Abstract
As one of the most abundant epigenetic modifications in RNA, N6-methyladenosine (m6A) affects RNA transcription, splicing, stability, and posttranscriptional translation. Methyltransferase-like 3 (METTL3), a key component of the m6A methyltransferase complex, dynamically regulates target genes expression through m6A modification. METTL3 has been found to play a critical role in tumorigenesis, tumor growth, metastasis, metabolic reprogramming, immune cell infiltration, and tumor drug resistance. As a result, the development of targeted drugs against METTL3 is becoming increasingly popular. This review systematically summarizes the factors that regulate METTL3 expression and explores the specific mechanisms by which METTL3 affects multiple tumor biological behaviors. We aim to provide fundamental support for tumor diagnosis and treatment, at the same time, to offer new ideas for the development of tumor-targeting drugs.
Video Abstract
Similar content being viewed by others
Background
m6A modification is the most abundant methylation modification of eukaryotic RNA [1,2,3] discovered in 1974 [4]. It can alter the stability of RNA, induce RNA conformational changes, regulate protein-RNA interactions, and manipulate microRNA maturation. Unlike the methylation modifications that occur at the 3’UTR or 5’Cap of RNA, the m6A modification deposited on the N6 position of RNA adenylate is highly selective and conserved as an internal RNA modification. Mostly, m6A modification occurs at the shared RNA motif of RRACH (R = A, G/U; R = A/G; H = A/U/C) [5]. m6A is mainly enriched in exons, near the stop codon, and at the 3’UTR. m6A modification is a dynamic and reversible process, mediated by the methyltransferase complex “writers”, the demethylase “erasers”, and the m6A binding protein “readers”.
Although m6A methyltransferase have been identified as a complex of several proteins, it was not until 1997 that MT-A70, a protein subunit of METTL3 with the methylation substrate S-adenosylmethionine (SAM), was first isolated from HeLa cells [6]. It is generally accepted that the m6A methyltransferase complex consists of seven evolutionarily conserved members, including METTL3, METTL14, WTAP, VIRMA, RBM15, ZC3H13 and Hakai.
METTL3, the core component of the catalytic methyltransferase complex, is recruited to the target RNA by the remaining components, using SAM as a donor to transfer methyl to the RRACH motif. METTL3 catalyzes the methylation of RNA, which further regulates the expression of target genes and influences cell biological behavior. Thus, METTL3 is involved in a wide range of physiological and pathological processes, such as haematopoiesis, immunity, viral infection and replication. In particular, METTL3 expression is elevated in a variety of tumor, enhancing the m6A levels of different target genes. The change in m6A content leads to altered expression of oncogenes/anti-oncogenes and activation of various pro-oncogenic signaling pathways. This study is dedicated to exploring the regulation of METTL3 and the specific mechanisms by which METTL3 affects tumor biological behavior, thus providing some reference for clinical development and application of drugs targeting METTL3.
Structure and distribution of METTL3
The human METTL3 gene is located at 14q11.2 and contains 11 exons. The full-length METTL3 protein consists of 580 amino acids. The primary structure contains 20 different amino acids, with 58 leucine, 46 serine, 43 aspartic acid, 42 alanine, and 38 glutamic acid, forming a protein monomer with a molecular weight of 64 kDa.
METTL3 is a highly conserved protein consisting of a leading helix (LH) (1–34), a nuclear localization signal (NLS) (209–215), a zinc finger domain (ZFD) and a methyltransferase domain (MTD) (369–580/357–580/358–580) (Fig. 1A, B). The LH and NLS of METTL3 work in concert with other members of the methyltransferase complex to facilitate the entry of METTL3 into the nucleus [7]. The ZFD in METTL3 consists of two CCCH-type zinc fingers, ZnF1 (259–298) and ZnF2 (299–336), which are responsible for the specific recognition of RNA and stabilization of the catalytic activity of methyltransferases [8]. The MTD of METTL3, the catalytic core of methylation, is a classical α-β-α sandwich fold consisting of four α helices (α1, α2, α4 on one side and α3 on the other) and eight β folds (in the order β1↑, β8↑, β7↑, β2↑, β3↑, β5↓, β4↑ and β6↑), three 310 helices and three loops with low sequence similarity, named gate loop 1 (residues 396–410), interface loop (residues 462–479) and gate loop 2 (residues 507–515).
Further studies showed that METTL3 contains one acetylation site Ser2 [9], eight phosphorylation sites Ser2, Ser43, Ser48, Ser50, Ser219, Ser243, Thr348, Ser350 [7] and four SUMOylation sites Lys177, Lys211, Lys212, and Lys21 [10].
In eukaryotes, the intracellular distribution of METTL3 varies among different cellular sources. METTL3 is predominantly found in the nucleus and a small amount of METTL3 is expressed in the cytoplasm [11] (Fig. 1C).
How METTL3 mediates m6A modification
Sequence analysis shows that METTL3 belongs to the class I MTase family and has weak methyltransferase activity in vitro. Therefore, METTL3 needs to cooperate with other members of “writers” to exert high catalytic activity. The formation of the complex effectively enhances the METTL3-mediated enzymatic reaction leading to m6A modification of target RNAs.
Compared with METTL3 alone, the METTL3-METTL14 complex greatly enhances methyltransferase activity through synergistic effects [12]. METTL3 and METTL14 form a butterfly-like antiparallel heterodimer of approximately 40 Å width and 70 Å length in an asymmetric unit, interacting with extensive hydrogen bonding and producing a positively charged groove. The complex binds to SAM. Of these, SAM is only visible in the METTL3 pocket, demonstrating that METTL3 acts as the catalytic core, while METTL14 acts as the RNA-binding platform [13]. The residues 357–580 of METTL3 (MTD3) and the residues 111–456 of METTL14 (MTD4) interact with each other to form a stable complex. METTL14 stabilizes the METTL3 structure and interacts with the substrate RNA [14], facilitating the transfer of methyl groups from METTL3 to the target RNA.
Additionally, METTL3-METTL14 forms a complex with other cofactors to achieve full enzymatic activity. The METTL3-METTL14 heterodimer accumulates in the nuclear speckles and binds to the target RNA under the guidance of WTAP [7]. Next, the METTL3-METTL14-WTAP complex is recruited by VIRMA and mediates preferential RNA methylation near the 3’UTR and stop codons [15]. At the same time, METTL3 binds to RBM15 and recruits the methyltransferase complex to specific sites in the RNA. This results in selectively methylation of adjacent RRACH motifs in the target RNA while distant ones are ignored [16].
Regulation of METTL3
The expression of METTL3 is regulated via several mechanisms, including gene activation, initiation of transcription, transcript modification and transport, transcript translation and posttranslational modifications. Among these, histone modification and DNA methylation modulate METTL3 gene activation. Transcription factors regulate the RNA level of METTL3 through transcription initiation. Noncoding RNAs then change the expression of METTL3 at the posttranscriptional level. Furthermore, posttranslational modifications such as phosphorylation alert the content of METTL3 at the protein level. In addition, some chemicals are involved in adjusting METTL3 expression, but the exact mechanism is not yet clear.
Histone modifications
In chromosomes, the proteins entangled in the DNA double strand are known as histones. Histones are octamers consisting of four components, H2A, H2B, H3 and H4. The N-terminal protein tails of the histone components have sites of modification such as methylation, acetylation and lactylation (Fig. 2A). Studies have shown that different modifications to the N-terminal protein tail of histones have different effects on the transcriptional activation of METTL3, which in turn affects the expression of METTL3.
In colorectal cancer, H3K4me3 is enriched in the METTL3 promoter region and promotes METTL3 expression through methylation [17]. In cervical cancer, WDR5 was found to mediate H3K4me3 histone modification of the METTL3 promoter and induce METTL3 transcriptional activation [18].
Wang et al. found abundant H3K27ac signals in the METTL3 promoter region in gastric cancer cells. Further experiments revealed that P300 mediated H3K27 acetylation of METTL3 and induced upregulation of METTL3 RNA expression at transcriptional level [19].
Xiong et al. found that METTL3 expression was elevated in tumor-infiltrating myeloid cells as lactate accumulated in the tumor microenvironment. Mechanistically, H3K18la, which is abundant in the METTL3 promoter region, elevates the expression of METTL3 by histone lactylation [20].
DNA methylation
In multicellular eukaryotes, DNA methylation is the covalent bonding of a methyl group at the cytosine 5 carbon position of a genomic CpG dinucleotide in the presence of DNA methyltransferases [21]. Due to the CpG-rich island in the promoter region of the METTL3 gene, METTL3 can be transcriptionally regulated by DNA methylation.
