Abstract
Background
One of the most common side effects of the immunosuppressive drug tacrolimus (FK506) is the increased risk of new-onset diabetes mellitus. However, the molecular mechanisms underlying this association have not been fully clarified.
Methods
We studied the effects of the therapeutic dose of tacrolimus on mitochondrial fitness in beta-cells.
Results
We demonstrate that tacrolimus impairs glucose-stimulated insulin secretion (GSIS) in beta-cells through a previously unidentified mechanism. Indeed, tacrolimus causes a decrease in mitochondrial Ca2+ uptake, accompanied by altered mitochondrial respiration and reduced ATP production, eventually leading to impaired GSIS.
Conclusion
Our observations individuate a new fundamental mechanism responsible for the augmented incidence of diabetes following tacrolimus treatment. Indeed, this drug alters Ca2+ fluxes in mitochondria, thereby compromising metabolism-secretion coupling in beta-cells.
Similar content being viewed by others
Background
Tacrolimus (also known as fujimycin and FK506) is a macrolide lactone isolated from Streptomyces tsukubaensis, currently used as potent immunosuppressant in organ transplantation to reduce rejection rates [1, 2]. One of its most common adverse effects is new-onset diabetes mellitus following transplantation [3,4,5,6,7], a serious complication that also increases the risk of infection and cardiovascular disease [8]. Indeed, a 5-year follow-up study monitoring patients treated with tacrolimus after transplant revealed an incidence of diabetes of 41% [9].
The exact mechanisms underlying the diabetogenic effects of tacrolimus have not been fully elucidated. Various studies have suggested that tacrolimus side effects are attributable to its peripheral action, engendering a markedly reduced insulin sensitivity [10,11,12]. We hypothesize that tacrolimus at therapeutic dosage has also a direct detrimental effect on beta-cells, in particular on mitochondrial Ca2+ dynamics. To test our hypothesis, we evaluated the specific effects of tacrolimus on beta-cells.
Methods
Cell culture and drugs
INS-1 beta-cells (AddexBio, San Diego, CA) were cultured in a humidified atmosphere (37 °C) containing 5% CO2 in RPMI-1640 medium, and insulin levels were determined as previously described and validated by our group [13,14,15]. In some experiments, cells were treated with glucose (Bio-Techne, Abingdon, UK), L-leucine and glutamine (both from MyBioSource, San Diego, CA, USA), KCl (Merck KGaA, Darmstadt Germany) or tacrolimus (LC Laboratories, Woburn, MA, dissolved in dymethylsulfoxide).
Cell viability assay
Cell viability was evaluated using the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, as described [16, 17].
Ca2+ measurements
Ca2+ imaging experiments were carried out as previously described [15, 18,19,20]. Briefly, to assess mitochondrial Ca2+, cells were loaded with Rhod-2 AM (3 μM, Thermo Fisher Scientific, Waltham, MA) at 37 °C for 30 min, followed by washout and 1 h rest at room temperature for de-esterification. Because of its delocalized positive charge, Rhod-2 AM accumulates preferentially within the mitochondrial matrix, where it is hydrolyzed and trapped [15, 20, 21]. Fluorescence was detected using a pass-band filter of 545–625 nm in response to excitation at 542 nm. Ca2+ mobilization from the ER following caffeine (10 mM, Biorbyt, Cambridge, UK) stimulation was assessed loading the cells with Fura-2 acetoxymethyl (AM) ester (Thermo Fisher Scientific, 5 μM, 15 min, 37 °C), as described [15, 18]; images were obtained using a dual excitation fluorescence imaging system: changes in intracellular Ca2+ were reflected in the ratio of fluorescence emission acquired above 510 nm in response to excitation at 340 nm and 380 nm. Intracellular Ca2+ leak was assessed spectrophotometrically in microsomes obtained from pancreatic beta-cells, as previously described [15]. Besides the above mentioned indirect evaluation of ER Ca2+ in response to caffeine, ER Ca2+ content was assessed using the FRET-based camaleon D1ER (Addgene, Cambridge, MA) [22].
Mitochondrial respiration
Mitochondrial respiration was assessed using the Seahorse Analyzer (Agilent Technologies, Santa Clara, CA, USA), adding to each well glucose (16.7 mM), oligomycin (1 μM, Merck KGaA), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, 0.5 μM, Merck KGaA), rotenone and antimycin A (both 1 μM, Merck KGaA). After each assay, cells were collected to quantify DNA using QuantiFluor dsDNA System (Promega, Madison, WI, USA).
