Abstract
As one of the most important human fungal pathogens, Candida albicans senses and adapts to host niches with different pH values through the pH-responsive Rim101 pathway. Its transcription factor Rim101 activates the expression of alkaline pH-induced genes including PHR1 that encodes a glycosylphosphatidylinsitol-anchored β(1,3)-glucanosyltransferase critical for hyphal wall formation. The calcium/calcineurin signaling pathway is mediated by the transcription factor Crz1 in yeasts and other lower eukaryotes. Here we report that deletion of PHR1 leads to calcium sensitivity of C. albicans cells. In addition, expression of Phr1 is induced by calcium stress and under the control of Crz1 in C. albicans. EMSA assay demonstrates that Crz1 binds to one CDRE element in the PHR1 promoter. Alkaline treatment induces two species of glycosylated Phr1 proteins with different degrees of glycosylation, which is independent of Crz1. In contrast, only one species of Phr1 protein with a low degree of glycosylation is induced by calcium stress in a Crz1-dependent fashion. Therefore, we have provided an evidence that regulation of cell wall remodeling is integrated through differential degrees of Phr1 glycosylation by both the pH-regulated Rim101 pathway and the calcium/calcineurin signaling pathway in C. albicans.
Video Abstract
Similar content being viewed by others
Background
As one of the most important human fungal pathogens, Candida albicans remains the primary cause of candidiasis, about 60% of the global candidiasis burden [1]. Its cell wall forms the interface with the host, gives the cell shape, and protects cells from stresses and damage. In response to the environmental conditions and host attack, the cell wall allows remodeling and changes in its components, which is composed of the outer mannoprotein coat and the inner polysaccharide-rich layer (chitin and β-glucan cross-linked together). Some cell wall proteins provide anchors for the mannan outer layer of the cell wall, whereas others are cell wall remodeling enzymes responsible for generating essential covalent linkages between cell wall components [2]. The cell wall is essential for the viability of fungal pathogens such as C. albicans. In addition, human cells lack a cell wall. Therefore, the fungal cell wall is an attractive target for the development of new antifungal drugs [3, 4].
C. albicans can adapt to host niches with different pH values, such as the stomach (pH2.0), the vagina (pH4.5), the kidney, the liver and the duodenum (pH7.0) as well as the blood (pH 7.3) [5, 6]. C. albicans cells sense environmental pH changes through the pH-responsive Rim101 pathway that is involved in both tolerance and resistance of antifungal drugs [7, 8]. This pathway is mediated by the transcription factor Rim101, which not only activates the expression of alkaline pH-induced genes such as PHR1 but also represses the expression of acidic pH-induced genes such as PHR2 [9, 10]. C. albicans Phr1 and Phr2 are members of the GH72 family of glycosylhydrolases, which exist in all fungi and are required for the formation of cell wall [11, 12]. Phr1 and Phr2 are required for proper cross-linking of β-1,3- and β-1,6-glucans in the cell wall of C. albicans [13, 14]. Phr1 is required for the virulence of C. albicans cells in the mouse model of systemic infection, but not in the rat model of vaginal infection; Contrarily, Phr2 is not required for the virulence of C. albicans cells in mouse systemic infection, but is essential in the rat vaginal infection [15]. These data indicate that Phr1 and Phr2 play differential roles in the virulence of C. albicans cells. CRZ1 encodes the transcription factor, a downstream target of the calcium/calcineurin signaling pathway in C. albicans [16, 17]. Our recent RNA sequencing work has revealed that Crz1 positively regulates expression of 219 genes, including PHR1, in response to calcium stress (Additional file 1: Figure S1; GEO Accession number: GSE123122; [18]). In this study, we have shown that Phr1 is glycosylated and its expression is induced and controlled by Crz1 in response to calcium stress in C. albicans.
