Abstract
Background
Diagnosis and intervention of prediabetes is an emerging method for preventing diabetic progression and complications. Periodontitis has been reported to strongly correlate with the dysregulation of glucose metabolism. Nonetheless, the relationship between periodontal status and the prevalence of prediabetes as well as its prognosis remains elusive. This study aimed to investigate the association of periodontitis with the prevalence of prediabetes and furtherly explore the all-cause mortality of different periodontal status among patients with prediabetes.
Methods
The dateset from the National Health and Nutrition Examination Survey (NHANES) was utilized for our study. Participants were divided into two groups (with or without periodontitis) and further assigned into subgroups by different grades of periodontitis to analyze the association between periodontitis and prevalence of prediabetes. Then we analyzed the association between all-cause mortality and periodontitis among patients with prediabetes. Weighted multivariate logistic/Cox regression models were adopted in our study.
Results
A total of 15390 participants were included and divided into a periodontitis group (n = 5033) and a nonperiodontitis group (n = 10357). The results showed that participants with periodontitis had a higher risk of prediabetes. After adjusting for covariables, more severe periodontitis was positively related to prediabetes (moderate vs. no periodontitis: OR = 1.46, 95% CI: 1.29–1.65; severe vs. no periodontitis: OR = 1.62, 95% CI 1.31–2.01). Furtherly, we explored the association between all-cause mortality and periodontal status among patients diagnosed with prediabetes (n = 4518) and found that severe (HR = 1.806, 95% CI 1.19–2.74) and moderate periodontitis (HR = 2.42, 95% CI 1.95–3.01) were associated with elevated all-cause mortality among patients with prediabetes.
Conclusions
In general, the results suggest that periodontitis is positively associated with the prevalence and mortality of prediabetes. These results suggest that good management of periodontal status could be a potential strategy to reduce the occurrence and development of prediabetes.
Similar content being viewed by others
Introduction
Prediabetes is the stage of intermediate hyperglycemia characterized by particular parameters, including impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and specific scope of hemoglobin A1c (HbA1c) by The American Diabetes Association (ADA) [1]. Over the past few decades, the prevalence of prediabetes has been continuously increasing, with rates reaching as high as 43.5% in America [2]. Studies have shown that prediabetes patients have a higher risk of developing diabetes, cardiovascular complications and even death [3]. A recent study demonstrated that early management of prediabetes can alleviate the risk of progression to diabetes [4]. Despite this, attention and research toward prediabetes remains inadequate when compared to diabetes. Therefore, it is essential to identify more early interventions to prevent the progression of prediabetes to diabetes and cardiovascular complications.
As is commonly understood, individuals experiencing oral diseases are predisposed to metabolic dysregulation [5]. Periodontitis, a gradual and symptomless ailment, is a prevalent oral disease that results in tooth destruction and is generally irreversible [6]. The effects of periodontitis have been reported to be associated with cardiovascular complications in patients with dysglycemia, adversely impacting glycemic control [7]. A two-year follow-up study on the Indian population revealed that periodontitis was associated with an increased risk of poor glycemic control [8]. In addition, studies investigating the impact of periodontal treatment on glycemic control reported that a reduction in HbA1c of approximately 0.4% was found in diabetic patients with periodontal treatment [9]. Nevertheless, whether patients afflicted with periodontitis have a high risk of developing prediabetes and death has yet to be ascertained.
Currently, there are no effective methods to avert the onset of prediabetes. Hence, we attempted to find an early marker for prediabetes and intervene accordingly. Based on the findings of the association between periodontitis and glycemic control, we hypothesize that periodontitis is associated with a greater prevalence of prediabetes and its poor prognosis. Consequently, the purpose of this investigation is to ascertain whether periodontitis serves as a risk factor for the incidence and adverse outcomes of prediabetes, utilizing data from the National Health and Nutrition Examination Survey (NHANES).
