrens SE, Mundt E. Nuclear factor NF45 interacts with viral proteins of infectious bursal disease virus and inhibits viral replication. J Virol. 2010;84(20):10592–605.
Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116(Pt 15):3051–60.
Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129(4):665–79.
Dieckman LM, Freudenthal BD, Washington MT. PCNA structure and function: insights from structures of PCNA complexes and post-translationally modified PCNA. Subcell Biochem. 2012;62:281–99.
Indiani C, O’Donnell M. The replication clamp-loading machine at work in the three domains of life. Nat Rev Mol Cell Biol. 2006;7(10):751–61.
Cardona-Felix CS, Lara-Gonzalez S, Brieba LG. Structure and biochemical characterization of proliferating cellular nuclear antigen from a parasitic protozoon. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 6):497–505.
Chia N, Cann I, Olsen GJ. Evolution of DNA replication protein complexes in eukaryotes and Archaea. PLoS One. 2010;5(6), e10866.
Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79(7):1233–43.
Matsumiya S, Ishino Y, Morikawa K. Crystal structure of an archaeal DNA sliding clamp: proliferating cell nuclear antigen from Pyrococcus furiosus. Protein Sci. 2001;10(1):17–23.
Roa S, Avdievich E, Peled JU, Maccarthy T, Werling U, Kuang FL, Kan R, Zhao C, Bergman A, Cohen PE, et al. Ubiquitylated PCNA plays a role in somatic hypermutation and class-switch recombination and is required for meiotic progression. Proc Natl Acad Sci U S A. 2008;105(42):16248–53.
Wang Q, Li Q, Liu R, Zheng M, Wen J, Zhao G. Host cell interactome of PA protein of H5N1 influenza A virus in chicken cells. J Proteomics. 2016;136:48–54.
Dembowski JA, DeLuca NA. Selective recruitment of nuclear factors to productively replicating herpes simplex virus genomes. PLoS Pathog. 2015;11(5), e1004939.
Sun Z, Jha HC, Robertson ES. Bub1 in Complex with LANA Recruits PCNA To Regulate Kaposi’s Sarcoma-Associated Herpesvirus Latent Replication and DNA Translesion Synthesis. J Virol. 2015;89(20):10206–18.
Acknowledgements
We are grateful to Dr. Rui Wang for her help in analyzing the data, and to Professor Changjiang Weng for his help in Co-IP technology.
Funding
This study was supported by the Heilongjiang National Funds for Distinguished Young Scientists (No. JC201314). The funder played no role in the design of the study, the collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional file.
Authors’ contributions
Conceived and designed the experiments: QW YWL ZJT XHC. Performed the experiments: QW YWL. Analyzed the data: QW YWL ZJT. Contributed reagents/materials/analysis tools: HD LW JMP TQA XFY. Contributed to the writing of the manuscript: QW YWL ZJT XHC. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Animal experiments were approved by the Animal Ethics Committee of Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences (CAAS) and performed in accordance with animal ethics guidelines and approved protocols. The Animal Ethics Committee approval number was SYXK (Hei) 2011022.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1:
Table S1. The list of the proteins interacting with the PRRSV M protein. (XLS 61 kb)
Additional file 2:
Table S2. The annotation of proteins interacting with M protein during PRRSV infection using Gene Ontology. (XLS 2143 kb)
Additional file 3:
Table S3. The list of the enriched KEGG Pathways of the PRRSV M protein interacting proteins. (XLS 277 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, Q., Li, Y., Dong, H. et al. Identification of host cellular proteins that interact with the M protein of a highly pathogenic porcine reproductive and respiratory syndrome virus vaccine strain. Virol J 14, 39 (2017). https://doi.org/10.1186/s12985-017-0700-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-017-0700-1