Abstract
Background
It is well established that lipoprotein(a)[Lp(a)] play a vital role in atherosclerosis. Whether Lp(a) can predict recurrence of cardiovascular events (CVEs) in prior CVEs patients is still unclear. We aim to investigate its association with subsequent long-term adverse events in this high-risk population.
Methods
A total of 4,469 patients with prior CVEs history after PCI were consecutively enrolled and categorized according Lp(a) values of < 10 (low), 10 to 30 (medium), and ≥ 30 mg/dL (high). The primary endpoint was MACCE, a composite of all-cause death, myocardial infarction, stroke and unplanned revascularization.
Results
During an average of 5.0 years of follow-up, 1,078 (24.1%) and 206 (4.6%) patients experienced MACCE and all-cause death with 134 (3.0%) of whom from cardiac death. The incidence of MACCE, all-cause death and cardiac death were significantly higher in the high Lp(a) group (p < 0.05). After adjustment of confounding factors, high Lp(a) level remained an independent risk factor for MACCE (adjusted HR 1.240, 95%CI 1.065–1.443, p = 0.006), all-cause death (adjusted HR 1.445, 95%CI 1.023–2.042, p = 0.037) and cardiac death (adjusted HR 1.724, 95%CI 1.108–2.681, p = 0.016). This correlation remained significant when treated as a natural logarithm-transformed continuous variable. This finding is relatively consistent across subgroups and confirmed again in two sensitivity analyses.
Conclusions
Our present study confirmed that Lp(a) was an independent predictor for recurrent CVEs in patients with established CVEs, illustrating that Lp(a) level might be a valuable biomarker for risk stratification and prognostic assessment in this high-risk population.
Similar content being viewed by others
Introduction
Coronary heart disease (CHD) is a common cardiovascular disease and is a main cause of disability and death. Patients with history of cardiovascular events (CVEs) entail heavy coronary atherosclerotic burden. Despite significant advances in diagnosis and management have improved prognosis of CVEs in the last decades, patients continue to experience CVEs and remain at high-risk for recurrence [1] . Moreover, previous studies reported that healthcare costs of subsequent CVEs are substantial and incremental costs remain elevated for several years after an event [2]. Therefore, identifying predictive biomarkers that would contribute to effective screening or early diagnosis in this high-risk population may reduce recurrent CVEs.
More recently, an increased Lipoprotein(a) [Lp(a)] was identified as a major cardiovascular lipid-related residual risk factor. Lp(a) consists of a low-density lipoprotein (LDL)-like particle that is bound to apolipoproteinB100(apoB), which is then linked with apolipoprotein(a)[apo(a)] [3]. Large prospective epidemiological studies confirmed that Lp(a) is an independent and causal risk factor for a variety of atherothrombotic disorders, most notably CHD through proatherogenic, prothrombotic and proinflammatory effects, it is also related with occurrences of peripheral arterial disease, myocardial infarction(MI), and ischemic stroke [4,5,6]. Moreover, our previous researches indicated that Lp(a) levels were strongly associated coronary severity and could increase risk of CVEs in patients with three-vessel coronary artery disease (CAD) or chronic kidney disease after percutaneous coronary intervention (PCI) [7,8,9].
To the best of our knowledge, its clinical implications have not been investigated in the setting of prior CVEs with advanced atherosclerotic burden. Therefore, we aim to evaluate whether Lp(a) is able to predict long-term CVEs recurrence in patients with established CVEs after PCI, based on the analysis from a real-world, prospective, observational cohort of Chinese patients.
Methods
Study population
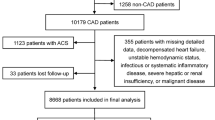
This study was based on a prospective, observational, single-center cohort. From January 2013 to December 2013, 10,724 CAD patients were consecutively enrolled undergoing PCI at Fuwai Hospital, Chinese Academy of Medical Sciences (Beijing, China). The study flow-chart was shown as Fig. 1. The inclusion criteria were patients who had experienced a history of prior CVEs [defined as MI, stroke, peripheral arterial disease, PCI and coronary artery bypass grafting (CABG)] before admission. The exclusion criteria were patients with significant hematologic disorders and infectious or systematic inflammatory disease; severe liver and/or renal dysfunction; decompensated heart failure or arrhythmia; malignant tumors, and patients with missing Lp(a) data. Finally, a total of 4,469 patients were enrolled in the current study. Finally, a total of 4,469 patients were enrolled in analysis. The study protocol was approved by the Institutional Review Board of Fuwai Hospital and complied with the Declaration of Helsinki. All patients provided written informed consent before the intervention.
