Abstract
Background
Rheumatic diseases are associated with an increased fracture risk. The tissue level characteristics of the bone involvement in children have not been well elucidated. Our objectives were to describe the bone micro-architectural characteristics in children with rheumatic diseases on chronic glucocorticoids, and to determine associations between micro-architectural findings with clinical and radiological variables.
Methods
Children on chronic glucocorticoids for an underlying rheumatic disease were referred for evaluation of bone fragility given the presence of vertebral compression fractures. A trans-iliac bone biopsy was performed as part of the clinical assessment. Histomorphometric analysis and quantitative backscattered electron imaging (qBSE) of the biopsy samples were undertaken.
Results
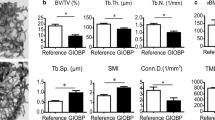
Data of 15 children (14.0 ± 3.2 years) with a duration of glucocorticoid exposure of 6.2 ± 4.1 years and average prednisone dose of 14.1 ± 6.2 mg/m2/day were assessed. Histomorphometric analyses demonstrated significant decrease in trabecular thickness (p = 0.01), osteoid thickness (p < 0.01), osteoblast surface (p = 0.02) and increase in trabecular separation (p = 0.04) compared to published age-matched normative data. Severity of the trabecular deficit was correlated to glucocorticoid dose, height and body mass index Z score, but not bone mineral density or measures of disease activity. Using qBSE to measure bone mineralization, the subjects were shown to have a heterogeneous and hypermineralized profile, with higher cumulative glucocorticoid dose being associated with greater mineralization (p < 0.01).
Conclusions
In children with rheumatic diseases presenting with vertebral fractures, there is evidence of abnormal bone matrix mineralization and impairments of bone micro-architecture that correlate to glucocorticoid dose.
Similar content being viewed by others
Background
Children and adolescents with rheumatic diseases, such as systemic lupus erythematosis (SLE) and juvenile idiopathic arthritis (JIA) are recognized to be at risk for impaired bone health. In those who develop overt osteoporosis, multiple risk factors are implicated, including increased concentrations of inflammatory cytokines, diminished nutrition, physical activity and muscle bulk [1, 2]. Use of glucocorticoids, in particular, is also known to compromise bone mass, quality and strength [3]. As a consequence, in children with rheumatic conditions, a 10 to 30 % prevalence rate of vertebral fractures has been reported [4–7], with a 6 % incidence of vertebral fractures within 12 months of glucocorticoid initiation [8].
Bone mineral density (BMD) as measured by dual-energy x-ray absorptiometry (DXA), is one of the most common tools used to assess bone health. Low BMD is associated with an increased risk for fractures in otherwise healthy children [9]. Children with rheumatic diseases including SLE and JIA have been shown to have reduced BMD compared to healthy age-matched controls [1, 10–12]. Decreased lumbar spine BMD Z score in the first 6 months of initiation of glucocorticoid treatment has been demonstrated to be an important predictor for increased vertebral fracture risk [13]. DXA has, however, limitations in the assessment of bone health in pediatric cohorts given that the results are influenced by bone size [14]. In addition bone strength is determined not only by bone density but also by the underlying bone quality and micro-architecture.
Histomorphometric analysis of trans-iliac bone samples allows characterization of bone microarchitecture including bone quantity, structure and metabolism. Histomorphometric studies in adults with rheumatoid arthritis have shown evidence of reduced trabecular bone volume with decreased trabecular thickness and bone turnover [15, 16]. Moreover, in adult cohorts, vertebral fractures have been demonstrated to relate to trabecular bone micro-architecture independent of the bone density [17].
The mineral content of bone has similarly been demonstrated to be an important determinant of bone stiffness and strength [18]. The degree and heterogeneity of bone mineralization can be measured from trans-iliac bone samples using quantitative backscattered electron imaging (qBSE). Deviations in bone mineralization have been demonstrated in patients with bone fragility, such as in children with osteogenesis imperfecta [19] and adults with postmenopausal osteoporosis [20], but have not been assessed to date in patients with rheumatic diseases.