Cigarette smoke condensate (CSC) decreases methylation within the CPG islets of the METTL3 gene. CSC increases transcription factor NFIC in the METTL3 promoter region and elevates METTL3 transcription in pancreatic ductal adenocarcinoma cells [22] (Fig. 2B).
Transcription factors
Reportedly, multiple potential transcription factor binding sites exist in the METTL3 promoter region. In tumors, a variety of transcription factors have been identified that regulate the initiation of METTL3 transcription and its expression at the RNA level.
In cervical cancer cells, the transcription factor TBP binds directly to the promoter of METTL3 and upregulates METTL3 expression [23]. In gastric cancer, phosphorylated Smad2/3 is increased in the nucleus and initiates transcription of METTL3 [24]. In colorectal cancer, FOXD3 acts as a transcription activator to increase METTL3 expression [25]. Further studies revealed that RUNX3 interacts with the METTL3 promoter and activates circMETTL3 transcription in colorectal cancer [26]. In acute myeloid leukaemia, YY1 binds to the promoter of METTL3 by liquid-liquid phase separation, leading to elevated METTL3 expression [27]. In glioblastoma, EGR1 contributes to the high expression of METTL3 by binding straightly to the promoter of METTL3 [28]. In bladder cancer, activated c-JUN is recruited to the METTL3 promoter to enhance METTL3 transcription [29] (Fig. 2C).
Noncoding RNA
Noncoding RNAs are a class of RNAs that do not encode proteins, including microRNAs (miRNAs), circular RNAs (circRNAs), tRNA-derived small RNA fragments (tRFs), and long noncoding RNAs (lncRNAs), etc. They can bind to the 3’UTR of target genes and affect gene expression. Noncoding RNAs are one of the important factors modulating METTL3 expression at the posttranscriptional level. For example, miR-302a directly targets the 3’UTR of METTL3 in M1-type macrophages and reduces the intracellular METTL3 RNA content [30]. In non-small cell lung cancer, circVMP1 acts as a sponge for miR-524-5p. circVMP1 releases METTL3 from the repression of miR-524-5p and enhances the protein expression of METTL3 [31]. In addition, the small RNA fragment tRF-1001 targets METTL3 and decreases the RNA level of METTL3 [32]. The effects of different noncoding RNAs on METTL3 are shown in the table below (Table 1).
Posttranslational modifications
The covalent binding of chemical groups or small molecules proteins to specific sites of amino acid sequences causes post-translational modification of proteins, which is essential for protein maturation and expression. Recent studies have shown that METTL3 is modified by phosphorylation, SUMOylation, and lactylation modifications.
ERK directly phosphorylates METTL3 at S43, S50 and S52. The zinc finger domain of ERK interacts with USP5 to reduce the level of ubiquitination-mediated degradation of METTL3, enhancing the stability of METTL3 [50]. Activated ataxia-telangiectasia mutated (ATM) kinase also upregulates METTL3 expression by phosphorylation [51].
SUMOylated sites K177, K211, K212 and K215 have been identified on the amino acid sequence of METTL3. The SUMOylation of METTL3 does not affect METTL3 expression, localization or binding to other methyltransferase complex components, but inhibits METTL3 methyltransferase activity [10]. Furthermore, Xv et al. found that the SUMO-conjugating enzyme E2 UBC9 promoted SUMO1-mediated SUMOylation of METTL3. Decreased methyltransferase activity of SUMOylated METTL3 results in reduced intracellular m6A content [52] (Fig. 2D).
In addition, the K281 and K345 sites of METTL3 can be directly modified by lactylation, which allows METTL3 to acquire stronger RNA binding capacity and promote m6A methylation of target RNAs [20].
Chemical substances
A variety of chemical substances, such as fatty acids and metal contaminants, are involved in regulating METTL3 expression. However, the underlying mechanism by which chemicals alter METTL3 expression is unclear. Endogenous arachidonic acid LXA4, a small lipid molecule secreted by prostate cancer cells, significantly inhibits the RNA and protein expression of METTL3 in mouse peritoneal macrophages [53]. Chronic hexavalent chromium exposure upregulates METTL3 expression in mouse and human lung tumors [54]. In addition, in lung cancer, β-elemene targets and inhibits METTL3 expression at both RNA and protein levels [55]. In oral squamous cell carcinoma, Oxymatrine targets METTL3 and suppresses its expression [56] (Fig. 2D).
Role of METTL3 in tumor cell proliferation
One of the fundamental features of tumors is the unlimited proliferative potential of tumor cells. The continuous release of growth signals within tumor cells promotes the cell cycle process and induces mitosis. Activation of oncogenes and inactivation of tumor suppressor genes accelerate cell cycle progression by regulating the distribution of growth signals. In a wide range of tumors, METTL3 relies on m6A modification to regulate the expression of classical oncogenes such as AKT and MYC to promote cell proliferation. Moreover, classical tumor suppressor genes such as p53 are under-expressed in tumors due to the negative regulation of m6A modification mediated by METTL3. Furthermore, METTL3 directly regulates the expression of cell cycle proteins and affects tumor growth (Fig. 3).
AKT signaling
AKT is a serine/threonine kinase that is a central node of many signaling pathways and can phosphorylate a variety of downstream proteins. A wide range of growth signals can activate AKT, and then activated AKT promotes proliferation and inhibits apoptosis. AKT has been previously reported to be modified by various posttranslational modifications, such as O-GlcNAcylation, SUMOylation, acetylation, and ubiquitination [57, 58]. Currently, METTL3-mediated m6A regulation of AKT signaling is of interest.
In endometrial cancer, reduced expression of METTL3 leads to increased proliferation and tumorigenicity. Mechanistically, reduced m6A mediated by METTL3 results in diminished expression of the AKT negative regulator PHLPP2 and increased expression of the AKT positive regulator mTORC2. These results identify METTL3 as a regulator of AKT signaling [59].
However, the regulation of AKT activity by METTL3 varies in different tumors. In bladder cancer, knockdown of METTL3 significantly inhibits proliferation in vivo and in vitro. This may be associated with the significant upregulation of the tumor suppressors LHPP and NKX3-1 at both the RNA and protein levels, which inhibits the phosphorylation of downstream AKT [60]. In uveal melanoma, METTL3 induces AKT phosphorylation and promotes cell cycle progression by upregulating the expression of the target gene c-Met via m6A [61]. In pancreatic cancer, METTL3 synergistically induces SMS expression with IGF2BP3 and promotes AKT phosphorylation, thus enhancing tumor cell proliferation [62].
In bladder cancer, METTL3 accelerates pri-miR221/222 maturation through m6A modification, which downregulates PTEN expression through miR221/222 binding to its 3’UTR and stimulates proliferation in vitro and in vivo [63].
METTL3 in ovarian cancer cells suppresses PTEN expression by accelerating miR-126-5p maturation. The decrease of PTEN content leads to the activation of PI3K/AKT signaling pathway, which in turn elevates phosphorylated AKT and its downstream effectors driving ovarian cancer growth [64]. The same mechanism is also found in lung cancer [42], retinoblastoma [65], and esophageal cancer [66].
In summary, the regulation of AKT signaling by METTL3-mediated m6A plays an important role in tumor cell proliferation, but the mechanisms involved remain to be further explored.
MYC regulation
MYC is a powerful oncogene that drives tumorigenesis and encodes a member of the bHLH-zip transcription factors that act as master transcription factors. In human cancers, dysregulated expression of MYC greatly promotes tumor cell proliferation.
Through m6A, METTL3 can either directly upregulate MYC expression or enhance the expression of AFF4, the transcription promoter of MYC, to regulate the expression of MYC in bladder cancer [67]. In non-small cell lung cancer, METTL3 enhances lncRNA DLGAP1-AS2 stability via m6A modification and interacts with YTHDF1 to improve MYC RNA stability [68]. In colorectal cancer, METTL3 cooperates with IGF2BP2 to increase the stability of SOX2 RNA, which promotes the transcription of MYC and enhances the self-renewal and proliferation of tumor cells [69].
Interestingly, the mechanism by which METTL3 enhances MYC expression in an m6A-dependent manner to regulate tumor growth also exists in cervical cancer [70], bladder cancer [71], acute myeloid leukaemia [72], and oral squamous carcinoma [73].