Cytochrome c oxidase activity assay
The cells were permeabilized by freeze-thaw cycle three times and mixed with detergent solution (0.1% bovine serum albumin, 250 mM sucrose, 10 mM KH2PO4 2.5 mM laurylmaltoside, all from Merck KGaA). The enzymatic activity of cytochrome c oxidase was spectrophotometrically measured at 550 nm.
ATP/ADP measurement
The genetically encoded sensors PercevalHR and pHRed (Addgene) were co-transfected in the cells in order to measure relative intracellular changes in ATP/ADP and pH, respectively. PercevalHR was excited at 405 nm (ADP) and 488 nm (ATP) and emission was collected at 530 nm. pHRed was excited using 405 nm and 546 nm and emission was collected at 630 nm band pass filter. Since PercevalHR has been reported to be sensitive to pH [23], a correction for pH was performed.
Statistical analysis
Experiments were performed in a blinded fashion at least three times, unless otherwise noted. All results are presented as mean ± SEM. Statistical analysis was performed via unpaired t test when comparing two groups (normal distribution was confirmed by Anderson-Darling test) and one-way ANOVA, followed by Tukey-Kramer post hoc correction, when comparing more than two groups. A P value <0.05 was considered statistically significant.
Results
Glucose-induced insulin secretion (GSIS) is reduced following tacrolimus treatment
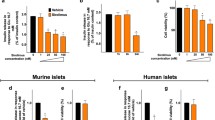
To evaluate the effects of tacrolimus on beta-cell function, we measured GSIS in INS-1 beta-cells. In a dose-response assay, we found that the treatment with 5 nM tacrolimus, which is the average level that has been measured in the blood of patients undergoing organ transplantation [24], was sufficient to determine a significant reduction of GSIS compared with vehicle (Fig. 1a). The same dose (5 nM) was then tested in a time-course assay, which revealed that 24-h incubation markedly impaired GSIS (Fig. 1b). Importantly, tacrolimus had a significantly detrimental effect on the metabolic response of beta-cells to leucine and glutamine (Fig. 1c), strongly suggesting an alteration in mitochondrial oxidative metabolism. Supporting this view, insulin secretion in response to cell depolarization obtained via KCl, thereby bypassing mitochondria, was comparable between groups (Fig. 1c).
Therapeutic doses of tacrolimus alter insulin secretion without affecting cell viability. Insulin release measured in beta-cells incubated for 24 h with vehicle or tacrolimus at the indicated doses (a) and with vehicle or 5 nM tacrolimus at the indicated times (b). Effect of fuel secretagogues leucine (Leu, 10 mM) and glutamine (Gln, 2 mM) on beta-cells treated for 24 h with vehicle or 5 nM tacrolimus (c). Cell viability assessed in beta-cells treated for 24 h with vehicle or 5 nM tacrolimus (d). Data are presented as mean ± S.E.M. of experiments performed at least in triplicate; *:p < 0.01 vs vehicle, ANOVA. In panel d, whiskers represent 1% to 99% spread of the data; NS: non-significant; t test
Therapeutic doses of tacrolimus do not affect beta-cell viability
To test the effect of 5 nM tacrolimus on cell viability, we performed an MTT assay and we did not observe any significant effect on beta-cell viability (Fig. 1d).
Tacrolimus alters mitochondrial Ca2+ uptake and mitochondrial respiration in beta-cells
Since mitochondrial Ca2+ has been shown to be a major determinant of beta-cell function, especially in terms of generation of metabolic coupling factors [25, 26], we evaluated the effect of tacrolimus on mitochondrial Ca2+. We found that tacrolimus significantly impairs mitochondrial Ca2+ uptake in beta-cells (Fig. 2a-b). Next, we assessed mitochondrial oxygen dynamics and we observed a marked decrease in oxygen consumption rate following incubation with tacrolimus (Fig. 3a-b), further supporting our hypothesis of a direct effect of this immunosuppressant on mitochondrial fitness.