Methods and participants
Study population
The NHANES (RRID:SCR_013201) is continuous survey research that provides population estimates related to the nutrition and health of adults and children in America. The survey employs a stratified, multistage probability design to recruit a representative sample of the American population. Data were obtained via personal structured interviews at home, health examinations at a mobile examination center, and specimen analyses in the laboratory. NHANES 1999–2004 and 2009–2014 were approved by The National Center for Health Statistics (NCHS) Ethics Review Board and conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from all participants. The acquisition and analysis of data was consistent with NHANES research requirements.
Identification of prediabetes and periodontitis
Prediabetes was diagnosed by one of the following four principles: (1) Self-reported prediabetes: participants answer “Yes” to the question “Have you ever been told prediabetes/IGT/IFG/borderline diabetes/blood sugar higher than normal but not high enough to be called diabetes in diabetes questionnaire”. (2) HbA1c: 5.7%–7.0%. (3) Fasting plasma glucose: 5.6–7.0 mmol/L. (4) oral glucose tolerance test: 7.8–11.0 mmol/L [10].
Periodontitis was defined as clinical attachment loss (CAL; the difference between pocket depth and gingival recession) ≥ 3 mm. This has been recommended for epidemiological studies as an extent measure of periodontitis and is henceforth referred to as CAL extent. The severity of periodontitis was defined by following Centers for Disease Control and Prevention in partnership with the American Academy of Periodontology (CDC–AAP) case definitions for surveillance of periodontitis [11].
Inclusion and exclusion process
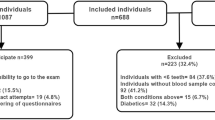
We used the data from the NHANES 1999–2004 and 2009–2014 cycles. First, 15390 patients with clear periodontal, prediabetes and other covariable data were included to explore the association between periodontitis status and the risk of prediabetes. Furthermore, we enrolled 4518 participants with prediabetes of 15390 sample to further explore the association between periodontitis status and all-cause mortality (Fig. 1).
Outcomes
The outcome of association analysis was diagnosed with prediabetes. The outcome of survival analysis was all-cause mortality with periodontitis or not among patients diagnosed with prediabetes. All-cause mortality was ascertained by linkage to National Death Index records through 2018. ICD-10 was used to determine the causes of death.
Assessment of covariates
Standardized questionnaires obtained information on sociodemographic characteristics, smoking status, and alcohol consumption. Never smokers were classified as those who reported smoking < 100 cigarettes during their lifetime. Those who smoked > 100 cigarettes in their lifetime were considered current smokers, and those who smoked > 100 cigarettes and had quit smoking were considered former smokers. Nondrinkers were defined as consuming no drinks per year. Heavy alcohol use was defined as ≥ 3 drinks per day for females, ≥ 4 drinks per day for males, or binge drinking [≥ 4 drinks on the same occasion for females, ≥ 5 drinks on the same occasion for males] on 5 or more days per month. Moderate alcohol use was defined as ≥ 2 drinks per day for females, ≥ 3 drinks per day for males, or binge drinking ≥ 2 days per month. Other frequencies of alcohol consumption were defined as mild alcohol use. Body mass index (BMI) was calculated from weight/height2 (kg/m2). Diabetes was defined as follows: (1) doctor diagnosed you as having diabetes; (2) HbA1c > 7%; (3) fasting glucose ≥ 7.0 mmol/L; (4) random blood glucose ≥ 11.1 mmol/L, (5) two-hour oral glucose tolerance test (OGTT) blood glucose ≥ 11.1 mmol/L; and (5) use of diabetes medication or insulin. Hyperlipidemia was defined as follows: (1) triglyceride ≥ 150 mg/dL, (2) cholesterol ≥ 200 mg/dL, (3) low-density lipoprotein (LDL)-cholesterol ≥ 130 mg/dL, (4) high-density lipoprotein (HDL)-cholesterol < 40 mg/dL (male); 50 mg/dL (female), and (5) use of hyperlipidemia medication. Coronary heart disease (CHD) was defined as self-reported CHD. Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated by the following formula: HOMA-IR = [fasting plasma glucose (mU/L) × fasting plasma glucose (mmol/L)]/22.5. Homeostasis model assessment-insulin sensitivity (HOMA-IS) was estimated by the following formula: HOMA-IS = 22.5/[fasting plasma glucose (mU/L) × fasting plasma glucose (mmol/L)]. In addition, strict laboratory analyses were performed, including the assessment of glucose, insulin, HbA1c, triglyceride, HDL, LDL, and C-reactive protein (CRP) levels at baseline. Further details of these measurements were documented in the NHANES Laboratory Medical Technologists Procedures Manual.