As a transcription factor, MYC is mainly located in the nucleus and lacks a specific small molecule active site. Therefore, it is difficult to inhibit its activity or to target MYC with specific monoclonal antibodies. Thus, studies on the regulatory effect of METTL3 on MYC expression, mediated either directly or indirectly by m6A, in the context of tumor growth are expected to provide a new therapeutic strategy for tumors.
p53 influence
As a classical tumor suppressor, wild-type p53 monitors the integrity of genes and regulates cell cycle progression. Once cellular DNA is damaged, the p53 protein binds to the corresponding part of the gene and inhibits the activity of the cell cycle proteins, arresting the cell in G1 phase and thus inhibiting malignant proliferation. It is now believed that p53 is regulated by METTL3 in different ways, which affects tumor growth.
METTL3, which is highly expressed in hepatocellular carcinoma cells, inhibits RDM1 expression by increasing the m6A modification of its RNA. In fact, RDM1 binds to p53, which enhances p53 protein stability and inhibits phosphorylation activation of the Ras/Raf/ERK pathway to induce G2/M cell cycle arrest and impair the capability of cell proliferation [74]. Similarly, METTL3 reduces the expression of the p53-binding protein BATF2 via m6A modification to accelerate the growth of gastric cancer. Specifically, the reduced binding of BATF2 to p53 induced downregulates the stability of p53 and inhibits the phosphorylation activation of ERK in gastric cancer cells [75].
Cell cycle protein impact
In addition to manipulating p53 expression levels, METTL3 also affects mitosis in eukaryotic cells by regulating the expression of cell cycle proteins.
The expression of METTL3 is significantly increased in the M-phase of cervical cancer cells. Enhanced m6A modification mediated by METTL3 accelerates translation of CDC25B and increases the proportion of tumor cells in G2/M stage, leading to malignant cancer growth [76]. The same mechanism is also observed in head and neck squamous cell carcinoma [77]. In glioblastoma, ADAR1 elevates CDK2 expression by binding to CDK2, while METTL3 upregulates ADAR1 protein expression and promotes G1/S phase transition [78]. In breast cancer, METTL3, a host gene for circMETTL3, regulates circMETTL3 expression in an m6A-dependent manner. CircMETTL3 is a competitive endogenous RNA of miR-31-5p that upregulates the expression of its target gene CDK1 and promotes tumor cell proliferation [79].
In colorectal cancer, METTL3 regulates long chain noncoding RNA HNF1A-AS1 expression, accelerating cell cycle progression and promoting proliferation. Mechanistically, HNF1A-AS1 enhances CCND1 expression by inhibiting PDCD4 or competitively sponging miR-93-5p [80].
METTL3 directly modulates the cell cycle to promote tumor cell proliferation, which undoubtedly provides a new perspective in our understanding of malignant tumor proliferation.
Role of METTL3 in tumor cell migration and invasion
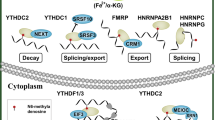
Due to genetic instability and heterogeneity, metastatic tumors physically disseminate from the primary site to distant tissues via capillaries or capillary lymphatics. Furthermore, metastatic tumors tolerate microenvironments that are not conducive to their growth and accomplish a variety of biological behaviors in the new microenvironment [81]. Significantly high expression of METTL3 was observed in a variety of metastatic tumors [82]. Mechanistically, METTL3 regulates the expression of epithelial-mesenchymal transition (EMT)-related genes through m6A modification. Moreover, METTL3 activates metastasis-associated oncogenic signaling pathways and upregulates related transcription factors. In addition, METTL3 regulates the ubiquitination of metastasis-related target genes, thereby promoting tumor metastasis (Fig. 4).
EMT- related proteins
EMT is an important step in cancer cell metastasis and is essentially characterized by the loss of E-cadherin. Snail, Slug, Twist, Zeb1, and Smad3 regulate E-cadherin expression as EMT-related transcription factors. TGFβ acts as a prominent inducer of EMT development.
Lin et al. found that METTL3 inhibited E-cadherin expression by regulating Snail splicing to promote Snail translation and enhance protein stability, which affected EMT in nasopharyngeal carcinoma [83]. Furthermore, Xv et al. demonstrated that SUMOylated METTL3 enhanced m6A modification to improve Snail RNA stability and expression, which promoted EMT progression in hepatocellular carcinoma cells [52]. In gastric cancer cells, METTL3 increases Snail and Slug expression to repress E-cadherin transcription, promoting the occurrence of EMT and tumor metastasis [24]. Interestingly, Li et al. found that METTL3 could induce EMT by promoting TGFβ1 dimer formation, activating the TGFβ1/Smad2/Snail signaling pathway [84].
JUNB is an important transcriptional regulator of EMT. In lung cancer, METTL3 affects the RNA expression and stability of JUNB through m6A modification, which stimulates the EMT process [29].
In colorectal cancer, expression of the non-coding RNA circ1662 is upregulated by METTL3-mediated m6A modification. Highly expressed circ1662 straightly binds to YAP1 and contributes to the accumulation of YAP1 in the nucleus. YAP1 negatively regulates Smad3 expression to mediate EMT and tumor metastasis [85]. Pan et al. further showed that METTL3 induced CRB3 degradation through m6A modification. Reduced CRB3 altered the state of the Hippo pathway, promoting YAP nuclear localization and EMT in colorectal carcinoma [86].
In gastric cancer, METTL3 cooperates with HuR to enhance the RNA and protein stability of ZMYM1 in an m6A-dependent manner. ZMYM1 recruits the CtBP/LSD1/CoREST complex to the E-cadherin promoter and suppresses E-cadherin expression through physical association, modulating EMT and metastasis [87]. These results suggest that METTL3 plays a vital role in EMT in tumor cells.
Classical oncogenic signaling pathways
In addition, the activation of some classical oncogenic signaling pathways such as NF-KB and AKT is essential for cancer metastasis.
In gliomas, METTL3 induces the m6A modification and degradation of UBXN2 RNA in concert with YTHDF2, activating the downstream NF-KB signaling pathway and boosting tumor metastasis [88]. Additionally, METTL3-mediated m6A modification enhances the stability and expression of the oncogenic lncRNA MALAT1. MALAT1 promotes NF-KB phosphorylation and nuclear ectopic expression, which in turn activates the NF-KB signaling pathway [89]. In non-small cell lung cancer, METTL3 downregulates DAPK2 expression, leading to enhanced tumor migration in vitro and in vivo following the activation of the NF-KB signaling pathway [90]. In bladder cancer, METTL3 upregulates PCAT6 expression and increases IGF1R RNA stability by forming the PCAT6/IGF2BP2/IGF1R complex [91], which activates the NF-KB and PI3K/AKT signaling pathways.
In pancreatic ductal adenocarcinoma, METTL3 accelerates the maturation of the miR-25-3p precursor. miR-25-3p targets and represses the expression of PHLPP2, which contributes to tumor metastasis by activating the downstream AKT-p70S6K signaling pathway [22]. Similarly, in esophageal squamous cell carcinoma, METTL3 enhances the maturation of miR-320b via m6A modification to downregulate the expression of PDCD4. The miR-320b-PDCD4 axis activates the AKT signaling pathway to drive tumor metastasis [92]. In cervical cancer, METTL3 induces m6A modified NR4A1 RNA degradation through the YTHDF2-DDX6 pathway to manipulate tumor metastasis. In detail, the role of NR4A1 in recruiting the LSD1/HDAC1/CoREST complex to the AKT1 promoter is weakened, and the transcriptional activity of AKT1 is promoted [93]. In papillary thyroid cancer, METTL3 stabilizes STEAP2 RNA and positively regulates STEAP2 expression in an m6A-dependent manner, which inhibits the Hedgehog signaling pathway and suppresses cancer metastasis [94].
Interestingly, in medulloblastoma, METTL3 acts directly on PTCH1 and GLI2, important factors in the Hedgehog pathway, to promote tumor progression [95]. The above results suggest that METTL3 also affects tumor metastasis by regulating important metastasis-related signaling pathways.
Metastasis-related transcription factors
Moreover, METTL3 relies on m6A modification to regulate some important metastasis-related transcription factors and promote the metastasis of various cancers.
In concert with IGF2BP2, METTL3 promotes m6A modification of the SOX2 CDS region to inhibit SOX2 RNA degradation, which increases the protein content of intracellular SOX2 and induces colorectal cancer metastasis [69].
Furthermore, METTL3 enhances SPHK2 expression to increase KLF2 ubiquitination-mediated degradation and promote malignant gastric cancer progression [96].
In multiple myeloma, METTL3 increases YY1 expression and promotes tumor progression by enhancing the RNA stability of YY1 [97].
It is thus suggested that the regulatory role of METTL3 in tumor metastasis is not limited to classical metastasis-related proteins and signaling pathways.