Tacrolimus impairs mitochondrial Ca2+ uptake. Representative traces (a) of glucose-induced mitochondrial Ca2+ uptake evaluated in beta-cells incubated for 24 h with vehicle or 5 nM Tacrolimus. b quantification of experiments (at least 35 cells per group were analyzed): whiskers represent 1% to 99% spread of the data; *:p < 0.001 vs vehicle
Tacrolimus impairs mitochondrial respiration in beta-cells. Analysis of oxygen consumption rate (OCR) in beta-cells incubated for 24 h with vehicle or 25 nM tacrolimus and then treated with glucose, oligomycin, phenylhydrazone (FCCP), antimycin A and rotenone, at the indicated times (a). Data represent mean ± S.E.M. of at least 4 independent experiments. b Quantification of the maximal respiratory capacity: whiskers indicate 1% to 99% spread of the data; *:p < 0.01 vs vehicle
Tacrolimus decreases cytochrome c oxidase activity and causes a decrease in ATP/ADP ratio in beta-cells
To further investigate the role of tacrolimus on mitochondrial function, we first measured the enzymatic activity of cytochrome c oxidase, a key component of the electron transport chain; compared with vehicle-treated cells, we detected a significant reduction in cytochrome c oxidase activity following incubation with tacrolimus (Fig. 4a). Then, we evaluated the dynamics of ATP/ADP levels within the cell, fundamental regulators of fuel-stimulated insulin release [27]. Interestingly, tacrolimus significantly reduced ATP/ADP ratio in response to glucose compared with vehicle-treated cells (Fig. 4b-c).
Tacrolimus at therapeutic dosage reduces mitochondrial ATP production. The enzymatic activity of cytochrome c oxidase was measured in beta-cells (at least 30 per group) incubated for 24 h with vehicle or 5 nM Tacrolimus (a); data represent mean ± S.E.M. The ATP/ADP ratio in response to 16.7 mM glucose was determined by PercevalHR ratio and corrected by pHRed ratio (b); the peak amplitude is quantified in panel c, where whiskers represent 1% to 99% spread of the data; *:p < 0.01 vs vehicle
Tacrolimus at therapeutic doses depletes intracellular Ca2+ stores
Given the anatomical and functional connection between mitochondria and endoplasmic reticulum (ER) [28,29,30,31], we tested the effects of tacrolimus on ER Ca2+ dynamics, and we observed that 24 h incubation with 5 nM tacrolimus caused significant ER Ca2+ depletion and intracellular Ca2+ leak, evaluated both indirectly, assessing Ca2+ dynamics in response to caffeine, and directly, using an ER-targeted probe, with consistent results (Fig. 5).
Tacrolimus causes ER Ca2+ depletion and ER Ca2+ leak. Endoplasmic reticulum (ER) Ca2+ stores (maximal peak amplitudes of caffeine-induced Ca2+ release, (a) and ER Ca2+ leak (b) were assessed following 24 h incubation with vehicle or 5 nM tacrolimus. c Direct evaluation of ER Ca2+ (representative curves, the arrow indicates thapsigargin, 1 μm) using the ER-targeted probe D1ER (left). The slope of the FRET ratio decrease after thapsigargin addition represents a bona fide measurement of the rate of Ca2+ leak (right), consistent with the results depicted in panel b. Data are presented as mean ± S.E.M. of at least 5 experiments performed in triplicate; *:p < 0.05 vs vehicle
Discussion
The main finding of the present study is that tacrolimus has a detrimental effect on mitochondrial Ca2+ dynamics and beta-cell function, determining a reduced mitochondrial Ca2+ uptake and significantly impairing GSIS. Our results are consistent with the previous demonstration by Wollheim and colleagues of the essential role played by mitochondrial Ca2+ in metabolism-secretion coupling [26]. Indeed, one of the key roles of Ca2+ within mitochondria is to serve as a signal for oxidative metabolism, activating at least three biochemical reactions in the Krebs cycle, producing reducing equivalents [32], thereby accelerating mitochondrial respiration and ATP generation [27].
Several studies investigating the potential mechanisms underlying the pathogenesis of new-onset diabetes following tacrolimus-based treatments had focused on insulin-resistance [8, 10, 33,34,35,36,37]. Alterations in peripheral insulin-signaling have been also reported in response to other immunomodulators, including rapamycin, cyclosporine A, and steroids [38,39,40,41,42]. In fact, ultrastructural analysis has revealed that cyclosporine A causes mitochondrial dysmorphology in renal allograft biopsies [43]. Herein, we sought instead to test the effects of tacrolimus in vitro, using the clonal INS-1 beta-cells, which provide one of the best systems to directly study the pharmacologic effects of a compound on beta-cell behavior, avoiding extracellular confounding factors and potential compensatory mechanisms present in whole organisms or in islet clusters [44], especially for the assessment of mitochondrial Ca2+ dynamics [26, 45,46,47]. Of course, we acknowledge that the lack of experimental evidence in islets or animal models can also be seen as a limitation of this work.