Studies of bone histomorphometry and mineralization in pediatric patients treated with glucocorticoids have contributed to the understanding of underlying pathology and clinical factors with the potential to predict severity of bone disease [21, 22]. Given these features have not to date been described in pediatric rheumatalogical patients, the aims of this pilot study were therefore to characterize bone histomorphometry and mineralization in a small cohort of children with rheumatic diseases and to assess associations between clinical and radiological indices to bone micro-architecture.
Methods
All patients with a rheumatic disease who had been referred to the Bone Health Clinic at the Hospital for Sick Children, Toronto between 2003 and 2011 and who had undergone a trans-iliac bone biopsy were included in this retrospective descriptive analysis. Patients had been referred to the clinic for assessment of bone health because of the finding of at least one vertebral compression fracture. All patients were on calcium and vitamin D supplements and had undergone baseline investigations including biochemical assessment of parameters of calcium homeostasis, as well as spinal radiographs and DXA. The bone biopsy was undertaken as part of the clinical evaluation for symptomatic osteoporosis. Exclusion criteria included children with impaired renal function or other chronic medical conditions (other than their rheumatic disease) that could affect bone health. This study was approved by the Hospital for Sick Children’s Research Ethics Committee.
Clinical data
Information was collected from the patients’ records regarding their underlying rheumatic disease, including age of diagnosis, duration of symptoms and treatment. All children were on glucocorticoid treatment (prednisone) at the time of the bone biopsy. Duration of glucocorticoid treatment, as well as cumulative and average daily dose was documented. All glucocorticoid doses were expressed as milligrams per body surface area (mg/m2) in prednisone equivalents. Clinical data including age, height and weight were collected from the time of the trans-iliac bone biopsy. Height was measured with a wall-mounted stadiometer to the nearest 0.1 cm and weight was taken on an electric digital scale to the nearest 0.1 kg. Height, weight and body mass index (BMI) [weight (kg)/height (m)2] z-score were calculated using reference data provided by the Centre for Disease Control 2000 standardized reference charts. Pubertal development was assessed according to Tanner criteria [23, 24]. As a measure of functional status of the subjects at the time of the bone biopsy, the Childhood Health Assessment Questionnaire (CHAQ) score was recorded. The CHAQ score is a validated, reliable tool for measuring functional status in children with juvenile idiopathic arthritis [25], and has also been demonstrated to reflect disease activity in other rheumatological conditions [26, 27]. CHAQ scores range from 0 (indicating no disability) to 3 (severe disability).
Biochemistry
Biochemical markers taken at the time of the biopsy were recorded. Serum calcium, phosphate and alkaline phosphatase were measured by spectophotometry using the VITROS 950 Chemistry System (Johnson and Johnson Ortho-Clinical Diagnostics, Rochester, NY). Serum intact parathyroid hormone (PTH) was measured by IMMULITE chemoiluminescent immunometric assay (Siemens Healthcare Diagnostics, Deerfield, IL). Serum 25 hydroxyvitamin D (25(OH)D) was measured by high-performance liquid chromatography tandem mass spectroscopy or radioimmunoassay (DiaSorin S,p,A, Saluggia, Italy). Erythrocyte sedimentation rate (ESR) was used as a biochemical marker of rheumatological disease activity.
Radiological studies
Lumbar spine (L1 to L4) areal BMD was measured by dual-energy X-ray absorptiometry (DXA) in the anterior-posterior direction (Lunar Prodigy; General Electric; Madison, WI, USA). Compressed lumbar vertebrae were excluded from the analysis. To account for bone size and height, bone mineral apparent density (BMAD) was derived using the method proposed by Kroger et al., where by BMAD = BMD × [4/(π x width)] [28]. Areal BMD and BMAD values were transformed into Z scores using the equipment specific age and sex-adjusted Canadian reference values for areal BMD and published reference data for BMAD [29].
Standard lateral thoracic and lumbar spine radiographs were obtained in an upright position. All radiographs were assessed for vertebral morphology and loss of vertebral height. Morphology was determined using the Genant semi-quantitative method by measuring the posterior, mid-body and anterior vertebral body height ratios as described previously [30]. Significant vertebral compression was defined as a height loss of greater than 20 %. Vertebral bodies with evidence of compression were assigned a severity score; grade 1 = mild (loss of height of > 20 to 25 %), grade 2 = moderate (loss of height of > 25 to 40 %), and grade 3 = severe (loss of height of > 40 %) [30].