Ubiquitination-related enzymes
In addition, the manipulation of ubiquitination-mediated degradation of multiple tumor metastasis-related genes by METTL3 to regulate tumor cell migration and invasion has attracted attention.
In bladder cancer, knockdown of METTL3 significantly inhibited cell migration and invasion. Mechanistically, METTL3 mediates the m6A modification of the deubiquitinating enzyme USP4 RNA at A2696, which in turn promotes the binding of YTHDF2 and HNRNPD to USP4 RNA, leading to USP4 degradation. The reduction in USP4 expression decreases the level of ELAVL1 protein deubiquitination, leading to decreased ELAVL1 protein expression and increased ARHGDIA expression, promoting bladder cancer cell migration and invasion [98].
In lung adenocarcinoma, significantly low expression of METTL3 inhibits m6A modification and translation of the E3 ubiquitin ligase FBXW7, resulting in reduced levels of ubiquitination and degradation of oncogenes such as MYC, accelerating tumor metastasis [99].
In gastric cancer, METTL3 enhances the expression of THAP7-AS1 by m6A modification to improve the migration and invasion capacity of tumor cells. Mechanistically, THAP7-AS1 facilitates E3 ubiquitin ligase CUL4B protein entry into the nucleus to suppress miR-22-3p and miR-320a expression and activate the PI3K/AKT signaling pathway [100].
The above studies suggest that METTL3 relies on m6A to regulate the expression of multiple tumor metastasis-associated proteins and activate oncogenic signaling pathways. However, whether crosstalk exists between these signaling pathways and the participants remains to be investigated in depth.
Role of METTL3 in tumor aerobic glycolysis
Aerobic glycolysis is one of the key hallmarks of tumors. On the one hand, aerobic glycolysis provides nutrients and energy to replenish the enormous energy gap needed for tumor growth and metastasis, permitting tumor cells to gain a competitive advantage in a threatened microenvironment; On the other hand, metabolites themselves, such as lactate, can be carcinogenic by altering cell signaling and preventing cell differentiation [101]. It is now believed that METTL3 regulates the metabolic reprogramming of tumors by modulating the expression of glucose transporters (GLUTs), lactate dehydrogenase (LDHA) and enolase 1 (ENO1) in tumor cells (Fig. 5).
In gastric cancer, high METTL3 expression significantly elevates glucose uptake and lactate production. Mechanistically, METTL3 enhances the stability of HDGF RNA in an m6A-dependent manner to promote HDGF entry into the nucleus. Nuclear HDGF acts as a transcription factor and induces the expression of target genes GLUT4 and ENO2, which enhances glycolysis and promotes tumor growth [19]. In hepatocellular carcinoma, METTL3 facilitates GLUT1 and GLUT3-mediated glycolysis through the upregulation of HIF1α [102]. Consistent with this, in gastric cancer, METTL3 enhances the stability of NDUFA4. NDUFA4 upregulates the expression of the HIF1-α target genes ENO1 and LDHA to promote glucose uptake, then increases ECAR and OCR as well as the cellular lactate and ATP levels [103]. METTL3 increases PDK4 RNA stability and translation to promote ATP production and glycolysis in cervical cancer cells [23]. In addition, METTL3 also acts directly on key glycolytic enzymes to regulate metabolic reprogramming. In colorectal cancer, METTL3 stabilizes HK2 and GLUT1 to activate aerobic glycolysis pathway via the deposited m6A in the 3’UTR/5’UTR [104]. In lung adenocarcinoma, METTL3-mediated m6A modification of ENO1 at 359A stimulates glycolysis and tumorigenesis [105].
Exploration of the role of METTL3 in tumor glycolysis facilitates the elucidation of the specifics of energy metabolism within tumor cells, which undoubtedly broadens our view of metabolic reprogramming in tumors.
Role of METTL3 in tumor immune escape
Tumor microenvironment is an indispensable part of tumor immune escape. The metabolic profile of tumor cells induces a hypoxic, hypoglycaemic and acidic tumor microenvironment that shifts the function of immune cells and cytokines from a tumor suppressive to a tumor promoting state. These alteration leads to tumor immune escape rather than the establishment of an effective host anti-tumor response. Most tumors shape the tumor microenvironment to promote tumor immune escape by recruiting immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs) and tumor-associated macrophages (TAMs), or by activating immunosuppressive signaling pathways such as those involving PD1/PDL1.It has been found that METTL3 plays an important role in tumor immune escape (Fig. 6).
In colorectal cancer, METTL3 depletion inhibits colorectal tumorigenesis. Silencing METTL3 exerts an inhibitory effect on the accumulation of MDSCs to maintain the activation and proliferation of CD4+ and CD8+ T cells. Mechanistically, METTL3 promotes the expression of BHLHE41 in an m6A-dependent manner and subsequently induces CXCL1 transcription to enhance MDSC migration in vitro [106]. Pathological tissue analysis of cervical cancer likewise showed that METTL3 expression positively correlates with the level of CD33+ MDSCs [107].
Meanwhile, in tumor-infiltrating myeloid cells (TIMs), METTL3 mediates the m6A modification of JAK1 RNA and enhances the translation efficiency of the JAK1 protein, followed by the activation of STAT3 phosphorylation to promote tumor cell growth [20].
In bladder cancer, m6A is enriched in the 3’UTR of PD-L1 RNA. High expression of METTL3 enhances PDL1 RNA stability and expression level, which facilitates resistance to CD8+ T-cell toxicity and boosts tumor immune escape in vitro and in vivo [108]. Similarly, METTL3 enhances immune escape by upregulating PDL1 expression and inhibiting the activation of antitumor T cells in breast cancer [109].
However, in papillary thyroid carcinoma, METTL3 directly destabilizes c-Rel mRNA by increasing m6A levels and, together with YTHDF2, inactivates the NF-KB pathway and increases IL-8 secretion to induce neutrophil infiltration [110]. The effect of METTL3 on the immune microfluidic response of the immune system has been discussed. The role of METTL3 in the immune microenvironment and immune infiltration remains to be further investigated, but there is no doubt that targeting METTL3 therapy is a novel means to overcome the challenge of tumor immune escape.
The role of METTL3 in tumor drug resistance
The causes of drug resistance in tumor cells are complex, with the involvement of genetic mutations, altered pharmacokinetics, activation of classical signaling pathways, and altered cellular adaptations. METTL3 relies on m6A modification to affect tumor cell drug resistance at multiple levels, including expression of anticancer drug targets and multidrug transporter proteins, classical signaling pathway switches, cellular antioxidant effects, DNA damage repair, cellular autophagy, and apoptosis (Fig. 7).
EGFR is an important cancer driver critical for tumor growth and survival. EGFR is a commonly used drug target in clinical practice. In melanoma, METTL3 elevates the m6A modification level of EGFR mRNA and increases its translational efficiency. High expression of EGFR activates the RAF/MEK/ERK pathway and induces resistance to PLX4032 in tumor cells [111].
In imatinib-resistant gastrointestinal stromal tumors, ETV1 activates METTL3 transcription and further promotes m6A modification mediated by METTL3 at the 5’UTR of MRP1 RNA, stimulating translation of MRP1 RNA and promoting imatinib resistance in tumor cells [112]. In breast cancer, METTL3 accelerates miR-221-3p precursor maturation, induces MDR1 and BCRP expression via the miR-221-3p/HIPK2/Che-1 axis, then promotes adriamycin resistance in cancer cells [113].
METTL3 and circKRT17 levels are elevated in osimertinib-insensitive lung adenocarcinoma tissues and cells. METTL3 enhances circKRT17 expression by promoting m6A modification. When it is overexpressed, circKRT17 recruites EIF4A3 to enhance YAP1 stabilization and nuclear import, upregulating osimertinib resistance in tumor cells [114]. In addition, METTL3 increases the RNA stability and expression of SNHG17 via m6A modification in gefitinib-resistant lung adenocarcinoma. Specifically, SNHG17 recruits EZH2 to the promoter of LATS2, epistemically repressing LATS2 and inducing gefitinib resistance [115].
One of the principles of chemotherapeutic drugs for tumor treatment is that they induce the production of reactive oxygen radicals in tumor cells, which rapidly depletes the antioxidant system, causing DNA damage and inducing programmed and nonprogrammed tumor death. In colorectal cancer, METTL3 inhibits TRAF5 expression by reducing TRAF5 stability. Downregulated intracellular TRAF5-mediated necrosis leads to enhanced antioxidant effects and increased resistance to oxaliplatin in tumor cells [116]. Moreover, METTL3 promotes resistance to 5-FU by upregulating the expression of RAD51AP1. Specifically, increased binding of RAD51AP1 to RAD51 results in an elevated ability to repair damaged DNA strands [117]. METTL3 enhances PARP1 RNA stability, which heightens the activity of the base excision repair pathway and effective repair of oxaliplatin-induced DNA damage in tumor cells, further promoting oxaliplatin resistance in CD133+ gastric cancer stem cells [118].