Statistical analysis
All analyses incorporated sample weights, strata, and primary sampling units to produce accurate national estimates. Sample characteristics are reported as the means (SEs) for normally distributed continuous variables, medians (interquartile ranges) for nonnormally distributed continuous variables, and percentages for categorical variables. Weighted multivariate logistic regression models were performed to estimate the OR and 95% confidence interval associated with periodontitis status and prediabetes with the first group as a reference. Weighted Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs of all-cause mortality in relation to periodontitis. Person-time was calculated as the interval between the NHANES interview date and the date of death or the end of the follow-up (31-December-2018), whichever occurred first. We constructed three statistical models. Model 1: no covariates were adjusted. Model 2: Age (continuous), gender (male or female), race/ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other Race), and education level (less than high school, high school, more than high school) were adjusted. Model 3: age (continuous), gender (male or female) and race/ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other Race), education level (less than high school, high school, more than high school), smoking status (never, former, or current), alcohol use (never, former, mild, moderate. severe), BMI (continuous), hypertension (no or yes), hyperlipidemia (yes or no), and CHD (yes or no) were adjusted. Any individuals with missing covariate data were not included in the analyses in Model 2 and Model 3 (listwise deletion). Furthermore, stratified analyses were conducted to examine whether the detected association differed by age, sex, and body mass index. All analyses were performed using R software (4.1.0). Two-sided P < 0.05 was considered statistically significant. The Bonferroni correction threshold was used to account for multiple comparisons.
Results
Participant characteristics
In this study, 15390 participants were enrolled in our analysis. There were 5033 participants with periodontitis and 10357 participants without periodontitis. Compared to participants without periodontitis, participants with periodontitis were more likely to be female, older, have a higher education level, hypertension, hyperlipidemia, CHD, be former alcohol user, smoke, have a higher BMI and glucose/insulin/HbA1c/HOMA-IR, have prediabetes, and have diabetes (Table 1).
Logistic regression between periodontitis and prediabetes
We conducted weighted multivariate logistic regression models to explore the association between periodontitis status and prediabetes (Table 2). Compared to nonperiodontitis, periodontitis was positively associated with prediabetes in all models (OR = 2.37, 95% CI 2.16–2.60; 1.45, 95% CI 1.30–1.61; 1.47, 95% CI 1.31–1.63). The p value of all models was < 0.001. We further stratified periodontitis into three statuses according to the CDC–AAP case definitions. In Model 1, all periodontitis statuses were significantly associated with prediabetes (OR = 1.42, 95% CI 1.15–1.77; 2.39, 95% CI 2.15–2.66; 2.74, 95% CI 2.26–3.32). The p value of all periodontitis statuses equaled 0.001. After adjusting for covariables, moderate and severe periodontitis were positively associated with prediabetes in Model 2 (OR = 1.46, 95% CI 1.30–1.65, 1.57, 95% CI 1.27–1.94) and Model 3 (OR = 1.46, 95% CI 1.29–1.65; 1.62, 95% CI 1.31–2.01). The p value of moderate and severe periodontitis was < 0.001 in Models 2 and 3. Furthermore, a significant association remained between periodontitis and prediabetes in most subgroups (Additional file 1: Table S1).