Laboratory analysis
When admission, all patients are required to take their venous blood after fasting for at least 12 h. Using the automated biochemical analyzer to achieve the concentration measurements of relevant indicators, which including low-density lipoprotein cholesterol (LDL-C), the serum total cholesterol (TC), triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) (The specific model is Hitachi 7150, Tokyo, Japan). Same as our previous researches [7,8,9], Lp(a) levels were assayed by an immunoturbidimetry method according to the manufacturer's guide, a latex turbidimetric method [LASAY Lp(a) auto; SHIMA laboratories] and presented in mg/dL with a normal value of < 30 mg/dL. All other laboratory measurements were conducted at the biochemistry center of FuWai Hospital by standard biochemical techniques.
Procedural details
As described in our previous study [7, 8], the PCI strategy and stent type were left to the discretion of the operating surgeon. Before the procedure, selected patients with PCI who were not on long-term aspirin and/or P2Y12 inhibitors received oral administration of aspirin 300 mg and clopidogrel (loading dose of 300 mg). Patients with acute coronary syndrome (ACS) who were scheduled for PCI received the same dose of aspirin and clopidogrel as soon as possible. During the procedure, unfractionated heparin (100 U/kg) was administered to all of the patients, and glycoprotein IIb/IIIa inhibitors were used according to the operator’s judgment. More than 50% stenosis of the left main artery, left anterior descending artery, left circumflex artery, right coronary artery, and main branch of these vessels was defined as coronary artery stenosis. More than 70% stenosis of these vessels was indicated for coronary stent implantation. After the procedure, aspirin was prescribed at a dose of 100 mg daily indefinitely, and clopidogrel 75 mg daily or ticagrelor 90 mg twice daily for at least 1 year was recommended after PCI.
Patient follow-up
All of the patients were evaluated at five time points after the discharge (1, 6 and 12-month, and 2-year, and thereafter up to 5-year). Follow-up data were collected through medical records, telephone calls, or clinical visits. Investigator training, blinded questionnaire filling, and telephone recording were performed to achieve high-quality data. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE), of which all-cause death, MI, unplanned revascularization and stroke during the follow-up were included. The secondary outcomes included all-cause death and cardiac death. Death that could not be attributed to a noncardiac etiology was considered cardiac death. As for myocardial infarction, the third general definition is chosen as its definition in this paper [10]. Unplanned revascularization specifically refers to the treatment of target vessels of ischemic symptoms and events by means of repetitive percutaneous access or surgical bypass. Stroke was defined as a loss of neurological function with residual symptoms at least 72 h after onset [11].
Statistical analysis
Continuous variables are reported as mean ± standard deviation or median with interquartile range and categorical variables were presented as number (percentage). Comparisons between continuous variables were performed by independent sample Student’s t test or Mann–Whitney U test and the chi-square test for categorical variables. The Lp(a) was subsequently analyzed as categorical variable, patients were stratified into three groups according to Lp(a) distribution [Low Lp(a)(< 10 mg/dL), Medium Lp(a)(10 mg/dL ≤ Lp(a) < 30 mg/dL), High Lp(a) (30 ≥ mg/dL)]. Survival curves were constructed by the Kaplan–Meier method and compared by the log-rank test according to Lp(a) groups. Univariable and multivariable Cox proportional hazard regressions were performed to calculate the hazard ratio (HR) and 95% confidence interval (CI) and evaluate the associations between Lp(a) levels (as a categorical or log-transformed continuous variable) and clinical outcomes. The multivariable model was adjusted for the following covariates in an all-enter way: age, sex, ACS, family history of CAD, hypertension, diabetes, smoking, SYNTAX score, Left ventricular eject fraction (LVEF), estimated glomerular filtration rate (eGFR), high sensitivity C-reactive protein (hsCRP), TG, LDL-C, statins because of their statistical significance in univariate analysis or clinical importance. Restricted cubic spline curves (RCS) were created to assess linearity assumptions of the relationship between Lp(a) and MACCE and all-cause death.