Methods
Strains and media
C. albicans strains and plasmids as well as primers used in this study were listed in Table 1 and Additional file 1: Table S1, respectively. LB medium was used for growing bacterial strains. YPD medium and SD medium (0.67% yeast nitrogen base without amino acids, 2% glucose, and auxotrophic amino acids as needed) were used for routine maintaining C. albicans strains. Alkaline and acidic treatment was carried out in YPD-150 mM HEPES buffered at pH 8.0 (with NaOH) and 4.0 (with HCl), respectively, as described previously [19]. In brief, cells were grown in YPD medium overnight. Overnight cultures were diluted into YPD-150 mM HEPES buffered at pH 8.0 or 4.0 at a final OD600nm of 0.3, which were allowed to further incubate with shaking for 4 h before cells were collected for protein extraction. It should be noted that the pH-related experiments were conducted steady-states and in adapted cells.
Construction of the homozygous mutant for PHR1
To construct the homozygous mutant for PHR1 in the C. albicans strain SN148 background, we PCR amplified the HIS1 and ARG4 cassettes from the plasmids pFA-HIS1 and pFA-ARG4, respectively, to subsequently replace two alleles of PHR1 through homologous recombination [23]. Genotypes of the homozygous mutants for PHR1 were confirmed by PCR (Additional file 1: Figure S2).
Gene cloning
The full-length gene PHR1 was PCR amplified with primers PHR1-clone-F/ PHR1-clone-R and cloned between KpnI and XhoI sites in the integration vector CIp10 [24], which generated CIp10-PHR1. This recombinant plasmid was confirmed by DNA sequencing, before it was StuI-linearized and integrated into the RPS1 locus of the homozygous mutant for PHR1. The vector CIp10 was StuI-linearized and integrated into the RPS1 loci of the wild type SN148 as well as its isogenic heterozygous and homozygous mutant for PHR1, respectively. Genotypes of these strains were confirmed by PCR with primer pairs CaRPS1-F/URA3-F and CaRPS1-R/CIp10-R [25].
Chromosomally C-terminal 3xHA tagging of PHR1
We PCR amplified the HA-URA3 cassette with primers PHR1-HA-UP/PHR1-HA-DOWN from the plasmid pFA-HA-URA3 [21, 22]. Through homologous recombination approach, we chromosomally integrated the HA tag at the C terminus of Phr1 in the wild type SN148 and the CRISPR homologous mutant for CaCRZ1 from our previous study [18]. Genotypes of these strains were confirmed by PCR with primer pairs URA3-DF/PHR1-DR and PHR1-DF/HA-DR (Additional file 1: Figures S3).
Protein extract preparation and deglycosylation reactions
Total protein extracts were prepared and Western blot analysis was carried out as described [26, 27]. In brief, cells were collected by centrifugation and broken by agitation with 5-mm glass beads at 4 °C in protein extraction buffer (50 mM Tris–HCl, pH 7.5/100 mM NaCl/1 mM EDTA/1% NP-40/2 mM PMSF) supplemented with complete protease inhibitor cocktail (Roche Diagnostics, Germany). Total cell lysates were clarified by centrifugation at 10,000 rpm for 10 min at 4 °C. Protein samples were fractionated by SDS-PAGE gels, and transferred onto PVDF membranes. PHR1-HA was detected by Western blot analysis with anti-HA monoclonal antibody. Expression of tubulin was detected with anti-tubulin antibody, which served as an internal expression control.
Protein deglycosylation under both non-denaturing reaction and denaturing reaction conditions was carried out as the manufacturer’s recommendations in the protocol of Protein Deglycosylation Mix II (NEB Biolabs, USA). Briefly, we mixed a total protein sample of 100 μg with 5 μl 10X Deglycosylation Mix Buffer 2, incubated the mixture at 75 °C for 10 min (for denaturing reaction), and cooled it down before the addition of 5 μl Protein Deglycosylation Mix II. For non-denaturing reaction, the mixture was not treated at 75 °C and directly mixed with 5 μl Protein Deglycosylation Mix II. This was followed by an incubation at 25 °C for 30 min and at 37 °C for further 16 h.
Electrophoretic mobility shift assay (EMSA)
We expressed and purified the recombinant His6-tagged CaCrz1 protein as described previously [18]. The double-stranded probe EMSA_PHR1_F/R was prepared by annealing its two complimentary oligonucleotides (Additional file 1: Table S1), and labeled at the 3′ end with the DIG Gel Shift Kit (Roche) according to the manufacturer’s recommendations.