We demonstrate here that 5 nM tacrolimus is sufficient to determine a marked reduction in GSIS, without affecting cell viability. We decided to test such dose because this is the average level of drug that has been actually measured in islet transplant recipients [24]. The lack of significant effect of therapeutic doses of tacrolimus on cell viability is consistent with earlier observations in HIT-T15 hamster beta-cells [48]. Instead, when used at high doses (e.g. 1 μM) tacrolimus does cause apoptosis, as shown ex vivo in experiments performed on rat islets [49]. The pro-apoptotic action of tacrolimus following prolonged exposures (> 24 h) has been also reported [50].
We and others have previously shown that mitochondrial function in pancreatic beta-cells and other tissues is mechanistically related to intracellular Ca2+ fluxes [15, 28, 31, 32, 51]. Here we demonstrate that tacrolimus treatment directly impairs mitochondrial Ca2+ uptake. This phenomenon could explain the reduced mitochondrial respiration observed in tacrolimus-treated beta-cells. Indeed, as mentioned above, Ca2+ activates dehydrogenases in the tricarboxylic acid cycle within the mitochondrion [32].
We also demonstrate here that an impaired mitochondrial Ca2+ uptake is mechanistically linked to mitochondrial dysfunction, as shown by the reduced enzymatic activity of cytochrome c oxidase following tacrolimus treatment. Our findings are consistent with the decreased mitochondrial bioenergetics observed in rat islets incubated with tacrolimus [52]. Moreover, tacrolimus has been shown to potentiate glucolipotoxicity in different models [53]. Crucially, the dynamic evaluation of ATP/ADP levels confirms that tacrolimus-treated cells generate less ATP compared with vehicle-treated cells, an observation that is in line with earlier reports suggesting that mitochondrial Ca2+ is essential for energy production [54,55,56]. The decreased insulin release detected in tacrolimus-treated beta-cells following stimulation with secretagogues that trigger insulin secretion via mitochondrial metabolism (e.g. leucine/glutamine), but not following KCl-induced membrane depolarization, corroborates this view, strongly indicating a defect in mitochondrial bioenergetics. Further studies are necessary to decipher in detail the exact processes linking tacrolimus pharmacodynamics to the observed effects on ER and mitochondrial Ca2+ reported here for the first time. Potential candidates, currently being investigated in our laboratory, include the immunophilin FK506 binding protein (FKBP), which forms a complex with tacrolimus [57,58,59], and ER stress, which has been recently reported in tacrolimus-treated lymphocytes [60].
Conclusion
Our findings individuate in impaired mitochondrial Ca2+ uptake a novel mechanism underlying the increased incidence of diabetes following tacrolimus treatment.
Abbreviations
- Ca2+ :
-
Calcium
- ER:
-
Endoplasmic reticulum
- FCCP:
-
Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- Fura-2 AM:
-
Fura-2-acetoxymethyl ester
- GSIS:
-
Glucose-stimulated insulin secretion
- Rhod2-AM:
-
1-[2-Amino-5-(3-dimethylamino-6- dimethylammonio- 9-xanthenyl) phenoxy]-2-(2-amino-5-methylphenoxy) ethane-N,N,N′,N′-tetra-acetic acid, tetra-acetoxymethyl ester
References
Wallemacq PE, Reding R. FK506 (tacrolimus), a novel immunosuppressant in organ transplantation: clinical, biomedical, and analytical aspects. Clin Chem. 1993;39:2219–28.
Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–33.
Chanchlani R, Joseph Kim S, Kim ED, Banh T, Borges K, Vasilevska-Ristovska J, Li Y, Ng V, Dipchand AI, Solomon M, et al. Incidence of hyperglycemia and diabetes and association with electrolyte abnormalities in pediatric solid organ transplant recipients. Nephrol Dial Transplant. 2017;32:1579–86.