Cox regression between periodontitis and mortality of patients with prediabetes
A total of 4518 participants with prediabetes were included to further explore the correlation between periodontitis status and diabetes mortality by weighted Cox proportional hazards regression. The characteristics of these participants are shown in Additional file 1: Table S2. The average follow-up time was 128.41 ± 1.67 months (Additional file 1: Table S2). Compared to the nonperiodontitis group, participants with periodontitis were pivotally correlated with mortality of prediabetes in all models (HR = 2.41, 95% Cl 1.98–2.94, p < 0.001; 1.47, 95% Cl 1.20–1.80, p < 0.001; 1.28, 95% Cl 1.04–1.57, p = 0.02). After stratifying the status of periodontitis, moderate and severe periodontitis were positively correlated with mortality of prediabetes (HR = 2.42, 95% Cl 1.95–3.01, p < 0.001; 1.806, 95% Cl 1.19–2.74, p = 0.005). After further adjustments for age, sex, race, education level, alcohol use, BMI, hypertension, CHD, moderate periodontitis was significantly associated with mortality of prediabetes in Model 2 (HR = 1.45, 95% CI 1.17–1.80, p < 0.001) and Model 3 (HR = 1.25, 95% CI 1.00–1.55, p = 0.047) (Table 3). The Kaplan–Meier survival curve presented similar results (Fig. 2). In the subgroup analysis, older age, female sex, high education level, mild alcohol use, former smoking, hypertension, hyperlipidemia, and higher BMI were positively correlated with all-cause mortality of prediabetes (Additional file 1: Table S3).
Discussion
With the discovery of pathophysiological processes, periodontitis is regarded as a chronic inflammatory disease that is associated with other noninfected systemic diseases, including cardiovascular and metabolic diseases, rheumatic arthritis, and Alzheimer’s disease [12]. Chiu [13], Demmer [14], and Saito [15] et al. reported that periodontitis was significantly associated with the elevation of HbA1c and the development of insulin tolerance. To further explore this relationship, our study aimed to investigate the association between periodontitis and prediabetes.
The findings of this study indicate a significant association between moderate and severe periodontitis and the occurrence of prediabetes. Similarly, Desvarieux [16] and Shiva [17] et al. reported that periodontal infection and inflammation were associated with insulin resistance and impaired glucose tolerance in the nationally representative American and Iranian sample. Nonetheless, Pérez [18] et al. established a 3-year oral health status cohort enrolled overweight adults and no association between periodontitis and the risk of prediabetes/diabetes were reported. But the median follow-up time of this longitudinal study was 2.96 years which may be too short to influence the results of the prevalence risk of prediabetes/diabetes. And in this study, patients with moderate or severe periodontitis had a significant improvement in periodontal health during follow-up, which could have a great influence on the results. Simultaneously, Amar [19] and Loos [20] et al. demonstrate that severe periodontitis was associated with the elevation of HbA1c and HOMA-IR. We found that moderate periodontitis was also positively associated with prediabetes. On the one hand, this may be due to the uniform quality control of periodontal examinations in the NHANES study. On the other hand, we utilized the CDC–AAP criterion to define the status of periodontitis. This criterion can effectively promote the clinical periodontal condition. But it excluded buccal and/or lingual sites which can impact periodontal condition. Therefore, large-scale prospective studies are still needed to investigate the impact of periodontitis on the development and prognosis of prediabetes.
Previous studies just suggested severe periodontitis was significantly associated with mortality of type 2 diabetes mellitus [21]. In our study, we found that moderate periodontitis was found to be positively correlated with all-cause mortality in individuals with prediabetes. We assumed that chronic systemic inflammation aroused by periodontitis and blood sugar dysregulation promotes the progression of various systemic diseases, including cardiovascular diseases, resulting in increased mortality [22].