Results
Phr1 is required for the response of C. albicans cells to calcium stress
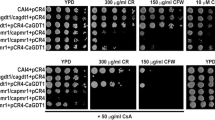
In order to examine if Phr1 is involved in the response of C. albicans cells to calcium stress, we generated the homozygous mutant for PHR1 by replacing the two alleles of PHR1 with the ARG4 cassette and the HIS1 cassette, respectively. Consistent with previous studies [28, 29], the homozygous mutant for PHR1 was sensitive to alkaline treatment but not to an acidic treatment (Additional file 1: Figures S4). As compared to the wild type and the heterozygous mutant for PHR1, the homozygous mutant for PHR1 was sensitive to 0.4 M CaCl2 and 0.6 M CaCl2, but not to 0.2 M CaCl2, and introduction of PHR1 back to its homozygous mutant reversed its calcium-sensitive phenotype (Fig. 1; Additional file 1: Figure S5). As compared to the wild type, we did not observe a change in the sensitivity of the homozygous mutant for PHR1 to 0.4 M MgCl2, 0.4 M MnCl2, 0.4 M LiCl, 0.4 M KCl, and 0.4 M NaCl (Data now shown). Taken together, these data indicate that Phr1 is required for the response of C. albicans cells to calcium stress.
Phenotypes of Candida albicans cells lacking a functional PHR1 gene. The wild type SN148 (WT; HHCA1), its isogenic heterozygous (PHR1/phr1::ARG4; HHCA1124) and homozygous (phr1::ARG4/phr1::HIS1; HHCA1091) mutants for PHR1 as well as the complemented strain (phr1::ARG4/phr1::HIS1 + CIp10-PHR1; HHCA1094) were grown overnight at 30 °C in liquid SD-URA medium, and overnight cultures were serially diluted and spotted onto YPD plates with or without CaCl2. Plates were incubated for 2–3 days before photos were taken
Calcium-induced expression of Phr1 is, but alkaline-induced expression of Phr1 is not, dependent of the transcription factor CaCrz1
PHR1 is known to be one of the downstream targets of the Rim101 signaling in C. albicans [9, 10]. Expression level of PHR1 is barely detectable at pH4.0 and significantly induced at pH8.0, and this alkaline-induction is controlled by Rim101 [30]. In our previous study [18], we have observed that transcripts of PHR1 is induced by calcium stress, which could be abolished in the absence of Crz1 (Additional file 1: Figure S1). To examine the protein expression of Phr1 in response to calcium stress and pH conditions, we chromosomally 3xHA tagged the C-terminus of one PHR1 allele in both the wild type SN148 and its isogenic homozygous mutant for CaCRZ1 (Additional file 1: Figure S3).
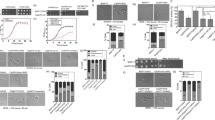
In both the wild type and the crz1/crz1 strains, two species of Phr1-HA proteins with approximately sizes of 88 kDa and 75 kDa were induced by alkaline treatment, but not by acidic treatment, with the 88-kDa protein being more abundant (Fig. 2A). Both Phr1-HA bands were bigger than its expected size of 63 kDa, suggesting posttranslational modifications for Phr1-HA proteins. This is consistent with a previous observation on the Phr1 protein tagged with GFP, showing a posttranslational modification with a possible glycosylation degree similar to the 88-kDa Phr1-HA band [19]. In addition, a mass spectrometric quantification approach detected the presence of Phr1 in the cell wall of cells growing under only alkaline but not acidic conditions [31]. Our result indicates that alkaline-induced expression of Phr1 is independent of CaCrz1. In contrast, in response to 0.2 M CaCl2, a Phr1-HA protein of about 75 kDa was also induced in the wild type strain, but not in the crz1/crz1 strain (Fig. 2B). This suggests that the calcium-induced expression of the 75-kDa Phr1-HA protein is dependent of CaCrz1.