Regelmann MO, Goldis M, Arnon R. New-onset diabetes mellitus after pediatric liver transplantation. Pediatr Transplant. 2015;19:452–9.
Andrade AR, Bittencourt PL, Codes L, Evangelista MA, Castro AO, Sorte NB, Almeida CG, Bastos JA, Cotrim HP. New onset diabetes and non-alcoholic fatty liver disease after liver transplantation. Ann Hepatol. 2017;16:932–40.
Rangel EB. Tacrolimus in pancreas transplant: a focus on toxicity, diabetogenic effect and drug-drug interactions. Expert Opin Drug Metab Toxicol. 2014;10:1585–605.
Santos AH Jr, Chen C, Casey MJ, Womer KL, Wen X. New-onset diabetes after kidney transplantation: can the risk be modified by choosing immunosuppression regimen based on pretransplant viral serology? Nephrol Dial Transplant. 2017; in press. https://www.ncbi.nlm.nih.gov/pubmed/29045704.
Sessa A, Esposito A, Giliberti A, Iavicoli G, Costa C, Bergallo M, Lettieri E, Rossano R, Capuano M. Immunosuppressive agents and metabolic factors of cardiovascular risk in renal transplant recipients. Transplant Proc. 2009;41:1178–82.
Weir M. Impact of immunosuppressive regimes on posttransplant diabetes mellitus. Transplant Proc. 2001;33:23S–6S.
Montero N, Pascual J. Immunosuppression and post-transplant hyperglycemia. Curr Diabetes Rev. 2015;11:144–54.
Bhat M, Pasini E, Copeland J, Angeli M, Husain S, Kumar D, Renner E, Teterina A, Allard J, Guttman DS, Humar A. Impact of immunosuppression on the metagenomic composition of the intestinal microbiome: a systems biology approach to post-transplant diabetes. Sci Rep. 2017;7:10277.
Rodriguez-Rodriguez AE, Trinanes J, Velazquez-Garcia S, Porrini E, Vega Prieto MJ, Diez Fuentes ML, Arevalo M, Salido Ruiz E, Torres A. The higher diabetogenic risk of tacrolimus depends on pre-existing insulin resistance. A study in obese and lean Zucker rats. Am J Transplant. 2013;13:1665–75.
Santulli G, Lombardi A, Sorriento D, Anastasio A, Del Giudice C, Formisano P, Beguinot F, Trimarco B, Miele C, Iaccarino G. Age-related impairment in insulin release: the essential role of beta(2)-adrenergic receptor. Diabetes. 2012;61:692–701.
Lombardi A, Ulianich L, Treglia AS, Nigro C, Parrillo L, Lofrumento DD, Nicolardi G, Garbi C, Beguinot F, Miele C, Di Jeso B. Increased hexosamine biosynthetic pathway flux dedifferentiates INS-1E cells and murine islets by an extracellular signal-regulated kinase (ERK)1/2-mediated signal transmission pathway. Diabetologia. 2012;55:141–53.
Santulli G, Pagano G, Sardu C, Xie W, Reiken S, D'Ascia SL, Cannone M, Marziliano N, Trimarco B, Guise TA, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest. 2015;125:1968–78.
De Vitis S, Sonia Treglia A, Ulianich L, Turco S, Terrazzano G, Lombardi A, Miele C, Garbi C, Beguinot F, Di Jeso B. Tyr phosphatase-mediated P-ERK inhibition suppresses senescence in EIA + v-raf transformed cells, which, paradoxically, are apoptosis-protected in a MEK-dependent manner. Neoplasia. 2011;13:120–30.
Fiory F, Lombardi A, Miele C, Giudicelli J, Beguinot F, Van Obberghen E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia. 2011;54:2941–52.
Xie W, Santulli G, Guo X, Gao M, Chen BX, Marks AR. Imaging atrial arrhythmic intracellular calcium in intact heart. J Mol Cell Cardiol. 2013;64:120–3.
Umanskaya A, Santulli G, Xie W, Andersson DC, Reiken SR, Marks AR. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci U S A. 2014;111:15250–5.
Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112:11389–94.
Xie W, Santulli G, Reiken SR, Yuan Q, Osborne BW, Chen BX, Marks AR. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep. 2015;5:11427.
Takeuchi A, Kim B, Matsuoka S. The mitochondrial Na+−Ca2+ exchanger, NCLX, regulates automaticity of HL-1 cardiomyocytes. Sci Rep. 2013;3:2766.
