Abstract
Background
When feasible, guidelines recommend mitral valve repair (MVr) over mitral valve replacement (MVR) to treat primary mitral regurgitation (MR), based upon historic outcome studies and transthoracic echocardiography (TTE) reverse remodeling studies. Cardiovascular magnetic resonance (CMR) offers reference standard biventricular assessment with superior MR quantification compared to TTE. Using serial CMR in primary MR patients, we aimed to investigate cardiac reverse remodeling and residual MR post-MVr vs MVR with chordal preservation.
Methods
83 patients with ≥ moderate-severe MR on TTE were prospectively recruited. 6-min walk tests (6MWT) and CMR imaging including cine imaging, aortic/pulmonary through-plane phase contrast imaging, T1 maps and late-gadolinium-enhanced (LGE) imaging were performed at baseline and 6 months after mitral surgery or watchful waiting (control group).
Results
72 patients completed follow-up (Controls = 20, MVr = 30 and MVR = 22). Surgical groups demonstrated comparable baseline cardiac indices and co-morbidities. At 6-months, MVr and MVR groups demonstrated comparable improvements in 6MWT distances (+ 57 ± 54 m vs + 64 ± 76 m respectively, p = 1), reduced indexed left ventricular end-diastolic volumes (LVEDVi; − 29 ± 21 ml/m2 vs − 37 ± 22 ml/m2 respectively, p = 0.584) and left atrial volumes (− 23 ± 30 ml/m2 and − 39 ± 26 ml/m2 respectively, p = 0.545). At 6-months, compared with controls, right ventricular ejection fraction was poorer post-MVr (47 ± 6.1% vs 53 ± 8.0% respectively, p = 0.01) compared to post-MVR (50 ± 5.7% vs 53 ± 8.0% respectively, p = 0.698). MVR resulted in lower residual MR-regurgitant fraction (RF) than MVr (12 ± 8.0% vs 21 ± 11% respectively, p = 0.022). Baseline and follow-up indices of diffuse and focal myocardial fibrosis (Native T1 relaxation times, extra-cellular volume and quantified LGE respectively) were comparable between groups. Stepwise multiple linear regression of indexed variables in the surgical groups demonstrated baseline indexed mitral regurgitant volume as the sole multivariate predictor of left ventricular (LV) end-diastolic reverse remodelling, baseline LVEDVi as the most significant independent multivariate predictor of follow-up LVEDVi, baseline indexed LV end-systolic volume as the sole multivariate predictor of follow-up LV ejection fraction and undergoing MVR (vs MVr) as the most significant (p < 0.001) baseline multivariate predictor of lower residual MR.
Conclusion
In primary MR, MVR with chordal preservation may offer comparable cardiac reverse remodeling and functional benefits at 6-months when compared to MVr. Larger, multicenter CMR studies are required, which if the findings are confirmed could impact future surgical practice.
Similar content being viewed by others
Introduction
Current guidelines on primary mitral regurgitation (MR) recommend mitral valve repair (MVr) over mitral valve replacement (MVR) whenever feasible [1, 2]. Supportive observational studies typically demonstrate worse early and long-term mortality post MVR [3, 4], however, numerous pre-date the routine use of chordal preservation techniques [3, 5, 6], which improves cardiac reverse remodeling post MVR [7,8,9]. Indeed, transthoracic echocardiographic (TTE) studies assessing left-ventricular (LV) reverse remodeling post MVr/MVR suggest it is comparable when chordal preservation is used [10, 11] and worse remodeling post-MVR when not [5, 6]. MVR is more frequently performed in older patients with more co-morbidities [12]. To date, no randomised trials have compared MVr and MVR in primary MR and studies using propensity matching in an attempt to overcome intrinsic bias present conflicting results [12, 13]. A randomised trial in ischaemic MR demonstrated comparable survival and LV reverse remodeling at 2 years, with greater recurrent MR and adverse events post MVr compared to post MVR with chordal preservation [14].
In primary MR, recurrent MR post-MVr is not uncommon, with moderate-severe MR reported in 13–17% in longitudinal studies [15, 16]. MVr typically results in equivalent [3, 12, 13] or more re-operations than MVR [17], however, the re-operation end-point may not account for all recurrent significant MR if patients are not amenable to, or deemed too high risk for, repeat surgery.
Accurate MR assessment is paramount to guide the need for surgical intervention and provide appropriate outcome comparisons between MVr/MVR. Cardiovascular magnetic resonance (CMR) is the reference standard for biventricular volume/function assessment [18] and compared to TTE, CMR MR quantification demonstrates superior reproducibility [19,20,21] and prognostication in primary MR [20, 22]. Additionally, CMR can accurately quantify the forward LV pump efficiency, despite the presence of severe MR, by assessing effective forward left ventricular ejection fraction (LVEF), calculated as aortic forward flow volume/left ventricular end diastolic volume (LVEDV) [23].
Ultimately, randomised trials comparing MVr vs MVR could guide future clinical decision making, especially in specific patient groups in whom MVR would not increase bleeding risk (patients in whom a tissue valve will last a lifetime or patients with a pre-existing indication for anticoagulation). Prior to this, rigorous hypothesis-generating observational data will be required; this study aimed to assess differences in cardiac reverse remodeling, residual MR (assessed by CMR) and functional capacity following surgical MVr and MVR with chordal preservation for primary MR, compared to a matched control group (moderate to/or severe MR patients on a watchful waiting pathway).
Methods
Study design
This single-center prospective observational cohort study recruited patients between February 2016 and February 2020 with primary MR at the Leeds Teaching Hospitals NHS Trust, Leeds, UK, during which time 313 patients had corrective surgery for MR, of which 168 patients without significant aortic valve disease had elective surgery for primary MR. All patients meeting inclusion/exclusion criteria were contacted, of which 83 were recruited. Inclusion criteria comprised moderate-severe or severe primary MR on echocardiography, aged > 18 years, suitable for elective surgical intervention, with capacity to provide written informed consent. Exclusion criteria comprised secondary (functional/ischaemic/atrial) MR, contraindications to CMR, significant (≥ moderate severity) aortic valve disease, uncontrolled atrial fibrillation (AF) > 120 bpm, New York Heart Association (NYHA) functional Class IV, terminal illness, haemodynamic instability, renal failure with an estimated glomerular filtration rate of < 30 ml/min/1.73m2, pregnancy or breast feeding, or inability to lie supine for 60 min.
At least moderate-severe MR was defined by an integrated echocardiographic assessment as per American Society of Echocardiography guidelines: vena contracta > 0.7cm2, proximal isovelocity surface area (PISA) radius > 0.8 cm, estimated regurgitant orifice area > 0.3cm2, MR regurgitant volume (Rvol) > 45 ml/beat, MR regurgitant fraction (RF) > 40% [24]. Surgical intervention (timing and technique) was decided by a multidisciplinary heart team, as per international guidance [1, 2], independent from the study. Pre-operative assessment commonly included transesophageal echocardiography and left ± right heart catheterisation where indicated. CMR imaging and 6-min walk tests (6MWT) were performed at baseline and 6-months post-surgery (MVR or MVr) or post-watchful waiting (control group). 6MWT followed American Thoracic Society guidelines [25]. Written informed consent was provided by all patients. The study was approved by local research ethics committee (Yorkshire & The Humber-South Yorkshire 15/YH/0503) and complied with the Declaration of Helsinki.