Trans-iliac histomorphometry
Utilizing ultrasound and fluoroscopic guidance, full thickness transiliac bone biopsies were obtained with a Rochester biopsy device under general anaesthetic from a site located 2 cm below and behind the anterior superior iliac spine for static histomorphometry. Informed consent was obtained from all subjects. Biopsy preparation and static histomorphometric analyses were performed as previously described [31]. In brief, the specimens were placed in 70 % ethanol, then dehydrated and embedded in polymethylmethacrylate. Histomorphometric parameters were evaluated in undecalcified sections treated with Goldner’s trichrome or Von Kossa stain. The total trabecular area was analyzed as serial fields using the Lietz Bioquant morphometry system (Bioquant Nova Prime, Version 6.50.10). Bone histology was reported using the terminology established by the Nomenclature Committee of the American Society of Bone and Mineral research [32]. Results were converted to percentage scores compared to published reference age and gender matched controls [31].
Bone mineralization
The embedded iliac crest biopsy blocks were used for evaluation of bone mineralization density distribution (BMDD) using quantitative backscattered electron imaging (qBSE). The blocks were cut, polished, carbon-coated and imaged using backscattered electron imaging (solid state BSE detector, FEI Company, Hillsboro, OR, USA) on a FEI XL30 ESEM. This technique allows quantification of the intensity of the backscattered electron signal from the surface of a sectioned bone by calibration of the brightness and contrast. Histograms of the grey level distribution were created for the trabecular bone of the iliac crest samples, with a range of grey levels from 0 to 255. Increasing brightness corresponds with increasing mineralization [33]. The signal is proportional to the weight fraction of calcium in the embedded bone tissue. Two parameters were derived for statistical analysis; mineralization peak, the peak position on the histogram which indicates the most frequently occurring grey level in the bone area, and the full width at half-maximum height (FWHMH) of the bone mineralization histogram curve which describes the mineral heterogeneity of the sample.
Statistical analysis
The data were analyzed using SPSS 21.0 for windows (SPSS Inc, Chicago, IL, USA). Anthropometry and densitometry Z scores were tested for significant deviation from zero using the one-sample t-test. Differences in histomorphometric measurements between the cohort and published normative data were assessed by comparing the subject’s results as expressed as a percentage of the reference mean versus 100 % using one-sample t-tests. Correlations between histomorphometric, bone mineralization and clinical variables were evaluated with Pearson and Spearman rank correlations. No adjustment for multiple comparisons was made. Statistical significance was inferred with a P value less than 0.05.
Results
Cohort clinical characteristics
Fifteen children (12 females) with a mean age of 14.0 ± 3.2 years were included in this analysis. Eight of the children had a diagnosis of SLE, 5 systemic JIA and 2 had a systemic vasculitis. The clinical characteristics of the cohort are displayed in Table 1. All patients had been commenced on glucocorticoid treatment within 2 months of clinical diagnosis.
Serum concentrations of calcium, phosphate, parathyroid hormone and creatinine were within the normal range for all subjects at time of the biopsy. The median 25(OH)D concentration was 69.5 (43 to 118) nmol/L, and only one patient had a concentration less than 50 nmol/L. Mean alkaline phosphatase was 133.9 ± 48.2 IU/L, with 6 patients having concentrations below the normal reference range for age and gender. ESR was mildly raised in most subjects with a median concentration of 13 (range 1 to 57) mm/h.
Radiological measures of bone health
Twelve of the fifteen patients were investigated for vertebral compression fractures due to the onset of back pain, while the remaining three had an X-ray of their spine performed following the finding of a lumbar BMD Z score less than −2 on DXA. There were a total of 68 vertebral fractures within the entire cohort, with the majority involving the thoracic region (71 %). Almost 50 % of the cohort had fractures involving 5 or more of the vertebrae, with over half of the subjects having grade 2 severity vertebral height loss (loss of height of > 25 to 40 %) (Table 1). Greater number of vertebral compression fractures was associated with higher BMI Z score (r = 0.78, p < 0.01). None of the patients had a history of peripheral fractures. Greater severity of vertebral height loss was associated with higher cumulative glucocorticoid dose (r = 0.56, p = 0.03), longer disease duration (r = 0.60, p = 0.02) and higher CHAQ score (r = 0.60, p = 0.02).