Expression and deglycosylation of Phr1-HA proteins induced by alkaline treatment and calcium stress. Log-phase growing CRZ1/CRZ1 PHR1/PHR1-HA and crz1/crz1 PHR1/PHR1-HA cells were treated in YPD-150 mM HEPES buffered at pH 4.0 or 8.0 (A) or in YPD containing 0.2 M CaCl2 (B) for one hour, before total protein samples were extracted from these cells and subjected for Western blot analysis with anti-HA or anti-tubulin (as internal control) antibodies. Two times of western blots were carried out. Total protein samples were also subjected for deglycosylation analysis under both non-denaturing and denaturing reaction conditions (C) and only under denaturing reaction condition (D). PD, partial deglycosylation
Alkaline- and calcium-induced Phr1 proteins are differentially glycosylated
Phr1 is a regulator of cell wall remodeling. We next tested if Phr1 proteins were glycosylated. When protein samples were not treated at 75 °C for 10 min (non-denaturing condition) before they were subjected for deglycosylation digestion, we observed that the alkaline-induced 88-kDa Phr1-HA product from both the wild type and the crz1/crz1 strains was largely deglycosylated to be a band of about 70 kDa (Lanes 1–4; Fig. 2C). When protein samples were pretreated at 75 °C for 10 min (denaturing condition), the 88-kDa Phr1-HA protein was deglycosylated to generate a smaller protein of about 73 kDa; whereas the 75-kDa Phr1-HA protein was deglycosylated to be a 70-kDa protein (Lanes 7–10; Fig. 2C and left panel; D). The 73-kDa band derived from the deglycosylation of the alkaline-induced 88-kDa Phr1-HA protein under denaturing condition seems to be not as sharp as the 70-kDa band (left panel; Fig. 2D), which indicates the 73-kDa product might be a partially deglycosylated form of the 88-kDa protein.
We did not observe any band from the deglycosylation reaction of two CaCl2-induced Phr1-HA samples under non-denaturing condition (lanes 5 and 6; Fig. 2C). This should be caused by the lower expression level of the 75-kDa Phr1-HA product induced by calcium stress and a possible protein degradation under non-denaturing condition, because a significant general protein degradation occurred during deglycosylation digestion if protein samples were not pretreated at 75 °C for 10 min (compare signal intensities between lanes 1–4 and lanes 7–10; Fig. 2C). After we increased the total protein amount to 200 μg in the deglycosylation reaction, a deglycosylated 70-kDa product was also observed in the calcium-induced Phr1-HA sample under denaturing condition (right panel; Fig. 2D). Taken together, these results suggest that both the 88-kDa and the 75-kDa Phr1-HA proteins are glycosylated.
CaCrz1 binds to the PHR1 promoter in vitro
We identified one potential CaCrz1-binding site from the complementary sequence of the 5’ CGACGCCTCA 3’, which locates at -244 to -253 from the ORF start, in the promoter of PHR1 according to the consensus motif in promoters of CaCrz1 target genes identified previously (Fig. 3A; [18]). To determine if CaCrz1 binds to the CDRE motif in the PHR1 promoter, we carried out EMSA experiment. The EMSA assay demonstrated that the recombinant 6xHis-Crz1 protein bound to the DIG-labeled probe (Lane 2 in Fig. 3B). The binding of 6xHis-Crz1 to the probe was abolished by its specific competitor, the unlabeled probe (Lane 3 in Fig. 3B). Taken together, these data indicate that CaCrz1 can bind in vitro to the CDRE motif in the PHR1 promoter.