CMR imaging
Baseline and 6-month follow-up CMR were performed (1.5T Philips Ingenia, Best, Netherlands). CMR protocol involved: (1) survey images, (2) LV short axis multi-slice, multi-phase cine imaging using a balanced steady-state free precession (bSSFP) sequence (TR 3 ms, TE 1.6 ms, flip angle 60°, SENSE factor 2, 10 mm thickness, 0 mm gap, in-plane spatial resolution 1.2 × 1.2 mm, 30 phases, matrix 192 × 131, typical FOV of 340 mm), (3) horizontal and vertical long axis cine imaging (4) transaxial right ventricular (RV) multi-slice, multi-phase bSSFP cine imaging (TR 2.8 ms, TE 1.41 ms, flip angle 60°, SENSE factor 1.8, 8 mm thickness, 0 mm gap, in-plane spatial resolution 1.88 × 1.88 mm, 20 phases, matrix 192 × 143, typical FOV 360 mm), (5) two orthogonal LV-outflow-tract and RV-outflow-tract views, (6) through-plane aortic and pulmonary phase contrast (PC)-CMR, planned at the aortic sinotubular junction, orthogonal to the aorta, to assess aortic flow and approximately 1 cm superior to the pulmonary valve, orthogonal to the main pulmonary artery to assess pulmonary flow. Velocity encoding was set to 150 cm/s and increased for repeat imaging if aliased. All PC sequences were planned with region of interest in the CMR scanner isocenter to reduce potential background phase-offset errors [26]. Other PC parameters: typical FOV 350 × 280 mm, TR 5.1 ms, TE 3.2 ms, flip angle 15°, temporal resolution 28 ms, number of signal averages 1, SENSE factor 2, turbo field echo factor 3, turbo field echo acquisition duration 30.8 ms, slice thickness 8 mm, 30 phases, phase percentage 100%, in-plane spatial resolution 2.5 × 2.5 mm, matrix 140 × 112, Cartesian sampling, and typical acquisition times 12–15 s for breath-held sequences. In patients with AF, two acquisitions of aortic/pulmonary PC-CMR imaging with the same parameters were obtained and the results averaged to account for heart rate variation, (7) Late-gadolinum enhancement (LGE) imaging, multi-slice LV-short axis covering base-apex (typical FOV 350 mm, 10 mm thickness, 0 mm gap) and (8) Modified Look-Locker inversion recovery sequence, single mid-ventricular short axis slice, pre and 15-min post gadolinium contrast (0.2 mmol/kg).
CMR analysis
Images were analysed using commercially available software (cvi42, Circle Cardiovascular Imaging, Calgary, Alberta, Canada). CMR acquisition and analysis was overseen and performed respectively by a dedicated experienced member of the CMR research team. For continuity, volumetric and phase contrast flow analysis was performed by the same individual, strictly using the methodology described below. Biventricular endocardial contours were manually traced; the papillary muscles and trabeculations were considered part of the ventricular blood pool and volumes calculated by summation of disks [27]. For sequential CMR analysis, careful comparison with baseline images was ensured to optimise basal slice selection and contouring around any post-surgical artefact. Maximal left atrial (LA) volume was calculated using the bi-plane area-length method from horizontal and vertical long-axis cines during ventricular systole [28] and maximal right atrial (RA) area measured, inclusive RA appendage, from horizontal long-axis cines during ventricular systole [28, 29]. As per prior studies [20,21,22] and Society of Cardiovascular Magnetic Resonance recommendations [30], mitral and tricuspid regurgitation were quantified indirectly using the following formulas respectively: Mitral regurgitant volume (MR-Rvol) = LV stroke volume − aortic stroke volume and tricuspid regurgitant volume (TR-Rvol) = RV stroke volume − pulmonary stroke volume. Effective forward LVEF was calculated as: aortic forward flow volume/LVEDV [23]. LGE short-axis images were visually assessed for LGE, if present quantitative assessment was performed by semi-automated signal intensity analysis according to the full width at half maximum technique [31]. Myocardial T1 relaxation times (ms) were assessed via a region of interest at the mid ventricular septum, with extracellular volume fraction (ECV) calculated using the patient’s haematocrit via standard formula [32].
Surgical technique
Surgical procedures were performed under general anaesthesia using a standard cardiopulmonary bypass technique via a 7–10 cm midline sternotomy incision and mild systemic hypothermia (30–34 °C). Intra-operative transesophageal echocardiography was utilised. Systemic heparinisation aorto-bicaval cannulation was performed. LA incision was made to expose and inspect the pathological mitral valve. Final decision to perform MVr or MVR was at discretion of the surgeon dependent upon feasibility of durable repair utilizing knowledge from prior imaging, heart team MDT discussions and intra-operative morphological assessment of the native valve/apparatus. All MVr were performed using Gore-Tex chordae sutures and supported by a Carpentier-Edwards Physio II annuloplasty ring (typical size 29–34 mm). MVR were performed using the St Jude mechanical valve, Edwards Perimount Magna bioprosthetic valve or St Jude Epic™ Mitral stented tissue valve (typical size 27–33 mm). At least partial chordal preservation was performed with MVR as routine practice. The type of prosthetic valve, preservation technique and suture placement technique were at the discretion of the surgeon. Mechanical MVR patients were treated with life-long anticoagulation (Vitamin K antagonist-warfarin) post-procedure. In selected cases AF was ablated with radiofrequency and coinciding LA appendage ligation performed.
Statistical analysis
Data were analysed using SPSS version 26 (IBM Corp, Armonk, New York, USA). All continuous data were assessed for normality using Shapiro–Wilk test. Baseline, follow-up/residual and the changes from baseline to follow up variables were compared between the three groups (control/MVr/MVR). Continuous variables are expressed as mean ± standard deviation (SD) and categorical variables expressed as frequencies and percentages. Continuous data were assessed between all groups by one-way analysis of variance (normally distributed variables) or Kruskal–Wallis (non-normally distributed variables), both performed with Bonferroni post-hoc analysis on statistically significant variables for subgroup comparison. Categorical data were compared by Fisher’s exact test, if a significant difference was found between all groups, Fisher’s exact tests were performed between each group to assess inter-group differences. p < 0.05 was considered statistically significant. Additional subgroup analysis was performed using one-way analysis of variance or Kruskal–Wallis and Fisher’s exact test as above: (1) comparing follow-up variables between surgical groups after exclusion of patients with residual MR-RF ≥ 30%; (2) comparing all groups after excluding patients whom underwent concomitant coronary artery bypass grafting (CABG); (3) comparing baseline variables between surgical groups whom achieved an LVEF ≥ 50% vs LVEF < 50% at follow-up [27, 33] and (4) comparing baseline/change/follow-up variables between surgical groups with baseline LGE (LGE+) vs those without (LGE−). Indices of myocardial fibrosis (T1/ECV/LGE) compared between control/MVr/MVR at baseline and follow-up (including subgroup analysis excluding CABG cases) utilized cases with available paired baseline/follow-up data. Predictors of post-surgical remodeling and residual MR were calculated using a stepwise linear regression model with baseline variables entered as covariates. For regression analysis, all volumetric indices were indexed to body surface area including valve regurgitant volumes and missing T1/ECV/LGE data were dealt with by listwise deletion. Variables with a univariate p < 0.1 were included in multivariate analysis. Post-surgical LV-end-diastolic reverse remodeling is calculated as the percentage reduction in LVEDV from baseline to follow-up imaging [(Follow-up LVEDV − Baseline LVEDV)/Baseline LVEDV x − 1], with values inverted so positive values indicate increased/improved reverse remodeling.