METTL3 induces the expression of genes such as ATG5 and ATG7 in gefitinib-resistant non-small cell lung cancer and regulates autophagy to promote drug resistance in tumor cells [119]. In addition, METTL3 induces autophagy to enhance the imatinib resistance in gastrointestinal stromal tumor cells by upregulating USP13 expression and promoting deubiquitination of ATG5 [120].
Tumor drug resistance has always been a risk factor for the prognosis of tumor patients. In-depth studies on METTL3 affecting the expression of various drug resistance-related genes through m6A modification can help us better understand the mechanism of tumor drug resistance and provide new therapeutic strategies for chemotherapy-resistant tumor patients.
Drug development and clinical treatment based on METTL3
METTL3, which is highly expressed in various primary and metastatic tumor tissues (Fig. 8), plays a crucial role in tumors through m6A modification. Consequently, several inhibitors targeting METTL3 have emerged, which undoubtedly provides new insights for tumor therapy. METTL3 requires a methyl group as a donor for m6A modification, therefore, effective reduction of METTL3 activity through competitive binding of small molecule complexes has become one of the ideas for the development of METTL3 inhibitors.
Substrate competitive inhibitors of METTL3 can be divided into nucleoside analogues and nonnucleoside analogues. However, despite the ability of nucleoside analogues to inhibit METTL3, they have low cell permeability and poor analytical selectivity, so research has progressively focused on the development of non-nucleoside analogues.
The currently disclosed nonnucleoside analogues, including UZH1a, UZH2 and STM2457, all exhibit high selectivity for METTL3 and have high inhibition efficiency. Among them, STM2457 has demonstrated excellent antitumor activity and therapeutic efficacy in vivo in AML patient-derived tumor models. Treatment of AML cell lines with STM2457 significantly inhibited tumor growth and increased the differentiation and apoptosis of key stem cell subpopulations. In vivo treatment of METTL3 with STM2457 is a promising therapeutic strategy for AML [121].
Moreover, the allosteric inhibitor 43n shows high selectivity and potent enzyme inhibitory activity for the METTL3-METTL14 complex based on the reversibility of the allosteric sites and the noncompetitive inhibition function [122].
Given the wide distribution of METTL3 in normal and tumor tissues, therapeutic agents targeting METTL3 may be of great clinical value and general applicability.
Conclusions
The expression and biological function of METTL3, one of the core catalases for m6A modification, have been studied extensively in recent years. Unsurprisingly, METTL3 is a crucial hub in tumor growth and progression, regulating the splicing, stability, and expression of a wide range of genes through m6A modifications. Trace it to its cause, the regulation of genes by METTL3 is multilayered. METTL3 can not only directly alter effector genes expression, but also indirectly manipulate effector genes by affecting upstream regulators or signaling pathways.
Flexible regulation enables METTL3 to function efficiently in tumors. As a result, it is extremely urgent and important to explore the factors that regulate the expression and role of METTL3 in tumor cells. Here, we summarize several factors affecting METTL3, such as histone modifications, DNA methylation, noncoding RNAs, transcription factors, and several posttranslational modifications. These results provide new ideas for us to gain insights into the mechanism by which METTL3 promotes tumor progression and, at the same time, provide certain clues for the development of anticancer drugs.
Drug development targeting METTL3 is undoubtedly promising, as METTL3 is highly expressed in a wide range of tumors. According to the structure of METTL3 and its mechanism of action, the following strategies have been proposed in drug development studies: (1) development of SAM competitive inhibitors based on the principle of substrate competitiveness; (2) inhibition of the methyltransferase activity of the MTD structural domain of METTL3; (3) disruption of the binding of METTL3 to METTL14; and (4) manipulation of the entry of METTL3 into the nucleus. Recently, several drugs developed based on the principle of substrate competitiveness have demonstrated certain effects. In the future, the pool of drugs targeting METTL3 will be greatly enriched.
However, it is worth noting that METTL3 is widely present in tissues, and drugs targeting METTL3 should have high tissue specificity; otherwise, they may have an impact on the biological behavior of normal tissues.
In summary, although the specific roles and mechanisms of METTL3 in different types of tumors require more detailed and comprehensive research, the treatment of tumors targeting METTL3 is an undoubtedly promising strategy. With further research, METTL3 may become a molecular marker for tumor diagnosis as well as a treatment target.
Availability of data and materials
Not applicable.
Abbreviations
- m6A:
-
N6-methyladenosine
- METTL3:
-
Methyltransferase-like 3
- SAM:
-
S-adenosylmethionine
- METTL14:
-
Methyltransferase 14
- WTAP:
-
WT1 associated protein
- VIRMA:
-
Vir like m6A methyltransferase associated
- RBM15:
-
RNA binding motif protein 15
- ZC3H13:
-
Zinc finger CCCH-type containing 13
- LH:
-
Leading helix
- NLS:
-
Nuclear localization signal
- ZFD:
-
Zinc finger domain
- MTD:
-
Methyltransferase domain
- CSC:
-
Cigarette smoke condensate
- TBP:
-
TATA-box binding protein
- Smad2/3:
-
SMAD family member 2/3
- FOXD3:
-
Forkhead box D3
- RUNX3:
-
RUNX family transcription factor 3
- YY1:
-
YY1 transcription factor
- EGR1:
-
Early growth response 1
- miRNAs:
-
MicroRNAs
- circRNAs:
-
Circular RNAs
- tRFs:
-
TRNA-derived small RNA fragments
- lncRNAs:
-
Long noncoding RNAs
- PHLPP2:
-
PH domain and leucine rich repeat protein phosphatase 2
- LHPP:
-
Phospholysine phosphohistidine inorganic pyrophosphate phosphatase
- NKX3-1:
-
NK3 homeobox 1
- IGF2BP3:
-
Insulin-like growth factor 2 mRNA binding protein 3
- CDC25B:
-
Cell division cycle 25B
- ADAR1:
-
Adenosine deaminase RNA specific 1
- PDCD4:
-
Programmed cell death 4
- RDM1:
-
RAD52 motif containing 1
- BATF2:
-
Basic leucine zipper ATF-like transcription factor 2
- AFF4:
-
ALF transcription elongation factor 4
- SPHK2:
-
Sphingosine kinase 2
- ZMYM1:
-
Zinc finger MYM-type containing 1
- JUNB:
-
AP-1 transcription factor subunit
- DAPK2:
-
Death-associated protein kinase 2
- UBXN2:
-
UBX domain-containing protein 2
- MALAT1:
-
Metastasis-associated lung adenocarcinoma transcript 1
- PCAT6:
-
Prostate cancer associated transcript 6
- NR4A1:
-
Nuclear receptor subfamily 4 group A member 1
- ELAVL1:
-
ELAV-like RNA binding protein 1
- USP4:
-
Ubiquitin specific peptidase 4
- FBXW7:
-
F-box and WD repeat domain containing 7
- IGF1R:
-
Insulin-like growth factor 1 receptor
- ENO1:
-
Enolase 1
- GLUT1:
-
Solute carrier family 2 member 1
- HK2:
-
Hexokinase 2
- PDK4:
-
Pyruvate dehydrogenase kinase 4
- LDHA:
-
Lactate dehydrogenase A
- HDGF:
-
Heparin binding growth factor
- HIF1-α:
-
Hypoxia inducible factor 1 subunit alpha
- PDL1:
-
Programmed cell death 1 ligand 1
- BHLHE41:
-
Basic helix-loop-helix family member e41
- CXCL1:
-
C-X-C motif chemokine ligand 1
- C-Rel:
-
Reticuloendotheliosis oncogene
- IL-8:
-
Interleukin 8
- EGFR:
-
Epidermal growth factor receptor
- TRAF5:
-
TNF receptor associated factor 5
- PARP1:
-
Poly (ADP-ribose) polymerase 1
- RAD51AP1:
-
RAD51-associated protein 1
- MRP1:
-
ATP binding cassette subfamily C member 1
- SNHG17:
-
Small nucleolar RNA host gene 17
- LATS2:
-
Large tumor suppressor kinase 2
- ATG5:
-
Autophagy related 5
- ATG7:
-
Autophagy related 7
References
Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5’ terminus of HeLa cell messenger RNA. Cell. 1975;4(4):379–86.