Although abundant research has revolved around periodontitis and IGT/insulin resistance, the mechanism remains unclear. A recent study demonstrated that the oral microbiota contained approximately 500–700 prevalent taxa [23]. Among them, Porphyromonas gingivalis (P. gingivalis) and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) were shown to be classical pathogens of periodontitis [24]. These pathogens activate the innate immune system in situ and secrete inflammatory factors [25]. Several studies have demonstrated that gingival crevicular fluid (GCF) and serum of patients with periodontitis contain high levels of TNF-α, IL-1β, IL-6, and other proinflammatory factors that play a pivotal role in the development of insulin secretion and resistance [26, 27]. Izumi [28] et al. built a periodontitis animal model affected by A. actinomycetemcomitans and performed an insulin/glucose tolerance test. The level of insulin resistance was significantly elevated. Furthermore, Luan et al. utilized P. gingivalis to establish an animal model [29]. They revealed that the levels of TNF-α and IL-6 were increased and the expression of PPARa, Irs1, and sitr1 was decreased, which suggested that glucose metabolism was dysregulated. Regarding clinical trials, Kocher [30] et al. conducted a multiple-center randomized study to explore the influence of nonsurgical periodontal treatment on prediabetes status. They revealed that treatment of periodontitis decreased the levels of HbA1c and CRP, which was consistent with our results. Kowall [31] et al. implied that periodontitis was only associated with poorly controlled diabetes, not prediabetes. The reason for the different conclusions may be the different races of the included participants and the different definitions of periodontitis. Therefore, further long-term follow-up is needed to clarify the role of root canal treatment of severe periodontitis in improving prediabetes.
In addition, these pathogens produce abundant virulence factors directly and via outer membrane vesicles (OMVs). These virulence factors, which activate macrophages and induce systemic inflammation, included lipopolysaccharide (LPS), protein, lipid, muramic acid, RNA, and DNA [32,33,34]. Subcutaneous chronic infusion of P. gingivalis LPS into high-fat diet-fed mouse enhanced insulin resistance of mouse [35]. Moreover, Ruiz [36] and Ivanovski [37] et al. noticed that GCF of patients with periodontitis had elevated concentrations of extracellular vesicles, especially OMV. OMV is a 50–250 nm particle derived from gram-negative bacteria [38]. OMV from P. gingivalis increased the expression of Toll-like receptors and nod-like receptors, which mediated insulin resistance signaling [38, 39]. Nonetheless, animal experiments and clinical trials to clarify the mechanism of virulence factors of these key pathogens in prediabetes are still lacking.
Clinical implication
The early diagnosis and intervention of prediabetes are important for preventing diabetic conversion and complications. Currently, the first-line therapy for prediabetes was lifestyle modification or metformin. Intensive lifestyle modification was more beneficial than metformin, such as diet, exercise, and weight loss [40]. However, there are no effective methods to decrease the morbidity of prediabetes. Our findings offer valuable insights into elucidating the association between periodontitis and prediabetes. As periodontitis is a common disease in the adult population, our findings provide a novel insight into the risk and treatment of prediabetes from the perspective of oral health status. Endocrinologists should pay attention to the oral condition of patients with prediabetes rather than only diabetes patients. Endocrinologists should also focus not only on oral health during the treatment of diabetes but also pay attention to the prevention of the development and deterioration of prediabetes through oral health management. Future studies are necessary to investigate whether therapeutic interventions aimed at addressing periodontitis can reverse or impede the progression of prediabetes to diabetes and thereby prevent subsequent negative health outcomes.
Limitations
This study explored the association between periodontitis and prediabetes. We conducted a large-scale sample retrospective study and adjusted for potential covariables to guarantee validity. However, some limitations remain. First, we only enrolled American adults in our study, which does not represent the population of another region. Second, although we considered every confounding variable, some variables still affected the prevalence and mortality of prediabetes. Third, the accuracy of periodontal pocket detection is limited by the type of probe. Therefore, the classification of periodontitis severity should be further demonstrated. We will conduct a further multicenter study and animal experiment to further validate our findings.