Results
Eighty-three patients were recruited and scanned at baseline. By group, 34 patients underwent MVr (1 death and 3 patient dropouts: 1 developed motor neurone disease, 2 declined due to the COVID-19 pandemic); 24 underwent MVR (2 deaths); 25 controls were observed with watchful waiting (3 deaths and 2 patient drop-outs: 1 claustrophobic and 1 developed lung cancer). This resulted in 72 patients with paired CMR scans: 30 MVr, 22 MVR (14 mechanical, 8 bio-prosthetic valves) and 20 controls with follow-up CMR at 188 ± 27, 194 ± 25 and 233 ± 8 days respectively (Fig. 1, CONSORT flow diagram), with duration to follow-up CMR comparable between surgical groups (p = 1).
CONSORT patient flow diagram—a figure demonstrating number of patients recruited against the inclusion/exclusion criteria and numbers completing follow-up imaging by group. *2 patients declined follow up imaging due to the COVID-19 pandemic. AF: atrial fibrillation; CMR: cardiovascular magnetic resonance; eGFR: estimated glomerular filtration rate; MND: motor neurone disease; MR: mitral regurgitation; MVr: mitral valve repair; MVR: mitral valve replacement; NYHA: New York Heart Association
Baseline patient characteristics
Baseline characteristics of the groups are presented in Table 1. There was no difference in age or sex between the groups. The underlying leaflet(s) affected differed between surgical groups (p = 0.014), with a greater proportion of posterior mitral valve leaflet (PMVL) disease in the MVr group and anterior mitral valve leaflet disease in the MVR group. Somewhat expectedly, the control group, compared with MVr and MVR groups, demonstrated a lower NYHA functional class (1.3 ± 0.6 vs 1.9 ± 0.7 and 2.2 ± 0.7 respectively, p = 0.001) and lower incidence of AF (20% vs, 53% and 59% respectively, p = 0.021). Otherwise, comorbidities and surgical risk scores (Log Euro/Log EuroII/ STS Mortality/morbidity) were comparable between groups.
Baseline CMR cardiac parameters
At baseline CMR assessment there were no significant differences in baseline biventricular volumes or quantified aortic, tricuspid, or pulmonary regurgitation between the groups (Table 2), except lower RVEF in the MVR and MVr groups when compared to controls at 46 ± 6.6% and 46 ± 9.4% vs 54 ± 8.0% respectively (p = 0.002). The control group had lower baseline quantitated MR volume compared to the MVr and MVR groups with an MR-Rvol of 49 ± 25 ml vs 66 ± 26 ml and 71 ± 29 ml (p = 0.002) and an MR-RF of 39 ± 13% vs 50 ± 10% and 52 ± 13% respectively (p = 0.001), resulting in a greater effective forward LVEF at 36 ± 8.3% vs 28 ± 7.6% and 27 ± 9.8 respectively (p = 0.002). There were no significant differences on baseline CMR between both surgical groups.
Surgical variables
The operation variables are compared between surgical groups in Additional file 1. Thirty patients underwent MVr and twenty-two patients underwent MVR (Prosthesis: mechanical = 14, tissue = 8). MVr and MVR groups were comparable in terms of concomitant coronary artery bypass grafting (2 vs 2 respectively, p = 1.00), tricuspid valve repair (5 vs 2 respectively, p = 0.685) and AF ablations (1 vs 2 respectively, p = 0.567). Cardiopulmonary bypass time and cross-clamp times were comparable between the MVr and MVR groups at 124 ± 26 min vs 132 ± 47 min (p = 0.837) and 96 ± 28 min vs 94 ± 41 min (p = 0.333) respectively. After dividing the MVR group into those with direct MVR (n = 16) and those with MVR after an attempted repair (MVRar) (n = 6), the MVRar group had longer surgical procedure times compared to direct MVR and MVr groups, with a cardiopulmonary bypass time of 190 ± 32 min vs 111 ± 31 min and 124 ± 26 min (p = 0.001) and cross-clamp time of 146 ± 39 min vs 74 ± 19 min and 96 ± 28 min (p = 0.001) respectively. On sub-group analysis, direct MVR patients had comparable bypass but shorter cross clamp times compared to the MVr group at 111 ± 32 min vs 124 ± 26 min (p = 0.216) and 74 ± 19 min vs 96 ± 28 min (p = 0.046) respectively.
Functional outcomes
Changes between the groups from baseline to follow up are presented in Table 3. At follow up, compared with controls, the MVr and MVR groups demonstrated improved 6MWT distances (+ 0.1 ± 55 m vs + 57 ± 54 m and + 64 ± 76 m respectively, p = 0.002) and significant improvement in NYHA functional class (p < 0.001), with no significant differences between both surgical groups in either outcome. At 6 months, there were no significant differences between all groups in 6MWT distances or NYHA functional class (Table 4).
Cardiac reverse remodeling and quantitated valve regurgitation
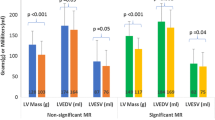
Changes to cardiac indices between baseline and follow up CMR are shown in Table 3 and the resultant residual cardiac indices are compared between groups in Table 4. Compared with controls, MVr and MVR resulted in comparable significant reductions in indexed LV end-diastolic volumes (− 1.3 ± 12 ml/m2 vs − 29 ± 21 ml/m2 and − 37 ± 22 ml/m2 respectively, p < 0.001), LVEF (+ 0.4 ± 3.9% vs − 8.7 ± 8.9% and − 8.8 ± 9.0% respectively, p < 0.001), indexed LA volumes (+ 1.2 ± 19 ml/m2 vs − 27 ± 30 ml/m2 and − 39 ± 26 ml/m2 respectively, p < 0.001) (Table 3) and improvements in effective forward LVEF (+ 0.2 ± 3.9% vs + 8.8 ± 8.3% and + 14 ± 8.7% respectively, p < 0.001). This resulted in lower LVEDVi (94 ± 28 ml/m2 and 94 ± 25 ml/m2 vs 117 ± 28 ml/m2 respectively, p = 0.005) and LVEF (47 ± 9.2% and 46 ± 8.1% vs 59 ± 5.0% respectively, p < 0.001) at 6-month follow-up in the MVr and MVR groups compared with controls and comparable effective forward LVEF between all groups (p < 0.241) (Table 4). There were no significant differences between surgical groups in terms of residual LV volumes/function or LA volume (Fig. 2). There were no significant differences between all groups in terms of change to RV volumes/function and RA areas, resulting in comparable residual right heart indices, except for lower residual RVEF in the MVr group compared with the controls (47 ± 6.1% vs 53 ± 8.0% respectively, p = 0.01). There was no significant difference in residual RVEF between MVr and MVR groups (47 ± 6.1% vs 50 ± 5.7% respectively p = 0.224). Both surgical groups demonstrated a significant reduction in and lower residual MR-Rvol and MR-RF compared with the control group (p < 0.001) (Tables 3 and 4). MVR resulted in a superior reduction in MR-RF (− 40 ± 14% vs − 29 ± 11%, p = 0.002), resulting in lower 6-month residual MR-RF compared with the MVr group (12 ± 8.0% vs 21 ± 11% respectively, p = 0.022). There were no significant differences between all three groups in changes to, or residual tricuspid regurgitation.