The lumbar spine areal BMD and BMAD were significantly below results expected in healthy subjects of the same age and gender (p < 0.001). There was, however, a spectrum of values, and 6 of the 15 of the subjects had a lumbar spine BMAD Z score greater than −2.0. Lower lumbar spine BMAD Z score was associated with greater average glucocorticoid dose (r = −0.50, p = 0.04), but not cumulative dose or duration of glucocorticoids. There was no significant association between number or severity of vertebral compression factures and BMD.
Trabecular iliac bone histomorphometry
Iliac bone histomorphometry demonstrated evidence of thinning of the trabecular with increased separation, although preservation of the trabecular number (Table 2). Bone formation activity was decreased with low markers of osteoid formation and percentage of osteoblast-covered surface. There was no significant difference in histomorphometric values in the subjects with JIA compared to those with SLE.
Univariate analysis showed that higher average glucocorticoid dose was associated with greater deficits in both bone structural and formation parameters. (Table 3). In particular, the amount of osteoblast surface per bone surface percentage strongly associated with both dose and duration of glucocorticoids. Systemic clinical effects of glucocorticoids (higher BMI and lower height Z score), also correlated with histomorphometric parameters. Lower bone volume and osteoblast surface associated with a greater number of vertebral compression fractures. There was no association between bone histomorphometry with lumbar spine areal BMD or BMAD Z scores, 25(OH)D or ESR concentrations.
The rheumatological patients had a mineralization peak of 167.4 ± 20.5 and a FWHMH of 34.3 ± 7.2. The bone mineralization of the patient cohort was compared to published normative values for healthy children [34]. The rheumatological patients had a shift in the mineral distribution towards a more hypermineralized profile (p <0.001) and had a greater heterogeneity in the mineralization distribution (p < 0.01). Greater cumulative glucocorticoid dose (r = 0.71, p < 0.01), lower histomorphometric bone formation parameters (r = −0.7, p < 0.01) and lower serum alkaline phosphatase concentration were associated with a more hypermineralized profile (r = −0.52, p = 0.04). Bone mineralization variables did not related to age, anthropometry or lumbar spine bone mineral density.
Discussion
This study demonstrated that children with rheumatic diseases on chronic glucocorticoids who presented with vertebral fractures, have evidence of significantly decreased bone structural and formation histomorphometric indices compared to healthy age matched peers. The bone mineralization findings revealed greater heterogeneity of mineralization as well as a significant shift towards hypermineralization within the trabecular bone.
Patients with rheumatic conditions are at increased risk for bone fragility and fractures. [5–7]. Higher glucocorticoid dose have been shown to correlate with the presence of vertebral fractures [7, 8]. Similarly in our study, those children with greater cumulative glucocorticoid dose had a more severe grade of vertebral height loss. The results from this study add to this literature by demonstrating that the underlying histomorphometric deficits in trabecular bone in these children also relate to glucocorticoid dose. In our cohort, structural and formation parameters were lower than the age-specific reference range. Adults with rheumatoid arthritis have been shown to have evidence of decreased trabecular bone volume and turnover [15, 35]. Reduced trabecular thickness and osteoblast surface have similarly been found in other cohorts of children treated with glucocorticoids such as those with Duchene muscular dystrophy and steroid dependent nephrotic syndrome [21, 22]. Unlike, however, the histomorphometry results from the pediatric patients with nephrotic syndrome, our subjects did not have evidence of osteomalacia, as seen by the reduced osteoid thickness and volume. The primary effect of the glucocorticoid treatment in our subjects would appear to be through the reduction in bone formation decreasing osteoblastogenesis, increasing osteoblast apoptosis and inhibiting osteoblast production of bone matrix [36–38].