CaCrz1 binds in vitro to the PHR1 promoter. A Location of the predicated CaCrz1-binding motif (boxed) in the promoter of PHR1. Location of the double-stranded probe EMSA_PHR1_F/R sequence is indicated underlined with a solid line. B DIG-labelled Probe EMSA_PHR1_F/R was added into samples in Lanes 1–3. The unlabelled Probe EMSA_PHR1_F/R was added into the sample in Lane 3. Purified 6xHis-Crz1 protein of 1 μg was added into samples in Lanes 2 and 3, respectively. Two EMSA replicates were performed
Discussion
It is known that in response to alkaline treatment, Rim101 directly binds to the promoter of the pH-responsive gene PHR1 to induce its transcriptional expression in C. albicans [10]. Here, we have demonstrated that C. albicans cells lacking PHR1 are sensitive to calcium stress. Furthermore, expression of Phr1 is also induced by calcium stress, which is controlled by the transcription factor CaCrz1 likely through its CDRE motif [5’ C253GACGCCTCA 3’] in the promoter of PHR1. In Saccharomyces cerevisiae, exposure of cells to an alkaline environment produces a robust transcriptional response involving hundreds of genes, part of which is triggered by an immediate burst of calcium and recruitment of Crz1 to the promoters of more than 100 genes [32, 33]. Immediate calcium influx has also been observed in the response of C. albicans cells to alkaline treatment, and expression of alkaline-induced PHO85 has been shown to be positively controlled by the calcium signaling transcription factor Crz1 [34]. In this study, we have shown that the Phr1 expression/glycosylation in alkaline pH is not dependent on CaCrz1, although alkaline pH can trigger calcium influx to the cell. This could be due to the possibility that Rim101-binding might somehow hinder the binding of CaCrz1 to its CDRE motif in the promoter of PHR1. In C. albicans, the Rim101 pathway acts in parallel to Crz1, via calcineurin, to adapt to alkaline pH, and acts in parallel to the Crz1 homologue Crz2, independent of calcineurin, to adapt to lithium stress and to repress filamentation at acidic pH [35]. Our study has provided an additional example that expression of an alkaline-induced cell wall-related gene PHR1 is also positively controlled by Crz1, the transcription factor of the calcium/calcineurin signaling pathway in C. albicans.
In addition, we have demonstrated that Phr1 proteins induced by both alkaline and calcium stresses are glycosylated. Two species of Phr1 proteins are observed to be induced by alkaline treatment. The smaller glycosylated species induced by alkaline treatment shows a similar size to the only one induced by calcium stress, but they might be different glycosylation species because they are controlled by Rim101 and Crz1, respectively (Fig. 2A). To our knowledge, this is the first report to demonstrate that Phr1 proteins are glycosylated in C. albicans. Our results are consistent with the bioinformatic analysis of Phr1 amino acid sequence by Psort II (https://psort.hgc.jp/form2.html), which predicts that Phr1 has a cleavable N-terminal signal peptide (M1 to A20) and is likely to be GPI anchored. In addition, there is a serine-rich region in the C-terminal region (486S-517S) of Phr1 protein (http://www.candidagenome.org/cgi-bin/locus.pl?locus=phr1&organism = C_albicans_ SC5314). These three characteristics meet all criteria for being GPI-anchored plasma membrane mannoproteins in S. cerevisiae [36, 37]. In this study, we have shown that the deglycosylated band of Phr1-HA is 70 kDa (Fig. 2D), which is still bigger than its expected size of about 63 kDa. This difference might be caused by another kind of posttranslational modification such as phosphorylation, which needs to be further addressed in the future study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- Crz1:
-
Calcineurin-responsive zinc finger 1
- PCR:
-
Polymerase chain reaction
- YPD:
-
Yeast peptone dextron
References
Ibe C, Munro CA. Fungal cell wall proteins and signaling pathways form a cytoprotective network to combat stresses. J Fungi (Basel). 2021;7(9):739.
Childers DS, Avelar GM, Bain JM, Larcombe DE, Pradhan A, Budge S, Heaney H, Brown AJP. Impact of the environment upon the Candida albicans cell wall and resultant effects upon immune surveillance. Curr Top Microbiol Immunol. 2020;425:297–330.
Li R, Zhu L, Liu D, Wang W, Zhang C, Jiao S, Wei J, Ren L, Zhang Y, Gou X, Yuan X, Du Y, Wang ZA. High molecular weight chitosan oligosaccharide exhibited antifungal activity by misleading cell wall organization via targeting PHR transglucosidases. Carbohydr Polym. 2022;285:119253.
Gow NAR, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 2017;5(3). https://doi.org/10.1128/microbiolspec.funk-0035-2016.
Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminting invasion from colonization. Nat Rev Microbiol. 2011;10:112–22.
Davis D. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr Genet. 2003;44:1–7.