Importantly, the findings of comparable cardiac reverse remodeling between surgical groups but greater residual MR post-MVr remained present when patients with at least moderate [24] residual MR (MR-RF ≥ 30% on follow-up CMR) were excluded (Additional file 2). Subgroup analysis excluding patients with MR-RF ≥ 30% was performed comparing post-MVr (n = 24) vs post-MVR (n = 22), demonstrating comparable residual biventricular and bi-atrial volumes between both surgical groups with greater residual MR-RF post MVr than MVR (18 ± 8.2% vs 12 ± 8.0% respectively, p = 0.018). Separate sub-group analysis was performed with comparison of patients from all groups after patients who underwent concomitant CABGwere excluded (Additional file 3). Similarly, this resulted in no significant changes to the key findings.
Subgroup analysis comparing the baseline parameters of surgical patients who achieved follow-up LVEF ≥ 50% vs LVEF < 50% is presented in Additional file 4. There were no significant differences between co-morbidities, aetiology, functional or surgical parameters or tissues characteristics (Native T1/ECV/LGE) between the groups. Post-surgical patients with follow-up LVEF < 50% vs ≥ 50% had greater baseline LVEDVi (139 ± 29 ml/m2 vs 112 ± 23 ml/m2, p = 0.001), LVESVi (67 ± 21 ml/m2 vs 47 ± 12 ml/m2, p = < 0.001), RVESVi (57 ± 13 ml/m2 vs 48 ± 15 ml/m2, p = 0.013) and MR severity (MR-RF: 54 ± 10% vs 47 ± 11%, p = 0.028) and lower baseline LVEF (52 ± 8.0% vs 59 ± 6.2%, p = 0.002).
Predictors of cardiac reverse remodeling in surgical groups
Baseline univariate and multivariate predictors of post-surgical LVEDV reverse remodeling are presented in Table 5 and predictors of follow-up indexed LVEDV, LVEF and MR-Rvol in Table 6. Baseline indexed MR-Rvol was the sole independent multivariate predictor of post-surgical LV-end-diastolic reverse remodeling. Baseline indexed LVEDV, MR-Rvol and NYHA were independent multivariate predictors of post-surgical indexed LVEDV. Baseline indexed LV end-systolic volume (LVESVi) was the sole independent multivariate predictor of post-surgical LVEF. Undergoing MVR (vs MVr) and baseline indexed left ventricular mass (LVMi) and LV stroke volume were independent multivariate predictors of indexed MR-Rvol at follow-up, with undergoing MVR (vs MVr) being the most significant (p < 0.001). Reductions in LVEDV post mitral valve correction demonstrate a moderate correlation with baseline MR-Rvol (r = 0.642, p < 0.001) and strong correlation with the reduction in MR-Rvol (r = 0.734, p < 0.001) (Fig. 3).
Myocardial fibrosis
The CMR protocol was generally well tolerated and of good quality however T1/LGE sequences were incomplete or insufficient quality in some cases. Sufficient quality baseline T1 & LGE images were acquired in all controls, 25 and 29 MVr respectively and 20 MVR. After follow up, available paired baseline/follow-up indices of LGE (control, n = 18; MVr, n = 27; MVR, n = 20) and T1/ECV (control, n = 16; MVr, n = 23; MVR, n = 20) demonstrated no significant difference between groups at baseline or follow-up, Tables 2 and 4 respectively. The single MVr with insufficient baseline image quality for LGE quantification, detailed above, was sufficient to determine presence/type of LGE. Therefore, 50 surgical patients (30 MVr, 20 MVR) were available for subgroup analysis comparing baseline LGE+ (n = 21) vs LGE− (n = 29) demonstrating comparable baseline, change and follow-up volumetric and functional parameters (Additional file 5), except for a lower RVEF in LGE+ vs LGE− (42 ± 6.0% vs 47 ± 8.9% respectively, p = 0.024). Finally, as demonstrated above (Tables 5 and 6), indices of myocardial fibrosis at baseline were not independently predictive of post-surgical change in LVEDV or follow-up indexed LVEDV, MR-RVol or LVEF after multiple linear regression.
Discussion
To our knowledge, this is the first CMR study comparing cardiac reverse remodeling and quantified residual MR between MVr and MVR, using a longitudinal control group for comparison. Importantly, at baseline the study had naturally well-matched surgical groups, with no significant differences in cardiac parameters and co-morbidities. The study has five important findings: 1, both MVr and MVR resulted in comparable LV reverse remodeling and functional improvement; 2, RVEF was worse post-MVr vs controls compared to post-MVR; 3, MVR was the most significant predictor of lower residual MR, resulting in a greater reduction in and lower residual MR than MVr; 4, the cardiac reverse remodeling and residual MR findings post-surgery remained unchanged on subgroup analysis when those with at least moderate residual MR were excluded and separate subgroup analysis with patients undergoing concomitant CABG excluded and 5, baseline LVESVi was the sole multivariate predictor of LVEF post mitral surgery.
LV reverse remodeling
Our LV reverse remodeling findings demonstrate equivalency between MVr and MVR. These findings are in keeping with prior echocardiographic studies [10, 11, 34] and the only prior CMR study [23] to directly compare remodeling between MVr and MVR with chordal preservation at 3-months (n = 20) and 27-months (n = 14). Like previous studies [10, 11, 35], we demonstrated a significant decrease in LVEF post-operatively, finding no significant difference between surgical groups. Compared with baseline, an initial reduction in LVEF is common post mitral valve correction [5, 10, 11, 23, 36,37,38] and then can improve for up to 3–4 years post-mitral valve correction before plateauing [38]. In significant mitral regurgitation, the LV offloads into the lower-pressure/more-compliant left atrium resulting in an augmented LVEF. However, the LV is less efficient, with less blood appropriately leaving via the aortic valve, as evidenced by reduced effective-forward-LVEF. Therefore, although LVEF reduces initially post-operatively, the LV becomes more efficient as evidenced by improved post-surgical effective-forward-LVEF in this and previous studies [23]. However, we demonstrate, somewhat expectedly, patients with greater MR severity, pre-surgical adverse remodeling and lower LVEF at baseline demonstrate poorer LVEF at 6-months post mitral valve correction. In comparison with prior CMR studies [23, 37, 39], our surgical cohorts demonstrate greater baseline MR severity (with definitively severe mean CMR quantified MR) and/or LV dilatation. Prior observations potentially explain the variability between cohorts. Uretsky et al. [37] discussed that several observational studies noted patients who underwent mitral correction for echocardiography derived significant MR did not have severe MR on further assessment (with a significant percentage having mild MR) [19, 37, 40], with echocardiography’s comparative suboptimal inter/intra-observer variability and potential to overestimate MR severity deemed potentially contributory [19, 20, 22, 37, 41,42,43].