Zhao W, Liu J, Wu J, Ma X, Wang X, Zhang L, et al. High-throughput microarray reveals the epitranscriptome-wide landscape of m6A-modified circRNA in oral squamous cell carcinoma. BMC Genomics. 2022;23(1):611.
Cui Y, Liu J, Liu L, Ma X, Gui Y, Liu H, et al. m6A-modified circFOXK2 targets GLUT1 to accelerate oral squamous cell carcinoma aerobic glycolysis. Cancer Gene Ther. 2023;30(1):163–71.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–5.
Krug RM, Morgan MA, Shatkin AJ. Influenza viral mRNA contains internal N6-methyladenosine and 5’-terminal 7-methylguanosine in cap structures. J Virol. 1976;20(1):45–53.
Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3(11):1233–47.
Schöller E, Weichmann F, Treiber T, Ringle S, Treiber N, Flatley A, et al. Interactions, localization, and phosphorylation of the m6A generating METTL3–METTL14–WTAP complex. RNA. 2018;24(4):499–512.
Huang J, Dong X, Gong Z, Qin LY, Yang S, Zhu YL, et al. Solution structure of the RNA recognition domain of METTL3-METTL14 N6-methyladenosine methyltransferase. Protein Cell. 2019;10(4):272–84.
Gauci S, Helbig AO, Slijper M, Krijgsveld J, Heck AJR, Mohammed S. Lys-N and trypsin cover complementary parts of the phosphoproteome in a refined SCX-based approach. Anal Chem. 2009;81(11):4493–501.
Du Y, Hou G, Zhang H, Dou J, He J, Guo Y, et al. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018;46(10):5195–208.
Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62(3):335–45.
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–5.
Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534(7608):575–8.
Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63(2):306–17.
Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, et al. VIRMA mediates preferential m6A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10.
Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–73.
Xu QC, Tien YC, Shi YH, Chen S, Zhu YQ, Huang XT, et al. METTL3 promotes intrahepatic cholangiocarcinoma progression by regulating IFIT2 expression in an m6A-YTHDF2-dependent manner. Oncogene. 2022;41(11):1622–33.
Du QY, Huo FC, Du WQ, Sun XL, Jiang X, Zhang LS, et al. METTL3 potentiates progression of cervical cancer by suppressing ER stress via regulating m6A modification of TXNDC5 mRNA. Oncogene. 2022;41(39):4420–32.
Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, et al. METTL3-mediated m6A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69(7):1193–205.
Xiong J, He J, Zhu J, Pan J, Liao W, Ye H, et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. 2022;82(9):1660-1677.e10.
Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9(16):2395–402.
Zhang J, Bai R, Li M, Ye H, Wu C, Wang C, et al. Excessive miR-25-3p maturation via N6-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10:1858.
Li Z, Peng Y, Li J, Chen Z, Chen F, Tu J, et al. N6-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat Commun. 2020;11(1):2578.
Song C, Zhou C. HOXA10 mediates epithelial-mesenchymal transition to promote gastric cancer metastasis partly via modulation of TGFB2/Smad/METTL3 signaling axis. J Exp Clin Cancer Res. 2021;40(1):62.
Chen S, Zhang L, Li M, Zhang Y, Sun M, Wang L, et al. Fusobacterium nucleatum reduces METTL3-mediated m6A modification and contributes to colorectal cancer metastasis. Nat Commun. 2022;13(1):1248.
Zhang F, Su T, Xiao M. RUNX3-regulated circRNA METTL3 inhibits colorectal cancer proliferation and metastasis via miR-107/PER3 axis. Cell Death Dis. 2022;13(6):550.
Li M, Li M, Xia Y, Li G, Su X, Wang D, et al. HDAC1/3-dependent moderate liquid-liquid phase separation of YY1 promotes METTL3 expression and AML cell proliferation. Cell Death Dis. 2022;13(11):992.
Lv D, Gimple RC, Zhong C, Wu Q, Yang K, Prager BC, et al. PDGF signaling inhibits mitophagy in glioblastoma stem cells through N6-methyladenosine. Dev Cell. 2022;57(12):1466-1481.e6.
Suphakhong K, Terashima M, Wanna-Udom S, Takatsuka R, Ishimura A, Takino T, et al. m6A RNA methylation regulates the transcription factors JUN and JUNB in TGF-β-induced epithelial-mesenchymal transition of lung cancer cells. J Biol Chem. 2022;298(11):102554.
Zhong C, Tao B, Yang F, Xia K, Yang X, Chen L, et al. Histone demethylase JMJD1C promotes the polarization of M1 macrophages to prevent glioma by upregulating miR-302a. Clin Transl Med. 2021;11(9):e424.
Xie H, Yao J, Wang Y, Ni B. Exosome-transmitted circVMP1 facilitates the progression and cisplatin resistance of non-small cell lung cancer by targeting miR-524-5p-METTL3/SOX2 axis. Drug Deliv. 2022;29(1):1257–71.
Jiang Q, Ma Y, Zhao Y, Yao MD, Zhu Y, Zhang QY, et al. tRNA-derived fragment tRF-1001: a novel anti-angiogenic factor in pathological ocular angiogenesis. Mol Ther Nucleic Acids. 2022;30:407–20.
Cheng L, Zhang X, Huang YZ, Zhu YL, Xu LY, Li Z, et al. Metformin exhibits antiproliferation activity in breast cancer via miR-483-3p/METTL3/m6A/p21 pathway. Oncogenesis. 2021;10(1):7.
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, et al. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–9.
Ruan HG, Gu WC, Xia W, Gong Y, Zhou XL, Chen WY, et al. METTL3 is suppressed by circular RNA circMETTL3/miR-34c-3p signaling and limits the tumor growth and metastasis in triple negative breast cancer. Front Oncol. 2021;11:778132.
Chang X, Lin YY, Bai LN, Zhu W. miR-302a-3p suppresses melanoma cell progression via targeting METTL3. J Chemother. 2022;34(1):55–66.
Hu H, Kong Q, Huang XX, Zhang HR, Hu KF, Jing Y, et al. Longnon-coding RNA BLACAT2 promotes gastric cancer progression via the miR-193b-5p/METTL3 pathway. J Cancer. 2021;12(11):3209–21.
He H, Wu W, Sun Z, Chai L. MiR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m6A-caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517(4):581–7.
Kang J, Huang X, Dong W, Zhu X, Li M, Cui N. MicroRNA-1269b inhibits gastric cancer development through regulating methyltransferase-like 3 (METTL3). Bioengineered. 2021;12(1):1150–60.
Wang G, Zhang Z, Xia C. Long non-coding RNA LINC00240 promotes gastric cancer progression via modulating miR-338-5p/METTL3 axis. Bioengineered. 2021;12(2):9678–91.
Du M, Zhang Y, Mao Y, Mou J, Zhao J, Xue Q, et al. MiR-33a suppresses proliferation of NSCLC cells via targeting METTL3 mRNA. Biochem Biophys Res Commun. 2017;482(4):582–9.
Wei W, Huo B, Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177–87.
Song Z, Jia G, Ma P, Cang S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021;276:119399.
Li M, Wang Q, Zhang X, Yan N, Li X. CircPUM1 promotes cell growth and glycolysis in NSCLC via up-regulating METTL3 expression through miR-590-5p. Cell Cycle. 2021;20(13):1279–94.
Cui X, Wang Z, Li J, Zhu J, Ren Z, Zhang D, et al. Cross talk between RNA N6-methyladenosine methyltransferase-like 3 and miR-186 regulates hepatoblastoma progression through Wnt/β-catenin signalling pathway. Cell Prolif. 2020;53(3):e12768.
Yang Y, Song S, Meng Q, Wang L, Li X, Xie S, et al. miR24-2 accelerates progression of liver cancer cells by activating Pim1 through tri-methylation of Histone H3 on the ninth lysine. J Cell Mol Med. 2020;24(5):2772–90.
Wang F, Xie Z, Zhang N, Ding H, Xiong K, Guo L, et al. Has_circ_0008583 modulates hepatocellular carcinoma progression through the miR-1301-3p/METTL3 pathway. Bioengineered. 2022;13(1):1185–97.
Zhang M, Bai M, Wang L, Lu N, Wang J, Yan R, et al. Targeting SNHG3/miR-186-5p reverses the increased m6A level caused by platinum treatment through regulating METTL3 in esophageal cancer. Cancer Cell Int. 2021;21(1):114.