Iyer KR, Robbins N, Cowen LE. The role of Candida albicans stress response pathways in antifungal tolerance and resistance. iScience. 2022;25(3):103953.
Serra-Cardona A, Canadell D, Ariño J. Coordinate responses to alkaline pH stress in budding yeast. Microb Cell. 2015;2(6):182–96.
Baek YU, Martin SJ, Davis DA. Evidence for novel pH-dependent regulation of Candida albicans Rim101, a direct transcriptional repressor of the cell wall beta-glycosidase Phr2. Eukaryot Cell. 2006;5(9):1550–9.
Ramón AM, Fonzi WA. Diverged binding specificity of Rim101p, the Candida albicans ortholog of PacC. Eukaryot Cell. 2003;2(4):718–28.
Kar B, Patel P, Ao J, Free SJ. Neurospora crassa family GH72 glucanosyltransferases function to crosslink cell wall glycoprotein N-linked galactomannan to cell wall lichenin. Fungal Genet Biol. 2019;123:60–9.
Popolo L, Degani G, Camilloni C, Fonzi WA. The PHR Family: the role of extracellular transglycosylases in shaping Candida albicans cells. J Fungi (Basel). 2017;3(4):59.
Degani G, Popolo L. The glucan-remodeling enzyme Phr1p and the chitin synthase Chs1p cooperate to maintain proper nuclear segregation and cell integrity in Candida albicans. Front Cell Infect Microbiol. 2019;9:400.
Fonzi WA. PHR1 and PHR2 of Candida albicans encode putative glycosidases required for proper cross-linking of beta-1,3- and beta-1,6-glucans. J Bacteriol. 1999;181(22):7070–9.
De Bernardis F, Mühlschlegel FA, Cassone A, Fonzi WA. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66(7):3317–25.
Karababa M, Valentino E, Pardini G, Coste AT, Bille J, Sanglard D. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol Microbiol. 2006;59(5):1429–51.
Santos M, de Larrinoa IF. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr Genet. 2005;48(2):88–100.
Xu H, Fang T, Omran RP, Whiteway M, Jiang L. RNA sequencing reveals an additional Crz1-binding motif in promoters of its target genes in the human fungal pathogen Candida albicans. Cell Commun Signal. 2020;18(1):1.
Ragni E, Calderon J, Fascio U, Sipiczki M, Fonzi WA, Popolo L. Phr1p, a glycosylphosphatidylinsitol-anchored β(1,3)-glucanosyltransferase critical for hyphal wall formation, localizes to the apical growth sites and septa in Candida albicans. Fungal Genet Biol. 2011;48(8):793–805.
Sun Y, Gadoury C, Hirakawa MP, Bennett RJ, Harcus D, Marcil A, Whiteway M. Deletion of a Yci1 domain protein of Candida albicans allows homothallic mating in MTL heterozygous cells. MBio. 2016;7:(2). https://doi.org/10.1128/mBio.00465-16.
Jiang L, Wang J, Asghar F, Snyder N, Cunningham KW. (2018) CaGdt1 plays a compensatory role for the calcium pump CaPmr1 in the regulation of calcium signaling and cell wall integrity signaling in Candida albicans. Cell Commun Signal. 2018;16(1):33.
Lavoie H, Sellam A, Askew C, Nantel A, Whiteway M. A toolbox for epitopetagging and genome-wide location analysis in Candida albicans. BMC Genomics. 2008;9:578.
Gola S, Martin R, Walther A, Dünkler A, Wendland J. New modules for PCR-based gene targeting in Candida albicans: rapid and efficient gene targeting using 100 bp of flanking homology region. Yeast. 2003;20(16):1339–47.
Jiang L, Pan H. Functions of CaPhm7 in the regulation of ion homeostasis, drug tolerance, filamentation and virulence in Candida albicans. BMC Microbiol. 2018;18(1):49.
Asghar F, Yan H, Jiang L. The putative transcription factor CaMaf1 controls the sensitivity to lithium and rapamycin and represses RNA polymerase III transcription in Candida albicans. FEMS Yeast Res. 2018;18(6). https://doi.org/10.1093/femsyr/foy068.