In keeping with prior research, we demonstrate baseline MR-Rvol as the most significant independent predictor of LV-end-diastolic reverse remodeling, showing a good correlation with reduction in LVEDV post mitral valve correction [37] and is also an independent predictor of post-surgical LVEDVi. We also found that patients with greater baseline MR-Rvol understandably have a greater reduction in MR-Rvol post-correction, resulting in greater LV reverse remodeling and lower residual LVEDVi. Additionally, we found patients with greater baseline LVMi prone to more residual MR. Increases in LVM occur as an adverse remodeling response to chronic LV volume overload in primary MR [36, 44]. Prior transthoracic echocardiography (TTE) studies assessing recurrent MR demonstrate numerically (but non-statistically significant) greater baseline LVMi in patients that develop recurrent primary MR post MVr [15, 45]. As CMR provides reference standard LVM and MR quantification, the superior accuracy afforded may demonstrate significant results that prior TTE studies could not. Larger CMR studies are now required to assess the reproducibility and significance of this finding. Reinforcing prior research [46], our findings demonstrate baseline LVESVi as the sole multivariate predictor of post-surgical LVEF at follow-up. This finding supports current international guidelines recommending surveillance of LV-end systolic dimensions/volumes in asymptomatic primary MR patients and its use to guide patient selection for early intervention. Further studies to clarify CMR thresholds for early intervention are now required.
RV reverse remodeling
In keeping with Uretksy et al. we found no significant change in indexed RVEDV/RVESV post-MVr/MVR [19, 37]. Two CMR studies by Uretsky et al., the majority of which had MVr, demonstrated no statistically significant change between pre- and post-operative RVEF. Unfortunately, small numbers of MVR operations resulted in no direct comparison between surgical techniques. Our study demonstrated lower RVEF post-MVr vs controls (p = 0.01), but no significant difference between the two surgical groups on direct comparison (p = 0.224). However, our MVr group underwent a proportionally greater number of tricuspid valve repairs compared to the MVR group (5 vs 2 respectively), which may have blunted the RVEF augmentation in the MVr group. There were, however, no significant differences in the quantified tricuspid regurgitant fraction between the groups pre-operatively or at follow-up to support this. Therefore, the lower RVEF in the MVr group vs controls may be because of a lower reduction in and greater residual MR-RF compared with the MVR group.
Differences in residual MR between surgical groups
MVR compared to MVr resulted in a greater reduction in MR-RF post-operatively and hence lower residual MR-RF. This finding is demonstrated on inter-group comparison and reinforced with regression analysis whereby undergoing MVR (vs MVr) was the most significant predictor of having less residual MR. Our findings of greater residual MR post-MVr are in keeping with prior echocardiographic studies [11, 47]. Importantly our central findings remain unchanged on subgroup analysis with direct comparison between surgical groups (Additional file 2) even after exclusion of patients with ≥ moderate residual MR.
Myocardial fibrosis
Our study demonstrates LGE prevalence comparable between groups and with cohorts of severe primary MR patients in prior studies, similarly demonstrating reduced baseline RVEF in patients with LGE + vs LGE− [48, 49]. Otherwise, we found no significant differences in volumetric/functional indices at rest/change/follow-up between LGE+ vs LGE−. Although mean baseline/follow-up LVEF was 5% lower in LGE+ vs LGE−, this was not statistically significant. Additionally on multiple linear regression, LGE presence was not shown to be an independent predictor of the change in LVEDVi or follow-up LVEDVi or LVEF. Prior studies vary, demonstrating reduced [49] and comparable LVEF between LGE+ vs LGE− MR patients [48]. Given LGE presence in MR patients correlates with MR severity and LV dilatation [48] and LVEF artificially increases with increasing MR severity [50, 51], this may impact results when comparing LVEF between LGE+ vs LGE− MR patients. However, as RVEF commonly decreases with increasing MR severity [50, 52] and CMR provides reference standard assessment [18], it is not unexpected that LGE+ MR patients demonstrate lower RVEF vs LGE− reproducibly across studies, whilst LVEF varies [48, 49], whereas mitral valve prolapse patients with ≤ mild MR demonstrate comparable RVEF and impaired LVEF in LGE+ vs LGE− patients [53].
Study strengths
To date, our study may provide the most accurate comparison of cardiac reverse remodeling and quantification of residual MR between MVr/MVR in primary MR by the use of CMR and naturally well-matched surgical groups at baseline.
CMR is the reference standard for biventricular assessment [18] and arguably more accurate at assessing MR severity than TTE [19, 20, 22, 37]. This disparity may increase post-operatively, as acoustic shadow artefacts from mitral prostheses can restrict MR assessment by TTE [54]. Guidelines therefore recommend combined transthoracic/transesophageal echocardiography for accurate prosthetic MR assessment [54], potentially reducing the accuracy of studies solely utilising TTE to compare residual MR between surgical groups. Using CMR, prosthesis-related distortions of the magnetic field can create the potential for volume and flow miscalculation. However, this was mitigated with consistent LV basal slice analysis and indirect MR quantification (LV-Aortic stroke volume method), as aortic PC-CMR, planned carefully to avoid artefact, increases the distance from the prosthesis and accuracy of volume/flow assessment [55].
Baseline cardiac indices, surgical risk scores and co-morbidities were similar between surgical groups, potentially minimising bias. To expand upon prior studies comparing MVr vs MVR [12, 13, 23], we examined indices of myocardial fibrosis, demonstrating parity between the groups, thereby providing additional evidence of minimal baseline bias between surgical groups. Patients undergoing MVR are typically older with more comorbidities than those referred for MVr. In primary MR, propensity matched studies performed to overcome this bias present conflicting results, with Gilinov et al.demonstrating no significant difference in long term survival and freedom from re-operation between MVr and MVR with chordal preservation [12], whilst Lazam et al. found lower operative mortality, better long term survival and fewer valve related complications post MVr, specifically in patients with flail leaflets [13].
Clinical implications
Perhaps controversially, our findings of comparable cardiac reverse remodeling following MVr and MVR and lower residual MR-RF post-MVR, could pose a potential challenge to the current recommendation of ‘repair whenever feasible’. However, this would need to be confirmed in much larger multi-center series. Given cardiopulmonary bypass time and cross-clamp time both correlate with post-operative mortality and morbidity [56, 57], relaxing the recommendations in selected cases (e.g. in patients with another indication for lifelong anticoagulation or in older patients where a tissue valve may last a lifetime) may not adversely affect cardiac reverse remodeling and might positively impact surgical outcomes. As MVR is arguably more durable, with less recurrent MR [11, 47] then our results, if replicated in larger studies or randomised trials could impact clinical practice.