As well as the relationship between glucocorticoid dose and trabecular bone deficit, we were able to demonstrate that those children with more pronounced systemic effects from their steroid treatment (specifically reduced height and increased BMI Z score) have lower trabecular thickness and osteoblast surface. This association between increased BMI and greater bone fragility has also been demonstrated in a prospective study of children with rheumatic conditions which assessed determinants of vertebral fractures [13]. Combined, these findings would support that clinicians should have heightened vigilance around bone health screening in those rheumatological patients with systemic glucocorticoid effects.
In addition to structural and formation deficits, children with rheumatic diseases on glucocorticoids have significant differences in trabecular bone mineralization compared to healthy controls. Trabecular bone consists of individual osteons and bone packets that have different mineral contents depending on when they were produced during the (re) modeling cycle. During secondary mineralization the mineral content of these bone packets increases with time as free water in the bone matrix is replaced by mineral [18]. Bone mineralization as measured by qBSE quantifies the degree and heterogeneity of mineralization across the cross-sectional area of the trans-iliac bone biopsy, and is determined by bone turnover rate and by the time course of mineral accumulation within the bone matrix [18]. In our subjects, the shift in the bone mineralization towards a more hypermineralized profile would be consistent with decreased bone turnover leading to a greater percentage of mineralized bone packets. The inverse association between mineralization peak and histomorphometric bone formation parameters as well as serum bone turnover marker alkaline phosphatase, would support this finding. The increased heterogeneity of the matrix mineralization is also likely a result of a significant decrease in bone turnover. In experimental models, decreased bone turnover has been shown to lead to increased heterogeneity in bone mineralization [39]. Increased degree of mineralization through decreased bone remodeling is probably a significant contributor to the underlying bone fragility in our subjects. In other conditions, bone hypermineralization has been demonstrated to contribute to bone brittleness and tendency to fracture [40, 41].
BMD as measured by DXA is a commonly used screening tool to assess bone health in children on chronic glucocorticoids. A meta-analysis demonstrated a difference of −0.18 [−0.25; −0.1] g/m2 lower spine BMD in children with JIA on glucocorticoids compared to healthy controls [42]. Similarly we demonstrated an association between average daily glucocorticoid dose and BMAD Z score. While low bone mass in pediatric patients has been defined by a BMD Z score of less than or equal to −2 [43], only 60 % of our cohort, all of whom had vertebral compression fractures, had a lumbar spine BMAD Z score that met this criteria. We also did not find a correlation of either lumbar spine areal BMD or BMAD to the histomorphometric or mineralization indices. While the density of bone is measured in BMD values, our findings would suggest that DXA BMD values do not always reflect some of the other underlying pathological mechanisms that glucocorticoids have on bone. In addition clinical variables such as short stature, delayed skeletal maturation, ethnicity and lean body mass can confound BMD results [43, 44]. The lack of association of bone histomorphometry to BMD highlights the limitations of relying on BMD values alone to evaluate bone health in children with chronic diseases. Given its invasive nature, trans-iliac bone biopsies are not suitable as a bone health screening tool, but should be limited to those patients who appear to have severe bone fragility out of keeping of their medical history or bone health risk factors. Instead, yearly lateral thoracolumbar spine X-rays to assess for vertebral height loss should be used in conjunction with BMD values to screen children with rheumatic conditions on glucocorticoids for evidence of bone fragility.
The treatment of bone fragility in children with rheumatic conditions is challenging, in part because of limited therapeutic options in the pediatric age range. Given higher rates of vitamin D deficiency in children with rheumatic conditions [45, 46], ensuring adequate vitamin D and calcium intake to maintain bone mineralization is an important first line therapy, although may not be sufficient to help prevent fragility fractures [13]. The use of biologic agents in JIA, has been demonstrated to help facilitate new bone formation after discontinuation of glucocorticoids [47]. These effects are however, attenuated in those patients who remain on glucocorticoids [48]. Bisphosphonates have been shown to improve BMD in children with secondary osteoporosis [49, 50]. Our findings of low baseline osteoblast activity in children on chronic glucocorticoids, emphasizes the need for cautious use of anti-resorptive agents such as bisphosphonates, which can lead to further suppression of bone turnover [21]. Consideration of commencing bisphosphonate therapy should be reserved for those patients presenting with significant fragility fractures or bone pain.