Zhao Y, Du J, Xiong B, Xu H, Jiang L. ESCRT components regulate the expression of the ER/Golgi calcium pump gene PMR1 through the Rim101/Nrg1 pathway in budding yeast. J Mol Cell Biol. 2013;5(5):336–44.
Zhao Y, Yan H, Happeck R, Peiter-Volk T, Xu H, Zhang Y, Peiter E, van Oostende TC, Whiteway M, Jiang L. The plasma membrane protein Rch1 is a negative regulator of cytosolic calcium homeostasis and positively regulated by the calcium/calcineurin signalling pathway in budding yeast. Eur J Cell Biol. 2016;95(3–5):164–74.
Mühlschlegel FA, Fonzi WA. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol Cell Biol. 1997;17(10):5960–7.
Saporito-Irwin SM, Birse CE, Sypherd PS, Fonzi WA. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15(2):601–13.
Davis D, Wilson RB, Mitchell AP. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20(3):971–8.
Sosinska GJ, de Koning LJ, de Groot PWJ, Manders EMM, Dekker HL, Hellingwerf KJ, de Koster CG, Klis FM. Mass spectrometric quantification of the adaptations in the wall proteome of Candida albicans in response to ambient pH. Microbiology (Reading). 2011;157(Pt 1):136–46.
Roque A, Petrezsélyová S, Serra-Cardona A, Ariño J. Genome-wide recruitment profiling of transcription factor Crz1 in response to high pH stress. BMC Genomics. 2016;17:662.
Serrano R, Ruiz A, Bernal D, Chambers JR, Arino J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol. 2002;46:1319–33.
Wang H, Liang Y, Zhang B, Zheng W, Xing L, Li M. Alkaline stress triggers an immediate calcium fluctuation in Candida albicans mediated by Rim101p and Crz1p transcription factors. FEMS Yeast Res. 2011;11(5):430–9.
Kullas AL, Martin SJ, Davis D. Adaptation to environmental pH: integrating the Rim101 and calcineurin signal transduction pathways. Mol Microbiol. 2007;66(4):858–71.
Hamada K, Fukuchi S, Arisawa M, Baba M, Kitada K. Screening for glycosylphosphatidylinositol (GPI)-dependent cell wall proteins in Saccharomyces cerevisiae. Mol Gen Genet. 1998;258(1–2):53–9.
Udenfriend S, Kodukula K. How glycosylphospatidylino-sitol-anchored membrane proteins are made. Annu Rev Biochem. 1995;64:563–91.
Acknowledgements
We gratefully acknowledge the help of Malcolm Whiteway for providing reagents.
Authors’ information
N/A
Funding
This work was funded by the National Key Laboratory of Non-Food Biomass Energy Technology, the National Natural Science Foundation of China to LJ (No. 81571966 and No. 81371784), the Guangxi Science and Technology Base and Talent Special project to LJ (Grant No. 2021AC18023) and the Guangxi Innovation-Driven Development Major Science and Technology Innovation Base Construction Project to LJ (Grant No. 2022–36-Z06).
Author information
Authors and Affiliations
Contributions
LJ designed the study, analyzed the data and wrote the manuscript. HX, YG and LW performed the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A.
Consent for publication
All authors approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Transcript levels of PHR1genein the wild type SN148 and its isogenic mutant crz1/crz1cells growing in log phase in the presence or absence of0.2M CaCl2for 2 hours. Figure S2. Knockoutstrategy of two alleles of PHR1and PCR confirmation of genotypes. Figure S3. Chromosomally C-terminal 3xHA tagging of PHR1. Figure S4. Deletion of PHR1leads to sensitivity of C. albicanscells toalkaline stress. Figure S5. Cation sensitivityofCandida albicanscells lacking a functional PHR1gene. Table S1. Primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, L., Xu, H., Gu, Y. et al. A glycosylated Phr1 protein is induced by calcium stress and its expression is positively controlled by the calcium/calcineurin signaling transcription factor Crz1 in Candida albicans. Cell Commun Signal 21, 237 (2023). https://doi.org/10.1186/s12964-023-01224-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01224-y