Conclusion
In summary, moderate and severe periodontitis were significantly associated with the risk of prediabetes. Moderate periodontitis was positively correlated with mortality in prediabetes patients. This suggests that periodontitis may be a new and valuable risk indicator of prediabetes. Early treatment of periodontitis may improve the prognosis of prediabetes.
Availability of data and materials
The data used in this study are from a public database at https://www.cdc.gov/nchs/nhanes/index.htm, which can be accessed by everyone through the links provided in the paper.
Abbreviations
- DM:
-
Diabetes mellitus
- NHANES:
-
National Health and Nutrition Examination Survey
- NCHS:
-
National Center for Health Statistics
- CAL:
-
Clinical Attachment Loss
- CDC–AAP:
-
Centers for Disease Control and Prevention in partnership with the American Academy of Periodontology
- BMI:
-
Body Mass Index
- OGTT:
-
Oral glucose tolerance test
- LDL:
-
Low-Density Lipoprotein
- HDL:
-
High-Density Lipoprotein
- CHD:
-
Coronary Heart Disease
- HOMA-IR:
-
Homeostasis Model Assessment-Insulin Resistance
- HOMA-IS:
-
Homeostasis Model Assessment-Insulin Sensitivity
- CRP:
-
C-Reactive Protein
- HR:
-
Hazard Ratio
- P. gingivalis:
-
Porphyromonas gingivalis
- A. actinomycetemcomitans:
-
Aggregatibacter actinomycetemcomitans
- GCF:
-
Gingival Crevicular Fluid
- OMV:
-
Outer membrane vesicles
- LPS:
-
Lipopolysaccharide
References
Rayburn WF. Diagnosis and classification of diabetes mellitus: highlights from the American Diabetes Association. J Reprod Med. 1997;42(9):585–6.
Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42:59–77.
Bansal N. Prediabetes diagnosis and treatment: a review. World J Diabetes. 2015;6(2):296–303.
Khetan AK, Rajagopalan S. Prediabetes. Can J Cardiol. 2018;34(5):615–23.
Nazir MA, AlGhamdi L, AlKadi M, et al. The burden of diabetes, its oral complications and their prevention and management. Open Access Maced J Med Sci. 2018;6(8):1545–53.
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–20.
Wu CZ, Yuan YH, Liu HH, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20(1):204.
Yadav A, Anmol MYG, Nk K, et al. Predictors of periodontal disease among rural Indian type 2 diabetic population. Diabetes Metab Syndr. 2020;14(4):463–7.
Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Investig. 2013;4(5):502–9.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(1):62–9.
Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–54.
Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31.
Chiu SY, Lai H, Yen AM, et al. Temporal sequence of the bidirectional relationship between hyperglycemia and periodontal disease: a community-based study of 5,885 Taiwanese aged 35–44 years (KCIS No. 32). Acta Diabetol. 2015;52(1):123–31.
Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: longitudinal results from the study of health in Pomerania (SHIP). Diabetes Care. 2010;33(5):1037–43.
Saito T, Shimazaki Y, Kiyohara Y, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res. 2004;83(6):485–90.
Demmer RT, Squillaro A, Papapanou PN, et al. Periodontal infection, systemic inflammation, and insulin resistance: results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 2012;35(11):2235–42.
Maboudi A, Akha O, Heidari M, et al. Relation between periodontitis and prediabetic condition. J Dent. 2019;20(2):83–9.
Joshipura KJ, Munoz-Torres FJ, Dye BA, et al. Longitudinal association between periodontitis and development of diabetes. Diabetes Res Clin Pract. 2018;141:284–93.
Benguigui C, Bongard V, Ruidavets JB, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. 2010;37(7):601–8.
Teeuw WJ, Kosho MX, Poland DC, Gerdes VE, Loos BG. Periodontitis as a possible early sign of diabetes mellitus. BMJ Open Diabetes Res Care. 2017;5(1): e000326.
Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1):27–32.
Polak D, Shapira L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2018;45(2):150–66.