Tantama M, Martinez-Francois JR, Mongeon R, Yellen G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun. 2013;4:2550.
Desai NM, Goss JA, Deng S, Wolf BA, Markmann E, Palanjian M, Shock AP, Feliciano S, Brunicardi FC, Barker CF, et al. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation. 2003;76:1623–5.
Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008;44:64–76.
Kennedy ED, Rizzuto R, Theler JM, Pralong WF, Bastianutto C, Pozzan T, Wollheim CB. Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J Clin Invest. 1996;98:2524–38.
Sweet IR, Cook DL, DeJulio E, Wallen AR, Khalil G, Callis J, Reems J. Regulation of ATP/ADP in pancreatic islets. Diabetes. 2004;53:401–9.
Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–7.
Gambardella J, Trimarco B, Iaccarino G, Santulli G. New insights in cardiac calcium handling and excitation-contraction coupling. Adv Exp Med Biol. 2017; in press. https://www.ncbi.nlm.nih.gov/pubmed/28956314
Santulli G. Mitochondrial dynamics in cardiovascular medicine. Berlin: Springer Nature; 2017.
Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta. 1837;2014:461–9.
Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol. 2015;8:206–22.
Li Z, Sun F, Zhang Y, Chen H, He N, Chen H, Song P, Wang Y, Yan S, Zheng S. Tacrolimus induces insulin resistance and increases the glucose absorption in the jejunum: a potential mechanism of the diabetogenic effects. PLoS One. 2015;10:e0143405.
Bogan JS. Endocytic cycling of glucose transporters and insulin resistance due to immunosuppressive agents. J Clin Endocrinol Metab. 2014;99:3622–4.
Chen QJ, Li J, Zuo SR, Zhang YP, Jia SJ, Yuan H, Liu SK, Cheng K, Ming YZ, Zuo XC, et al. Tacrolimus decreases insulin sensitivity without reducing fasting insulin concentration: a 2-year follow-up study in kidney transplant recipients. Ren Fail. 2015;37:601–6.
Sharif A, Ravindran V, Moore RH, Dunseath G, Luzio S, Owens DR, Baboolal K. Insulin resistance indexes in renal transplant recipients maintained on tacrolimus immunosuppression. Transplantation. 2010;89:327–33.
Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648–54.
Pereira MJ, Palming J, Rizell M, Aureliano M, Carvalho E, Svensson MK, Eriksson JW. The immunosuppressive agents rapamycin, cyclosporin A and tacrolimus increase lipolysis, inhibit lipid storage and alter expression of genes involved in lipid metabolism in human adipose tissue. Mol Cell Endocrinol. 2013;365:260–9.
Garcia-Casarrubios E, de Moura C, Arroba AI, Pescador N, Calderon-Dominguez M, Garcia L, Herrero L, Serra D, Cadenas S, Reis F, et al. Rapamycin negatively impacts insulin signaling, glucose uptake and uncoupling protein-1 in brown adipocytes. Biochim Biophys Acta. 2016;1861:1929–41.
Fuhrmann A, Lopes P, Sereno J, Pedro J, Espinoza DO, Pereira MJ, Reis F, Eriksson JW, Carvalho E. Molecular mechanisms underlying the effects of cyclosporin A and sirolimus on glucose and lipid metabolism in liver, skeletal muscle and adipose tissue in an in vivo rat model. Biochem Pharmacol. 2014;88:216–28.
Galindo RJ, Wallia A. Hyperglycemia and diabetes mellitus following organ transplantation. Curr Diab Rep. 2016;16:14.
Kockx M, Glaros E, Leung B, Ng TW, Berbee JF, Deswaerte V, Nawara D, Quinn C, Rye KA, Jessup W, et al. Low-density lipoprotein receptor-dependent and low-density lipoprotein receptor-independent mechanisms of cyclosporin A-induced dyslipidemia. Arterioscler Thromb Vasc Biol. 2016;36:1338–49.
Nacar A, Kiyici H, Ogus E, Zagyapan R, Demirhan B, Ozdemir H, Haberal M. Ultrastructural examination of glomerular and tubular changes in renal allografts with cyclosporine toxicity. Ren Fail. 2006;28:543–7.