Optimising the timing to intervene for asymptomatic primary MR is challenging. Our findings, that baseline LVESVi was the sole multivariate predictor of post-surgical LVEF, reinforces current international guidance advising monitoring cardiac remodeling by assessing the LV at end-systole [1, 2]. Further CMR studies are required to derive prognostic CMR thresholds to supplement decisions on early intervention in primary MR.
Study limitations
This was a single center, non-randomised, prospective observational study with the potential for bias as with all studies of this design, therefore larger multicenter studies are required to validate the findings. We specifically recruited patients with primary MR and those undergoing elective surgery, therefore our results may not be generalizable to those with secondary MR or undergoing emergency surgery. As a non-randomised study intrinsic baseline differences between the groups could not be controlled. However, as demonstrated in Table 1, there were no significant differences between the surgical groups in terms of age, sex, or comorbidities. Despite differences in the underlying leaflet pathology between surgical groups there was no significant difference in cardiac reverse remodeling. The group sizes are modest by comparison with prior longitudinal MVR and MVr outcome studies, however the use of CMR and its high reproducibility for volumes [18] and flow quantification [19,20,21] means that much smaller sample sizes are required to detect a change compared to standard TTE. Except for 2 patients who had complete chordal preservation with MVR, partial chordal preservation was performed as routine practice in our study; however, complete chordal preservation is the optimal technique [8], which if performed may have led to greater remodeling differences between the groups, potentially in favour of MVR. Six patients in the MVr group had residual MR-RF ≥ 30%, potentially biasing results against the MVr group, however our central findings comparing MVr and MVR remained unchanged following subgroup analysis after exclusion of these patients. T1/LGE sequences were incomplete/insufficient image quality in some cases, resulting in analysis on a reduced cohort, potentially introducing bias/reducing accuracy. Finally, reverse remodeling can potentially continue until 3–4 years post mitral valve correction [38] and our study specifically assessed cardiac reverse remodeling and functional changes after 6-months, so the study is unable to confirm that residual differences between surgical groups would result in different long term clinical outcomes.
There were differences in leaflets affected between groups, with the MVr group more typically having PMVL disease than the other groups. This is unsurprising given PMVL prolapse is more amenable to successful surgical repair [58] and international guidelines advise repair whenever feasible [1, 2], making this difficult to control for in an observational study. At baseline, compared to the watchful waiting control group, both surgical groups demonstrated worse NYHA functional class, quantitated MR, RVEF and had a greater proportion of patients in AF, demonstrating as expected that the surgical groups were at a more advanced stage on the MR severity spectrum.
Conclusion
In primary MR, MVR with chordal preservation may offer comparable cardiac reverse remodeling benefits at 6-months compared to MVr. Larger, multicenter CMR studies are required, which if the findings are confirmed, may have implications for future surgical practice.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Dr. Seth Uretsky served as the JCMR Guest Editor for this manuscript.
Abbreviations
- 6MWT:
-
6-Minute walk test
- AF:
-
Atrial fibrillation
- AMVL:
-
Anterior mitral valve leaflet
- CMR:
-
Cardiovascular magnetic resonance
- ECV:
-
Extracellular volume
- EDV:
-
End-diastolic volume
- EF:
-
Ejection fraction
- ESV:
-
End-systolic volume
- I:
-
Indexed to body surface area
- LA:
-
Left atrium
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- MR:
-
Mitral regurgitation
- MVr:
-
Mitral valve repair
- MVR:
-
Mitral valve replacement
- MVRar:
-
Mitral valve replacement after attempted repair
- NYHA:
-
New York Heart Association
- PC:
-
Phase contrast
- PMVL:
-
Posterior mitral valve leaflet
- RA:
-
Right atrium
- RF:
-
Regurgitant fraction
- RV:
-
Right ventricle
- R-vol:
-
Regurgitant volume
- TR:
-
Repetition time
- TTE:
-
Transthoracic echocardiography
References
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, Group ESCSD. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–91.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70(2):252–89.
Suri RM, Schaff HV, Dearani JA, Sundt TM 3rd, Daly RC, Mullany CJ, Enriquez-Sarano M, Orszulak TA. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg. 2006;82(3):819–26.
Shuhaiber J, Anderson RJ. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur J Cardiothorac Surg. 2007;31(2):267–75.
Kouris N, Ikonomidis I, Kontogianni D, Smith P, Nihoyannopoulos P. Mitral valve repair versus replacement for isolated non-ischemic mitral regurgitation in patients with preoperative left ventricular dysfunction. A long-term follow-up echocardiography study. Eur J Echocardiogr. 2005;6(6):435–42.
Goldman ME, Mora F, Guarino T, Fuster V, Mindich BP. Mitral valvuloplasty is superior to valve replacement for preservation of left ventricular function: an intraoperative two-dimensional echocardiographic study. J Am Coll Cardiol. 1987;10(3):568–75.
Rozich JD, Carabello BA, Usher BW, Kratz JM, Bell AE, Zile MR. Mitral valve replacement with and without chordal preservation in patients with chronic mitral regurgitation. Mechanisms for differences in postoperative ejection performance. Circulation. 1992;86(6):1718–26.
Yun KL, Sintek CF, Miller DC, Pfeffer TA, Kochamba GS, Khonsari S, Zile MR. Randomized trial comparing partial versus complete chordal-sparing mitral valve replacement: effects on left ventricular volume and function. J Thorac Cardiovasc Surg. 2002;123(4):707–14.
Okita Y, Miki S, Ueda Y, Tahata T, Sakai T, Matsuyama K. Comparative evaluation of left ventricular performance after mitral valve repair or valve replacement with or without chordal preservation. J Heart Valve Dis. 1993;2(2):159–66.
Senechal M, MacHaalany J, Bertrand OF, O’Connor K, Parenteau J, Dubois-Senechal IN, Costerousse O, Dubois M, Voisine P. Predictors of left ventricular remodeling after surgical repair or replacement for pure severe mitral regurgitation caused by leaflet prolapse. Am J Cardiol. 2013;112(4):567–73.
Pandis D, Grapsa J, Athanasiou T, Punjabi P, Nihoyannopoulos P. Left ventricular remodeling and mitral valve surgery: prospective study with real-time 3-dimensional echocardiography and speckle tracking. J Thorac Cardiovasc Surg. 2011;142(3):641–9.
Gillinov AM, Blackstone EH, Nowicki ER, Slisatkorn W, Al-Dossari G, Johnston DR, George KM, Houghtaling PL, Griffin B, Sabik JF 3rd, Svensson LG. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg. 2008;135(4):885–93.
Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, de Meester C, Barbieri A, Rusinaru D, Russo A, Pasquet A, Michelena HI, Huebner M, Maalouf J, Clavel MA, Szymanski C, Enriquez-Sarano M, Investigators M. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation. 2017;135(5):410–22.
Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, Thourani V, Argenziano M, Gammie JS, Mack M, Demers P, Atluri P, Rose EA, O’Sullivan K, Williams DL, Bagiella E, Michler RE, Weisel RD, Miller MA, Geller NL, Taddei-Peters WC, Smith PK, Moquete E, Overbey JR, Kron IL, O’Gara PT, Acker MA. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374(4):344–53.