Bui FQ, Almeida-da-Silva C, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27–35.
Zambon JJ. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1(1):879–925.
Santos TR, Foss-Freitas MC, Nogueira-Filho GR. Impact of periodontitis on the diabetes-related inflammatory status. J Can Dent Assoc. 2010;76: a35.
Liu X, Giza CC, Vrana KE. High efficiency transient expression of eukaryotic genes: use of an HSV-1 immediate early promoter (ICP4). Biotechniques. 1990;9(2):168–73.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46.
Komazaki R, Katagiri S, Takahashi H, et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci Rep. 2017;7(1):13950.
Kang N, Zhang Y, Xue F, et al. Periodontitis induced by Porphyromonas gingivalis drives impaired glucose metabolism in mice. Front Cell Infect Microbiol. 2022;12: 998600.
Kocher T, Holtfreter B, Petersmann A, et al. Effect of periodontal treatment on HbA1c among patients with prediabetes. J Dent Res. 2019;98(2):171–9.
Kowall B, Holtfreter B, Volzke H, et al. Pre-diabetes and well-controlled diabetes are not associated with periodontal disease: the SHIP Trend Study. J Clin Periodontol. 2015;42(5):422–30.
Cecil JD, O’Brien-Simpson NM, Lenzo JC, et al. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front Immunol. 2017;8:1017.
Koeppen K, Hampton TH, Jarek M, et al. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. Plos Pathog. 2016;12(6): e1005672.
Veith PD, Chen YY, Gorasia DG, et al. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res. 2014;13(5):2420–32.
Blasco-Baque V, Garidou L, Pomie C, et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut. 2017;66(5):872–85.
Chaparro PA, Weber AL, Realini FO, et al. Molecular signatures of extracellular vesicles in oral fluids of periodontitis patients. Oral Dis. 2020. https://doi.org/10.1111/odi.13338.
Han P, Bartold PM, Ivanovski S. The emerging role of small extracellular vesicles in saliva and gingival crevicular fluid as diagnostics for periodontitis. J Periodontal Res. 2022;57(1):219–31.
Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19(22):2645–55.
Cecil JD, O’Brien-Simpson NM, Lenzo JC, et al. Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens. PLoS ONE. 2016;11(4): e0151967.
Echouffo-Tcheugui JB, Perreault L, Ji L, Dagogo-Jack S. Diagnosis and management of prediabetes: a review. JAMA. 2023;329(14):1206–16.
Acknowledgements
The authors thank all the staff and participants in NHANES 1999–2004 and 2009–2014 for their contribution to the donation, collection, and sharing of data.
Funding
The work was supported by grants from the National Natural Science Foundation of China (Nos. 82000301 to L.Z. Y), Natural Science Foundation of Hunan Province, China (Nos. 2021JJ40938 to L.Z.Y).
Author information
Authors and Affiliations
Contributions
LT analyzed the data and drafted the manuscript. JL drafted the manuscript. ZL ensured that the descriptions were accurate and agreed upon by all the authors. All authors made substantial contributions to the interpretation of the data and provided critical revisions to the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. NHANES 1999-2004 and 2009-2014 were approved by The NCHS Ethics Review Board (Protocol #98-12, Continuation of protocol #2005-06, Protocol #2011-17, and Continuation of protocol #2011-07), and informed consent was obtained from all participants. This study was based on publicly available data. The acquisition and analysis of data were consistent with NHANES research requirements.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary tables: Table S1. Subgroup analyses of the associations between periodontitis and the risk of prediabetes. Table S2. Baseline characteristics of included participants with prediabetes in NHANES 1999–2004 and 2009-2014. Table S3. Subgroup analyses of the associations between periodontitis and the mortality of prediabetes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tan, L., Liu, J. & Liu, Z. Association between periodontitis and the prevalence and prognosis of prediabetes: a population-based study. J Transl Med 21, 484 (2023). https://doi.org/10.1186/s12967-023-04340-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04340-y