Poitout V, Olson LK, Robertson RP. Insulin-secreting cell lines: classification, characteristics and potential applications. Diabetes Metab. 1996;22:7–14.
Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5:253–64.
Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic beta-cells. J Biol Chem. 2012;287:34445–54.
Nishi Y, Fujimoto S, Sasaki M, Mukai E, Sato H, Sato Y, Tahara Y, Nakamura Y, Inagaki N. Role of mitochondrial phosphate carrier in metabolism-secretion coupling in rat insulinoma cell line INS-1. Biochem J. 2011;435:421–30.
Redmon JB, Olson LK, Armstrong MB, Greene MJ, Robertson RP. Effects of tacrolimus (FK506) on human insulin gene expression, insulin mRNA levels, and insulin secretion in HIT-T15 cells. J Clin Invest. 1996;98:2786–93.
Grunnet LG, Aikin R, Tonnesen MF, Paraskevas S, Blaabjerg L, Storling J, Rosenberg L, Billestrup N, Maysinger D, Mandrup-Poulsen T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes. 2009;58:1807–15.
Choi SJ, You HS, Chung SY. Tacrolimus-induced apoptotic signal transduction pathway. Transplant Proc. 2008;40:2734–6.
Granatiero V, De Stefani D, Rizzuto R. Mitochondrial calcium handling in physiology and disease (Editor: G. Santulli). Adv Exp Med Biol. 2017;982:25–47.
Rostambeigi N, Lanza IR, Dzeja PP, Deeds MC, Irving BA, Reddi HV, Madde P, Zhang S, Asmann YW, Anderson JM, et al. Unique cellular and mitochondrial defects mediate FK506-induced islet beta-cell dysfunction. Transplantation. 2011;91:615–23.
Trinanes J, Rodriguez-Rodriguez AE, Brito-Casillas Y, Wagner A, De Vries APJ, Cuesto G, Acebes A, Salido E, Torres A, Porrini E. Deciphering tacrolimus-induced toxicity in pancreatic beta cells. Am J Transplant. 2017;17:2829.
Tarasov AI, Semplici F, Li D, Rizzuto R, Ravier MA, Gilon P, Rutter GA. Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic beta cells. Pflugers Arch. 2013;465:543–54.
Vandecasteele G, Szabadkai G, Rizzuto R. Mitochondrial calcium homeostasis: mechanisms and molecules. IUBMB Life. 2001;52:213–9.
Liu JC, Parks RJ, Liu J, Stares J, Rovira II, Murphy E, Finkel T. The in vivo biology of the mitochondrial calcium uniporter. Adv Exp Med Biol. 2017;982:49–63.
Tong M, Jiang Y. FK506-binding proteins and their diverse functions. Curr Mol Pharmacol. 2015;9:48–65.
Yuan Q, Chen Z, Santulli G, Gu L, Yang ZG, Yuan ZQ, Zhao YT, Xin HB, Deng KY, Wang SQ, Ji G. Functional role of Calstabin2 in age-related cardiac alterations. Sci Rep. 2014;4:7425.
Chiasson VL, Pakanati AR, Hernandez M, Young KJ, Bounds KR, Mitchell BM. Regulatory T-cell augmentation or Interleukin-17 inhibition prevents calcineurin inhibitor-induced hypertension in mice. Hypertension. 2017;70:183–91.
Lee HK, Chung MW, Chung YW, Choi SK, Choi SJ, Chung SY. Expression of endoplasmic reticulum-mediated stress proteins in FK506-treated T-lymphocytes. Transplant Proc. 2016;48:1292–6.
Acknowledgements
We thank Drs. X. Du, TV. McDonald, RN. Kitsis, and Y. Tomer (Albert Einstein College of Medicine) for helpful discussions.
Funding
G.S. is supported by the NIH (R00DK107895) and ES-DRC P30DK20541.
Availability of data and materials
The datasets supporting the conclusions of this article are included within this article.
Author information
Authors and Affiliations
Contributions
AL designed and performed experiments, analyzed data and wrote the paper; BT and GI analyzed data and contributed to discussion; GS conceived of the project and experimental plans, analyzed data, and wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lombardi, A., Trimarco, B., Iaccarino, G. et al. Impaired mitochondrial calcium uptake caused by tacrolimus underlies beta-cell failure. Cell Commun Signal 15, 47 (2017). https://doi.org/10.1186/s12964-017-0203-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-017-0203-0