Kim JH, Lee SH, Joo HC, Youn YN, Yoo KJ, Chang BC, Lee S. Effect of recurrent mitral regurgitation after mitral valve repair in patients with degenerative mitral regurgitation. Circ J. 2017;82(1):93–101.
David TE, David CM, Tsang W, Lafreniere-Roula M, Manlhiot C. Long-term results of mitral valve repair for regurgitation due to leaflet prolapse. J Am Coll Cardiol. 2019;74(8):1044–53.
Mohty D, Orszulak TA, Schaff HV, Avierinos JF, Tajik JA, Enriquez-Sarano M. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation. 2001;104(12 Suppl 1):I1–7.
Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21(16):1387–96.
Uretsky S, Gillam L, Lang R, Chaudhry FA, Argulian E, Supariwala A, Gurram S, Jain K, Subero M, Jang JJ, Cohen R, Wolff SD. Discordance between echocardiography and MRI in the assessment of mitral regurgitation severity: a prospective multicenter trial. J Am Coll Cardiol. 2015;65(11):1078–88.
Penicka M, Vecera J, Mirica DC, Kotrc M, Kockova R, Van Camp G. Prognostic implications of magnetic resonance-derived quantification in asymptomatic patients with organic mitral regurgitation: comparison with doppler echocardiography-derived integrative approach. Circulation. 2018;137(13):1349–60.
Lopez-Mattei JC, Ibrahim H, Shaikh KA, Little SH, Shah DJ, Maragiannis D, Zoghbi WA. Comparative assessment of mitral regurgitation severity by transthoracic echocardiography and cardiac magnetic resonance using an integrative and quantitative approach. Am J Cardiol. 2016;117(2):264–70.
Myerson SG, d’Arcy J, Christiansen JP, Dobson LE, Mohiaddin R, Francis JM, Prendergast B, Greenwood JP, Karamitsos TD, Neubauer S. Determination of clinical outcome in mitral regurgitation with cardiovascular magnetic resonance quantification. Circulation. 2016;133(23):2287–96.
Gelfand EV, Haffajee JA, Hauser TH, Yeon SB, Goepfert L, Kissinger KV, Delatorre R, Manning WJ. Predictors of preserved left ventricular systolic function after surgery for chronic organic mitral regurgitation: a prospective study. J Heart Valve Dis. 2010;19(1):43–50.
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–71.
Crapo RO, Casaburi R, Coates AL, Enright PL, MacIntyre NR, McKay RT, Johnson D, Wanger JS, Zeballos RJ. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Kilner PJ, Gatehouse PD, Firmin DN. Flow measurement by magnetic resonance: a unique asset worth optimising. J Cardiovasc Magn Reson. 2007;9(4):723–8.
Petersen SE, Aung N, Sanghvi MM, Zemrak F, Fung K, Paiva JM, Francis JM, Khanji MY, Lukaschuk E, Lee AM, Carapella V, Kim YJ, Leeson P, Piechnik SK, Neubauer S. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson. 2017;19(1):18.
Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17(1):29.
Maceira AM, Cosín-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference right atrial dimensions and volume estimation by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15(1):29.
Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22(1):17.
Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4(2):150–6.
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18(1):89.
Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B, Captur G, Francois CJ, Jerosch-Herold M, Salerno M, Teague SD, Valsangiacomo-Buechel E, van der Geest RJ, Bluemke DA. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson. 2020;22(1):87.
Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, Argenziano M, Gammie JS, Mack M, Ascheim DD, Bagiella E, Moquete EG, Ferguson TB, Horvath KA, Geller NL, Miller MA, Woo YJ, D’Alessandro DA, Ailawadi G, Dagenais F, Gardner TJ, O’Gara PT, Michler RE, Kron IL. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370(1):23–32.
Phillips HR, Levine FH, Carter JE, Boucher CA, Osbakken MD, Okada RD, Akins CW, Daggett WM, Buckley MJ, Pohost GM. Mitral valve replacement for isolated mitral regurgitation: analysis of clinical course and late postoperative left ventricular ejection fraction. Am J Cardiol. 1981;48(4):647–54.
Shafii AE, Gillinov AM, Mihaljevic T, Stewart W, Batizy LH, Blackstone EH. Changes in left ventricular morphology and function after mitral valve surgery. Am J Cardiol. 2012;110(3):403–8.
Uretsky S, Shah DJ, Lasam G, Horgan S, Debs D, Wolff SD. Usefulness of mitral regurgitant volume quantified using magnetic resonance imaging to predict left ventricular remodeling after mitral valve “correction.” Am J Cardiol. 2020;125(11):1666–72.
Joung KW, Kim SO, Nam JS, Moon YJ, Bae HJ, Chin JH, Jung SH, Choi IC. Changes in left ventricular ejection fraction after mitral valve repair for primary mitral regurgitation. J Clin Med. 2021;10(13):2830.
Liu B, Neil DAH, Bhabra M, Patel R, Barker TA, Nikolaidis N, Billing JS, Hayer M, Baig S, Price AM, Vijapurapu R, Treibel TA, Edwards NC, Steeds RP. Reverse myocardial remodeling following valve repair in patients with chronic severe primary degenerative mitral regurgitation. JACC Cardiovasc Imaging. 2022;15(2):224–36.
Acker MA. Clinical results with the Acorn cardiac restraint device with and without mitral valve surgery. Semin Thorac Cardiovasc Surg. 2005;17(4):361–3.
Biner S, Rafique A, Rafii F, Tolstrup K, Noorani O, Shiota T, Gurudevan S, Siegel RJ. Reproducibility of proximal isovelocity surface area, vena contracta, and regurgitant jet area for assessment of mitral regurgitation severity. JACC Cardiovasc Imaging. 2010;3(3):235–43.
Aplin M, Kyhl K, Bjerre J, Ihlemann N, Greenwood JP, Plein S, Uddin A, Tonder N, Host NB, Ahlstrom MG, Hove J, Hassager C, Iversen K, Vejlstrup NG, Lav MP. Cardiac remodelling and function with primary mitral valve insufficiency studied by magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2016;17(8):863–70.
Hassan AKM, Algowhary MI, Kishk AYT, Youssef AAA, Razik NA. Cardiac magnetic resonance assessment of mitral regurgitation severity appears better than echocardiographic imaging. Int J Cardiovasc Imaging. 2020;36(5):889–97.
Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol. 2008;52(5):319–26.
Suri RM, Clavel MA, Schaff HV, Michelena HI, Huebner M, Nishimura RA, Enriquez-Sarano M. Effect of recurrent mitral regurgitation following degenerative mitral valve repair: long-term analysis of competing outcomes. J Am Coll Cardiol. 2016;67(5):488–98.
Seldrum S, de Meester C, Pierard S, Pasquet A, Lazam S, Boulif J, Vanoverschelde JL, Gerber BL. Assessment of left ventricular reverse remodeling by cardiac mri in patients undergoing repair surgery for severe aortic or mitral regurgitation. J Cardiothorac Vasc Anesth. 2019;33(7):1901–11.