There are limitations to our study that deserve consideration, in particular the small number of subjects included in this analysis. Given the small subject numbers further data from a larger number of subjects is needed to confirm the findings from this pilot study. In addition the subjects in our study presented with a significant degree of bone fragility and vertebral compression fractures. It is uncertain to what degree similar histomorphological and mineralization deficits would be seen in a cohort of asymptomatic children on equivalent glucocorticoid doses. While we demonstrated a linear relationship between glucocorticoid dose and trabecular structural deficits, we cannot comment as to whether there is a threshold glucocorticoid dose below which there would not be a significant detrimental effect on bone quality. Only static and not dynamic histomorphometry was able to be performed, as the subjects had not received tetracycline prior to the biopsy being taken.
Conclusions
In conclusion, our results from this small pilot study demonstrate that children with rheumatic diseases on chronic glucocorticoid treatment who present with vertebral compression fractures have evidence of decreased trabecular bone volume and formation with increased and more heterogeneous mineralization. The association between glucocorticoid dose to the bone micro-architectural deficits, highlights the importance of decreasing the steroid dose in chronic disease states where possible. Treatment with bisphosphonates is sometimes used in children with glucocorticoid induced osteoporosis, however can lead to further suppression of bone formation [21]. Our findings support the need for further research in anabolic treatment options for children, in order to address the underlying bone pathology.
Abbreviations
- 25(OH)D:
-
25 hydroxyvitamin D
- BMAD:
-
Bone mineral apparent density
- BMD:
-
Bone mineral density
- BMDD:
-
Bone mineralization density distribution
- BMI:
-
Body mass index
- CHAQ:
-
Childhood Health Assessment Questionnaire
- DXA:
-
Dual-energy X-ray absorptiometry
- ESR:
-
Erythrocyte sedimentation rate
- FWHMH:
-
Full width at half-maximum height
- JIA:
-
Juvenile idiopathic arthritis
- qBSE:
-
Quantitative backscattered electron imaging
- SLE:
-
Systemic lupus erythematosis
References
Alsufyani KA, Ortiz-Alvarez O, Cabral DA, Tucker LB, Petty RE, Nadel H, Malleson PN. Bone mineral density in children and adolescents with systemic lupus erythematosus, juvenile dermatomyositis, and systemic vasculitis: relationship to disease duration, cumulative corticosteroid dose, calcium intake, and exercise. J Rheumatol. 2005;32:729–33.
Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J Bone Miner Res. 2007;22:646–55.
van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913–8.
Reyes ML, Hernandez MI, King A, Vinet AM, Vogel A, Lagomarsino E, Mericq MV, Mendez C, Gederlini A, Talesnik E. Corticosteroid-induced osteoporosis in children: outcome after two-year follow-up, risk factors, densitometric predictive cut-off values for vertebral fractures. Clin Exp Rheumatol. 2007;25:329–35.
Varonos S, Ansell BM, Reeve J. Vertebral collapse in juvenile chronic arthritis: its relationship with glucocorticoid therapy. Calcif Tissue Int. 1987;41:75–8.
Valta H, Lahdenne P, Jalanko H, Aalto K, Makitie O. Bone health and growth in glucocorticoid-treated patients with juvenile idiopathic arthritis. J Rheumatol. 2007;34:831–6.
Nakhla M, Scuccimarri R, Duffy KN, Chedeville G, Campillo S, Duffy CM, Azouz EM, Shenouda N, Sharma AK, Rodd C. Prevalence of vertebral fractures in children with chronic rheumatic diseases at risk for osteopenia. J Pediatr. 2009;154:438–43.
Rodd C, Lang B, Ramsay T, Alos N, Huber AM, Cabral DA, Scuccimarri R, Miettunen PM, Roth J, Atkinson SA, et al. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: a national observational study. Arthritis Care Res (Hoboken). 2012;64:122–31.
Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117:e291–7.