Zhang H, Li X, Li Y, Yang X, Liao R, Wang H, et al. CREB ameliorates osteoarthritis progression through regulating chondrocytes autophagy via the miR-373/METTL3/TFEB Axis. Front Cell Dev Biol. 2022;9:778941.
Sun HL, Zhu AC, Gao Y, Terajima H, Fei Q, Liu S, et al. Stabilization of ERK-phosphorylated METTL3 by USP5 increases m6A methylation. Mol Cell. 2020;80(4):633-647.e7.
Cheng Z, Gao S, Liang X, Lian C, Chen J, Fang C. Inhibiting PP2Acα promotes the malignant phenotype of gastric cancer cells through the ATM/METTL3 Axis. Biomed Res Int. 2021;2021:1015293.
Xu H, Wang H, Zhao W, Fu S, Li Y, Ni W, et al. SUMO1 modification of methyltransferase-like 3 promotes tumor progression via regulating Snail mRNA homeostasis in hepatocellular carcinoma. Theranostics. 2020;10(13):5671–86.
Jia G, Wang X, Wu W, Zhang Y, Chen S, Zhao J, et al. LXA4 enhances prostate cancer progression by facilitating M2 macrophage polarization via inhibition of METTL3. Int Immunopharmacol. 2022;107:108586.
Wang Z, Uddin MB, Xie J, Tao H, Zeidler-Erdely PC, Kondo K, et al. Chronic hexavalent chromium exposure upregulates the RNA methyltransferase METTL3 expression to promote cell transformation, cancer stem cell-like property, and tumorigenesis. Toxicol Sci. 2022;187(1):51–61.
Feng Y, Li C, Liu S, Yan F, Teng Y, Li X, et al. β-Elemene restrains PTEN mRNA degradation to restrain the growth of lung cancer cells via METTL3-mediated N6 methyladenosine modification. J Oncol. 2022;2022:3472745.
Luo R, Xie L, Lin Y, Shao J, Lin Z. Oxymatrine suppresses oral squamous cell carcinoma progression by suppressing CXC chemokine receptor 4 in an m6A modification decrease dependent manner. Oncol Rep. 2022;48(4):177.
Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79(6):1019–31.
Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Semin Cancer Biol. 2019;59:80–91.
Liu J, Eckert MA, Harada BT, Liu SM, Lu Z, Yu K, et al. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20(9):1074–83.
Li J, Xie H, Ying Y, Chen H, Yan H, He L, et al. YTHDF2 mediates the mRNA degradation of the tumor suppressors to induce AKT phosphorylation in N6-methyladenosine-dependent way in prostate cancer. Mol Cancer. 2020;19(1):152.
Luo G, Xu W, Zhao Y, Jin S, Wang S, Liu Q, et al. RNA m6 A methylation regulates uveal melanoma cell proliferation, migration, and invasion by targeting c-Met. J Cell Physiol. 2020;235(10):7107–19.
Guo Z, Zhang X, Lin C, Huang Y, Zhong Y, Guo H, et al. METTL3-IGF2BP3-axis mediates the proliferation and migration of pancreatic cancer by regulating spermine synthase m6A modification. Front Oncol. 2022;12:962204.
Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18(1):110.
Bi X, Lv X, Liu D, Guo H, Yao G, Wang L, et al. METTL3-mediated maturation of miR-126-5p promotes ovarian cancer progression via PTEN-mediated PI3K/Akt/mTOR pathway. Cancer Gene Ther. 2021;28(3–4):335–49.
Zhang H, Zhang P, Long C, Ma X, Huang H, Kuang X, et al. m6A methyltransferase METTL3 promotes retinoblastoma progression via PI3K/AKT/mTOR pathway. J Cell Mol Med. 2020;24(21):12368–78.
Hou H, Zhao H, Yu X, Cong P, Zhou Y, Jiang Y, et al. METTL3 promotes the proliferation and invasion of esophageal cancer cells partly through AKT signaling pathway. Pathol Res Pract. 2020;216(9):153087.
Cheng M, Sheng L, Gao Q, Xiong Q, Zhang H, Wu M, et al. The m6A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-κB/MYC signaling network. Oncogene. 2019;38(19):3667–80.
Zhang Q, Zhang Y, Chen H, Sun LN, Zhang B, Yue DS, et al. METTL3-induced DLGAP1-AS2 promotes non-small cell lung cancer tumorigenesis through m6A/c-Myc-dependent aerobic glycolysis. Cell Cycle. 2022;21(24):2602–14.
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, et al. METTL3 facilitates tumor progression via an m6A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18(1):112.
Xiang S, Liang X, Yin S, Liu J, Xiang Z. N6-methyladenosine methyltransferase METTL3 promotes colorectal cancer cell proliferation through enhancing MYC expression. Am J Transl Res. 2020;12(5):1789–806.
Yuan Y, Du Y, Wang L, Liu X. The M6A methyltransferase METTL3 promotes the development and progression of prostate carcinoma via mediating MYC methylation. J Cancer. 2020;11(12):3588–95.
Wang A, Chen Y, Shi L, Li M, Li L, Wang S, et al. Tumor-suppressive MEG3 induces microRNA-493-5p expression to reduce arabinocytosine chemoresistance of acute myeloid leukemia cells by downregulating the METTL3/MYC axis. J Transl Med. 2022;20(1):288.
Zhao W, Cui Y, Liu L, Ma X, Qi X, Wang Y, et al. METTL3 facilitates oral squamous cell carcinoma tumorigenesis by enhancing c-Myc stability via YTHDF1-mediated m6A modification. Mol Ther Nucleic Acids. 2020;20:1–12.
Chen SL, Liu LL, Wang CH, Lu SX, Yang X, He YF, et al. Loss of RDM1 enhances hepatocellular carcinoma progression via p53 and Ras/Raf/ERK pathways. Mol Oncol. 2020;14(2):373–86.
Xie JW, Huang XB, Chen QY, Ma YB, Zhao YJ, Liu LC, et al. m6A modification-mediated BATF2 acts as a tumor suppressor in gastric cancer through inhibition of ERK signaling. Mol Cancer. 2020;19(1):114.
Li H, Zhong Y, Cao G, Shi H, Liu Y, Li L, et al. METTL3 promotes cell cycle progression via m6A/YTHDF1-dependent regulation of CDC25B translation. Int J Biol Sci. 2022;18(8):3223–36.
Guo YQ, Wang Q, Wang JG, Gu YJ, Song PP, Wang SY, et al. METTL3 modulates m6A modification of CDC25B and promotes head and neck squamous cell carcinoma malignant progression. Exp Hematol Oncol. 2022;11(1):14.
Tassinari V, Cesarini V, Tomaselli S, Ianniello Z, Silvestris DA, Ginistrelli LC, et al. ADAR1 is a new target of METTL3 and plays a pro-oncogenic role in glioblastoma by an editing-independent mechanism. Genome Biol. 2021;22:51.
Li Z, Yang HY, Dai XY, Zhang X, Huang YZ, Shi L, et al. CircMETTL3, upregulated in a m6A-dependent manner, promotes breast cancer progression. Int J Biol Sci. 2021;17(5):1178–90.
Bian Y, Wang Y, Xu S, Gao Z, Li C, Fan Z, et al. m6A modification of long non-coding RNA HNF1A-AS1 facilitates cell cycle progression in colorectal cancer via IGF2BP2-mediated CCND1 mRNA stabilization. Cells. 2022;11(19):3008.
Nguyen DX, Massagué J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341–52.
Liu L, Wu Y, Li Q, Liang J, He Q, Zhao L, et al. METTL3 promotes tumorigenesis and metastasis through BMI1 m6A methylation in oral squamous cell carcinoma. Mol Ther. 2020;28(10):2177–90.
Lin X, Chai G, Wu Y, Li J, Chen F, Liu J, et al. RNA m6A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun. 2019;10:2065.
Li J, Chen F, Peng Y, Lv Z, Lin X, Chen Z, et al. N6-methyladenosine regulates the expression and secretion of TGFβ1 to affect the epithelial-mesenchymal transition of cancer cells. Cells. 2020;9(2):296.
Chen C, Yuan W, Zhou Q, Shao B, Guo Y, Wang W, et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11(9):4298–315.
Pan J, Liu F, Xiao X, Xu R, Dai L, Zhu M, et al. METTL3 promotes colorectal carcinoma progression by regulating the m6A–CRB3–Hippo axis. J Exp Clin Cancer Res. 2022;41:19.
Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, et al. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18(1):142.