Grapsa J, Dawson D, Pandis D, Ntalarizou E, Cheung WS, Efthimiadis I, Zimbarra Cabrita I, Punjabi P, Nihoyannopoulos P. Mitral valve repair results in better right ventricular remodelling than valve replacement for degenerative mitral regurgitation: a three-dimensional echocardiographic study. Hellenic J Cardiol. 2012;53(4):279–86.
Constant Dit Beaufils AL, Huttin O, Jobbe-Duval A, Senage T, Filippetti L, Piriou N, Cueff C, Venner C, Mandry D, Sellal JM, Le Scouarnec S, Capoulade R, Marrec M, Thollet A, Beaumont M, Hossu G, Toquet C, Gourraud JB, Trochu JN, Warin-Fresse K, Marie PY, Schott JJ, Roussel JC, Serfaty JM, Selton-Suty C, Le Tourneau T. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation. 2021;143(18):1763–74.
Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, Little SH, Quinones MA, Lawrie GM, Zoghbi WA, Shah DJ. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72(8):823–34.
Tokodi M, Nemeth E, Lakatos BK, Kispal E, Toser Z, Staub L, Racz K, Soltesz A, Szigeti S, Varga T, Gal J, Merkely B, Kovacs A. Right ventricular mechanical pattern in patients undergoing mitral valve surgery: a predictor of post-operative dysfunction? ESC Heart Fail. 2020;7(3):1246–56.
Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, Scientificdocument committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14(7):611–44.
Le Tourneau T, Deswarte G, Lamblin N, Foucher-Hossein C, Fayad G, Richardson M, Polge AS, Vannesson C, Topilsky Y, Juthier F, Trochu JN, Enriquez-Sarano M, Bauters C. Right ventricular systolic function in organic mitral regurgitation: impact of biventricular impairment. Circulation. 2013;127(15):1597–608.
Figliozzi S, Georgiopoulos G, Lopes PM, Bauer KB, Moura-Ferreira S, Tondi L, Mushtaq S, Censi S, Pavon AG, Bassi I, Servato ML, Teske AJ, Biondi F, Filomena D, Pica S, Torlasco C, Muraru D, Monney P, Quattrocchi G, Maestrini V, Agati L, Monti L, Pedrotti P, Vandenberk B, Squeri A, Lombardi M, Ferreira AM, Schwitter J, Aquaro GD, Chiribiri A, Rodriguez Palomares JF, Yilmaz A, Andreini D, Florian A, Leiner T, Abecasis J, Badano LP, Bogaert J, Masci PG. Myocardial fibrosis at cardiac mri helps predict adverse clinical outcome in patients with mitral valve prolapse. Radiology. 2023;306(1):112–21.
Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, Khandheria BK, Levine RA, Marx GR, Miller FA Jr, Nakatani S, Quinones MA, Rakowski H, Rodriguez LL, Swaminathan M, Waggoner AD, Weissman NJ, Zabalgoitia M, American Society of Echocardiography’s Guidelines and Standards Committee, Task Force on Prosthetic Valves, American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, European Association of Echocardiography, European Society of Cardiology, Japanese Society of Echocardiography, Canadian Society of Echocardiography, American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, European Society of Cardiology, Japanese Society of Echocardiography, Canadian Society of Echocardiography. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22(9):975–1014.
Richau J, Dieringer MA, Traber J, von Knobelsdorff-Brenkenhoff F, Greiser A, Schwenke C, Schulz-Menger J. Effects of heart valve prostheses on phase contrast flow measurements in Cardiovascular Magnetic Resonance—a phantom study. J Cardiovasc Magn Reson. 2017;19(1):5.
Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F, Sisillo E. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(6):814–22.
Nissinen J, Biancari F, Wistbacka JO, Peltola T, Loponen P, Tarkiainen P, Virkkila M, Tarkka M. Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion. 2009;24(5):297–305.
Coutinho GF, Antunes MJ. Mitral valve repair for degenerative mitral valve disease: surgical approach, patient selection and long-term outcomes. Heart. 2017;103(21):1663–9.
Acknowledgements
We thank the CMR team at Leeds General Infirmary for their help in recruiting and scanning patients and the organisation of running this study. Special thanks to research nurse Fiona Richards for her assistance in recruitment and study organization. No grant support to declare.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TC: subject recruitment, data acquisition, analysis, and interpretation, drafting of manuscript and revisions. PC: subject recruitment, data acquisition and critical and intellectual revision of manuscript. LD: study design, subject recruitment, data acquisition and critical and intellectual revision of manuscript. MG: data analysis and critical and intellectual revision of manuscript. MP: data analysis and critical and intellectual revision of manuscript. LB: critical and intellectual revision of manuscript. CS: critical and intellectual revision of manuscript. AD: critical and intellectual revision of manuscript. AC: critical and intellectual revision of manuscript. NJ: critical and intellectual revision of manuscript. DH: critical and intellectual revision of manuscript. ED: critical and intellectual revision of manuscript. EL: critical and intellectual revision of manuscript. DS: critical and intellectual revision of manuscript. PS: critical and intellectual revision of manuscript. SP: critical and intellectual revision of manuscript. JPG: study design, critical and intellectual revision of manuscript and study supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by local research ethics committee (Yorkshire & The Humber-South Yorkshire 15/YH/0503) and complied with the Declaration of Helsinki with all participants providing informed written consent.
Consent for publication
Not applicable.
Competing interests
Dr. David Higgins is an employee of Philips Healthcare. All other authors have no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Operation variable comparisons between surgical groups. Surgical variables between groups and differences between groups when mitral valve replacement group was divided into those that received direct replacement and had mitral valve replacement after attempted repair.
Additional file 2.
Subgroup analysis of surgical groups after exclusion of patients with moderate residual MR. Subgroup analysis of follow up cardiac, haemodynamic and functional indices between mitral valve repairand mitral valve replacement after excluding cases with at least moderate residual mitral regurgitation.
Additional file 3.
Subgroup analysis with CABG patients excluded. Subgroup analysis comparing groups after exclusion of patients who underwent CABG: demonstrating the baseline patient characteristics (Table S3), baseline CMR parameters (Table S4), change in functional, haemodynamic and cardiac parameters from baseline to 6 month follow up assessment (Table S5) and residual functional, haemodynamic and cardiac parameters at 6 month follow up assessment (Table S6).
Additional file 4.
Subgroup analysis of surgical groups—Comparison of baseline parameters of surgical patients when divided into groups by follow-up left ventricular ejection fraction. Subgroup analysis of surgical groups demonstrating baseline parameters between those achieving LVEF ≥ 50% vs LVEF < 50% at follow-up (Table S7).
Additional file 5.
Subgroup analysis of surgical groups by baseline presence or absence of late gadolinium enhanced myocardium. Subgroup analysis of surgical groups comparing those with and without late gadolinium enhancement at baseline—baseline patient characteristics and surgical parameters (Table S8) and baseline, change and follow-up CMR and functional parameters (Table S9).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Craven, T.P., Chew, P.G., Dobson, L.E. et al. Cardiac reverse remodeling in primary mitral regurgitation: mitral valve replacement vs. mitral valve repair. J Cardiovasc Magn Reson 25, 43 (2023). https://doi.org/10.1186/s12968-023-00946-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-023-00946-9