Compeyrot-Lacassagne S, Tyrrell PN, Atenafu E, Doria AS, Stephens D, Gilday D, Silverman ED. Prevalence and etiology of low bone mineral density in juvenile systemic lupus erythematosus. Arthritis Rheum. 2007;56:1966–73.
Stagi S, Masi L, Capannini S, Cimaz R, Tonini G, Matucci-Cerinic M, de Martino M, Falcini F. Cross-sectional and longitudinal evaluation of bone mass in children and young adults with juvenile idiopathic arthritis: the role of bone mass determinants in a large cohort of patients. J Rheumatol. 2010;37:1935–43.
Lim LS, Benseler SM, Tyrrell PN, Harvey E, Herbert D, Charron M, Silverman ED. Predicting longitudinal trajectory of bone mineral density in paediatric systemic lupus erythematosus patients. Ann Rheum Dis. 2012;71:1686–91.
LeBlanc CM, Ma J, Taljaard M, Roth J, Scuccimarri R, Miettunen P, Lang B, Huber AM, Houghton K, Jaremko JL, et al. Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. J Bone Miner Res. 2015;30:1667–75.
Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. Bone densitometry in pediatric populations: discrepancies in the diagnosis of osteoporosis by DXA and CT. J Pediatr. 2005;146:776–9.
Perez-Edo L, Diez-Perez A, Marinoso L, Valles A, Serrano S, Carbonell J. Bone metabolism and histomorphometric changes in rheumatoid arthritis. Scand J Rheumatol. 2002;31:285–90.
Hanyu T, Arai K, Takahashi HE. Structural mechanisms of bone loss in iliac biopsies: comparison between rheumatoid arthritis and postmenopausal osteoporosis. Rheumatol Int. 1999;18:193–200.
Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, Basle MF, Audran M. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res. 2000;15:13–9.
Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–66.
Boyde A, Travers R, Glorieux FH, Jones SJ. The mineralization density of iliac crest bone from children with osteogenesis imperfecta. Calcif Tissue Int. 1999;64:185–90.
Roschger P, Gupta HS, Berzlanovich A, Ittner G, Dempster DW, Fratzl P, Cosman F, Parisien M, Lindsay R, Nieves JW, Klaushofer K. Constant mineralization density distribution in cancellous human bone. Bone. 2003;32:316–23.
Misof BM, Roschger P, McMillan HJ, Ma J, Klaushofer K, Rauch F, Ward LM. Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with duchenne muscular dystrophy: a paired transiliac biopsy study. J Bone Miner Res. 2016;31:1060–9.
Freundlich M, Jofe M, Goodman WG, Salusky IB. Bone histology in steroid-treated children with non-azotemic nephrotic syndrome. Pediatr Nephrol. 2004;19:400–7.
Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303.
Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23.
Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761–9.
Moorthy LN, Harrison MJ, Peterson M, Onel KB, Lehman TJ. Relationship of quality of life and physical function measures with disease activity in children with systemic lupus erythematosus. Lupus. 2005;14:280–7.
Feldman BM, Ayling-Campos A, Luy L, Stevens D, Silverman ED, Laxer RM. Measuring disability in juvenile dermatomyositis: validity of the childhood health assessment questionnaire. J Rheumatol. 1995;22:326–31.
Kroger H, Kotaniemi A, Vainio P, Alhava E. Bone densitometry of the spine and femur in children by dual-energy x-ray absorptiometry. Bone Miner. 1992;17:75–85.
van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM. Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch Dis Child. 2002;87:341–7. discussion −7.
Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48.
Glorieux FH, Travers R, Taylor A, Bowen JR, Rauch F, Norman M, Parfitt AM. Normative data for iliac bone histomorphometry in growing children. Bone. 2000;26:103–9.
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17.
Boyde A, Jones SJ. Back-scattered electron imaging of skeletal tissues. Metab Bone Dis Relat Res. 1983;5:145–50.
Fratzl-Zelman N, Roschger P, Misof BM, Pfeffer S, Glorieux FH, Klaushofer K, Rauch F. Normative data on mineralization density distribution in iliac bone biopsies of children, adolescents and young adults. Bone. 2009;44:1043–8.