Chai RC, Chang YZ, Chang X, Pang B, An SY, Zhang KN, et al. YTHDF2 facilitates UBXN1 mRNA decay by recognizing METTL3-mediated m6A modification to activate NF-κB and promote the malignant progression of glioma. J Hematol Oncol. 2021;14:109.
Chang YZ, Chai RC, Pang B, Chang X, An SY, Zhang KN, et al. METTL3 enhances the stability of MALAT1 with the assistance of HuR via m6A modification and activates NF-κB to promote the malignant progression of IDH-wildtype glioma. Cancer Lett. 2021;511:36–46.
Jin M, Li G, Liu W, Wu X, Zhu J, Zhao D, et al. Cigarette smoking induces aberrant N6-methyladenosine of DAPK2 to promote non-small cell lung cancer progression by activating NF-κB pathway. Cancer Lett. 2021;518:214–29.
Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, et al. m6 A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11(6):e426.
Liu T, Li P, Li J, Qi Q, Sun Z, Shi S, et al. Exosomal and intracellular miR-320b promotes lymphatic metastasis in esophageal squamous cell carcinoma. Mol Ther Oncolytics. 2021;23:163–80.
Yu T, Wu F, Jia Y, Zhang X, Qi X, Jin Z, et al. RNA N6-methyladenosine modification mediates downregulation of NR4A1 to facilitate malignancy of cervical cancer. Cell Biosci. 2022;12:207.
Zhu Y, Peng X, Zhou Q, Tan L, Zhang C, Lin S, et al. METTL3-mediated m6A modification of STEAP2 mRNA inhibits papillary thyroid cancer progress by blocking the Hedgehog signaling pathway and epithelial-to-mesenchymal transition. Cell Death Dis. 2022;13(4):358.
Zhang ZW, Teng X, Zhao F, Ma C, Zhang J, Xiao LF, et al. METTL3 regulates m6A methylation of PTCH1 and GLI2 in Sonic hedgehog signaling to promote tumor progression in SHH-medulloblastoma. Cell Rep. 2022;41(4):111530.
Huo FC, Zhu ZM, Zhu WT, Du QY, Liang J, Mou J. METTL3-mediated m6A methylation of SPHK2 promotes gastric cancer progression by targeting KLF2. Oncogene. 2021;40(16):2968–81.
Che F, Ye X, Wang Y, et al. METTL3 facilitates multiple myeloma tumorigenesis by enhancing YY1 stability and pri-microRNA-27 maturation in m6A-dependent manner. Cell Biol Toxicol. 2023;39(5):2033–50.
Chen Y, Pan C, Wang X, Xu D, Ma Y, Hu J, et al. Silencing of METTL3 effectively hinders invasion and metastasis of prostate cancer cells. Theranostics. 2021;11(16):7640–57.
Wu Y, Chang N, Zhang Y, Zhang X, Xu L, Che Y, et al. METTL3-mediated m6A mRNA modification of FBXW7 suppresses lung adenocarcinoma. J Exp Clin Cancer Res. 2021;40(1):90.
Liu HT, Zou YX, Zhu WJ, Sen-Liu, Zhang GH, Ma RR, et al. lncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell Death Differ. 2022;29(3):627–41.
Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21(3):297–308.
Yang N, Wang T, Li Q, Han F, Wang Z, Zhu R, et al. HBXIP drives metabolic reprogramming in hepatocellular carcinoma cells via METTL3-mediated m6A modification of HIF-1α. J Cell Physiol. 2021;236(5):3863–80.
Xu W, Lai Y, Pan Y, Tan M, Ma Y, Sheng H, et al. m6A RNA methylation-mediated NDUFA4 promotes cell proliferation and metabolism in gastric cancer. Cell Death Dis. 2022;13(8):715.
Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X, et al. m6A-dependent glycolysis enhances colorectal cancer progression. Mol Cancer. 2020;19(1):72.
Ma L, Xue X, Zhang X, Yu K, Xu X, Tian X, et al. The essential roles of m6A RNA modification to stimulate ENO1-dependent glycolysis and tumorigenesis in lung adenocarcinoma. J Exp Clin Cancer Res. 2022;41(1):36.
Chen H, Pan Y, Zhou Q, Liang C, Wong CC, Zhou Y, et al. METTL3 inhibits antitumor immunity by targeting m6A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology. 2022;163(4):891–907.
Ni HH, Zhang L, Huang H, Dai SQ, Li J. Connecting METTL3 and intratumoural CD33+ MDSCs in predicting clinical outcome in cervical cancer. J Transl Med. 2020;18:393.
Ni Z, Sun P, Zheng J, Wu M, Yang C, Cheng M, et al. JNK signaling promotes bladder cancer immune escape by regulating METTL3-mediated m6A modification of PD-L1 mRNA. Cancer Res. 2022;82(9):1789–802.
Wan W, Ao X, Chen Q, Yu Y, Ao L, Xing W, et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N6-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol Cancer. 2022;21(1):60.
He J, Zhou M, Yin J, Wan J, Chu J, Jia J, et al. METTL3 restrains papillary thyroid cancer progression via m6A/c-Rel/IL-8-mediated neutrophil infiltration. Mol Ther. 2021;29(5):1821–37.
Bhattarai PY, Kim G, Poudel M, Lim SC, Choi HS. METTL3 induces PLX4032 resistance in melanoma by promoting m6A-dependent EGFR translation. Cancer Lett. 2021;522:44–56.
Xu K, Zhang Q, Chen M, Li B, Wang N, Li C, et al. N6-methyladenosine modification regulates imatinib resistance of gastrointestinal stromal tumor by enhancing the expression of multidrug transporter MRP1. Cancer Lett. 2022;530:85–99.
Pan X, Hong X, Li S, Meng P, Xiao F. METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. Exp Mol Med. 2021;53(1):91–102.
Ji Y, Zhao Q, Feng W, Peng Y, Hu B, Chen Q. N6-methyladenosine modification of CIRCKRT17 initiated by METTL3 promotes osimertinib resistance of lung adenocarcinoma by EIF4A3 to enhance YAP1 stability. Cancers (Basel). 2022;14(22):5582.
Zhang H, Wang SQ, Wang L, Lin H, Zhu JB, Chen R, et al. m6A methyltransferase METTL3-induced lncRNA SNHG17 promotes lung adenocarcinoma gefitinib resistance by epigenetically repressing LATS2 expression. Cell Death Dis. 2022;13(7):657.
Lan H, Liu Y, Liu J, Wang X, Guan Z, Du J, et al. Tumor-associated macrophages promote oxaliplatin resistance via METTL3-mediated m6A of TRAF5 and necroptosis in colorectal cancer. Mol Pharm. 2021;18(3):1026–37.
Li M, Xia M, Zhang Z, Tan Y, Li E, Guo Z, et al. METTL3 antagonizes 5-FU chemotherapy and confers drug resistance in colorectal carcinoma. Int J Oncol. 2022;61(3):106.
Li H, Wang C, Lan L, Yan L, Li W, Evans I, et al. METTL3 promotes oxaliplatin resistance of gastric cancer CD133+ stem cells by promoting PARP1 mRNA stability. Cell Mol Life Sci. 2022;79(3):135.
Liu S, Li Q, Li G, Zhang Q, Zhuo L, Han X, et al. The mechanism of m6A methyltransferase METTL3-mediated autophagy in reversing gefitinib resistance in NSCLC cells by β-elemene. Cell Death Dis. 2020;11(11):969.
Gao Z, Li C, Sun H, et al. N6-methyladenosine-modified USP13 induces pro-survival autophagy and imatinib resistance via regulating the stabilization of autophagy-related protein 5 in gastrointestinal stromal tumors. Cell Death Differ. 2023;30(2):544–59.
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593(7860):597–601.
Lee JH, Kim S, Jin MS, Kim YC. Discovery of substituted indole derivatives as allosteric inhibitors of m6 A-RNA methyltransferase, METTL3-14 complex. Drug Dev Res. 2022;83(3):783–99.
Acknowledgements
The authors would like to thank the editors of Springer Nature Author Services for providing high quality language editing.
Funding
This work was supported by the Jilin Provincial Department of Science and Technology, International Science and Technology Cooperation Project (No.20220402083GH).
Author information
Authors and Affiliations
Contributions
JQ drafted the manuscript and generated the figures. QHN reviewed the article and contributed to article writing. QCS supervised the writing, revised the article structure and acquired funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to submit the article for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jin, Q., Qu, H. & Quan, C. New insights into the regulation of METTL3 and its role in tumors. Cell Commun Signal 21, 334 (2023). https://doi.org/10.1186/s12964-023-01360-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01360-5