Lund B, Storm TL, Melsen F, Mosekilde L, Andersen RB, Egmose C, Sorensen OH. Bone mineral loss, bone histomorphometry and vitamin D metabolism in patients with rheumatoid arthritis on long-term glucocorticoid treatment. Clin Rheumatol. 1985;4:143–9.
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82.
Chavassieux P, Pastoureau P, Chapuy MC, Delmas PD, Meunier PJ. Glucocorticoid-induced inhibition of osteoblastic bone formation in ewes: a biochemical and histomorphometric study. Osteoporos Int. 1993;3:97–102.
Kim HJ. New understanding of glucocorticoid action in bone cells. BMB Rep. 2010;43:524–9.
Ruffoni D, Fratzl P, Roschger P, Klaushofer K, Weinkamer R. The bone mineralization density distribution as a fingerprint of the mineralization process. Bone. 2007;40:1308–19.
Farlay D, Armas LA, Gineyts E, Akhter MP, Recker RR, Boivin G. Nonenzymatic glycation and degree of mineralization Are higher in bone from fractured patients with type 1 diabetes mellitus. J Bone Miner Res. 2016;31:190–5.
Fratzl-Zelman N, Morello R, Lee B, Rauch F, Glorieux FH, Misof BM, Klaushofer K, Roschger P. CRTAP deficiency leads to abnormally high bone matrix mineralization in a murine model and in children with osteogenesis imperfecta type VII. Bone. 2010;46:820–6.
Hansen KE, Kleker B, Safdar N, Bartels CM. A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children. Semin Arthritis Rheum. 2014;44:47–54.
Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM. Dual-energy X-Ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD pediatric official positions. J Clin Densitom. 2014;17:225–42.
Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–73.
Stagi S, Cavalli L, Bertini F, de Martino M, Cerinic MM, Brandi ML, Falcini F. Vitamin D levels in children, adolescents, and young adults with juvenile-onset systemic lupus erythematosus: a cross-sectional study. Lupus. 2014;23:1059–65.
Stagi S, Bertini F, Cavalli L, Matucci-Cerinic M, Brandi ML, Falcini F. Determinants of vitamin D levels in children, adolescents, and young adults with juvenile idiopathic arthritis. J Rheumatol. 2014;41:1884–92.
Brabnikova Maresova K, Jarosova K, Pavelka K, Stepan JJ. Bone status in adults with early-onset juvenile idiopathic arthritis following 1-year anti-TNFalpha therapy and discontinuation of glucocorticoids. Rheumatol Int. 2013;33:2001–7.
Okano T, Koike T, Tada M, Sugioka Y, Mamoto K, Wakitani S, Nakamura H. The limited effects of anti-tumor necrosis factor blockade on bone health in patients with rheumatoid arthritis under the use of glucocorticoid. J Bone Miner Metab. 2014;32:593–600.
Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatology (Oxford). 2005;44:813–8.
Thornton J, Ashcroft DM, Mughal MZ, Elliott RA, O’Neill TW, Symmons D. Systematic review of effectiveness of bisphosphonates in treatment of low bone mineral density and fragility fractures in juvenile idiopathic arthritis. Arch Dis Child. 2006;91:753–61.
Acknowledgements
Not applicable.
Funding
No external funding was secured for this study.
Availability of data and materials
Patients have not consented on presenting their full data, therefore the dataset on supporting the conclusions of this article is not attached as an additional file.
Authors’ contributions
JH was involved in the design of the study, the acquisition and analysis of the data and wrote the first draft of the manuscript. Authors DH and EaS were involved in the acquisition of the data. Authors EtS and MG were involved in the design of the study and interpretation of the data. All authors revised the paper critically for intellectual content and approved the final version.
Competing interests
The authors declare they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Hospital for Sick Children’s Research Ethics Committee, REB number 1000028038.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Harrington, J., Holmyard, D., Silverman, E. et al. Bone histomorphometric changes in children with rheumatic disorders on chronic glucocorticoids. Pediatr Rheumatol 14, 58 (2016). https://doi.org/10.1186/s12969-016-0119-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-016-0119-z