Exploratory subgroup analyses of the primary outcome were performed according to age (< 65 or ≥ 65 years), sex (male or female), diabetes (yes or no), hypertension(yes or no), presentation (SAP or ACS), statins (yes or no), LDL-C leves (< 1.8 or ≥ 1.8 mmol/L) and hsCRP levels(≤ 3 or > 3 mg/L) between plasma Lp(a) level (< 30 or ≥ 30 mg/dL) and these covariates were tested to interpret potential subgroup differences. The above-described multivariable Cox proportional hazards models were used for the interaction and subgroup analyses.
Meanwhile, we performed sensitivity analysis of to confirm the association of plasma Lp(a) concentration for risk prediction of MACCE by 2 methods, which excluding subjects with Lp(a) levels in the top or the bottom 5% and excluding participants with prior CABG, stroke and peripheral arterial disease. The prevalence of all-cause mortality is 2% of the population in Beijing, China. The present desired significance level was P < 0.05, and the study power was 0.80, thus, the overall required sample size was calculated to be 3000 cases to be able to detect statistical significance. Two-sided p-value of < 0.05 were considered statistically significant. Analyses were performed using SPSS software version 25.0 (IBM Corporation, Chicago, IL) and R language (version 3.5.2, Feather Spray; The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
As presented in Fig. 1, A total of 4,469 patients eligible were ultimately included in the study. According to Lp(a) concentrations, patients were divided into three subgroups as low Lp(a) group (< 10 mg/dL, n = 1,402), medium Lp(a) group (≥ 10 and < 30 mg/dL, n = 1,458), high Lp(a) group (≥ 30 mg/dL,n = 1,609), respectively. In the overall population, Lp(a) levels had a skewed distribution with a tail toward the highest levels, which was consistent with previous researches [12] (Fig. 2).
The baseline clinical and laboratory characteristics of the study participants were shown in Table 1. In general, the mean age of the study was 59.51 ± 10.27 years, and 3,577(80.0%) were male. Participants in high Lp(a) group were more older and female, with higher TC, LDL-C, but lower plasma TG, LVEF, BMI, and tend to have lower proportion of current smoker. The baseline glucose, HbA1c and eGFR did not differ significantly among the three groups. Notably, baseline Lp(a) and hsCRP levels were significantly elevated from low to high Lp(a) subjects (p < 0.001). Additionally, the prevalence of cardiovascular risk profiles such as hypertension, dyslipidemia, diabetes, family history of CAD and chronic obstructive pulmonary disease(COPD) did not differ significantly across the three groups. Moreover, the prevalence of left main disease and STNTAX score were similar among groups. With regard to secondary prevention medication, there was no significant differences in the proportion of aspirin, P2Y12 inhibitor, statins, ACEI/ARB, β-blockers, and CCB among groups.
Relation of Risk factors and Recurrent Adverse Events
The distribution of clinical parameters with and without MACCE was summarized in Supplementary Table 1. Compared to those free of events, those with MACCE were older, with higher levels of baseline glucose, HbA1c and hsCRP level, but with lower LVEF and estimated GFR. The proportion of hypertension and diabetes were significantly higher in patients who developed MACCE. The mean concentration of TG, TC, LDL-C and HDL-C did not differ significantly between the groups. Of note, MACCE patients had higher STNTAX score compared with the controls (p = 0.005). While, there was no significant difference between the two groups in the presence rate of dyslipidemia, family history of CAD, smoking and COPD, as while as proportion of Left main involvement and ACS presentation. Moreover, there was no difference in terms of medication prescriptions between the event and non-event ones. Specially, the MACCE-present group had statistically higher admission Lp(a) levels than the MACCE-absent group [21.56 (8.6.2–46.98) vs. 18.12 (7.68–41.72) mg/dL, p = 0.002].
Lipoprotein(a) and Recurrent Adverse Events
The overall median follow-up time was 5.0 years (interquartile range 3.0–5.1 years), and the response rate was 92.3% (Fig. 1). During the period, 1,078 (24.1%) and 206 (4.6%) patients experienced MACCE and all-cause death with 134 (3.0%) of whom from cardiac death. In total cohort, the incidence of MACCE in low, medium and high Lp(a) group was 22.1%, 22.4% and 27.4%, based on the cut-off value of 10 and 30 mg/dL, respectively.
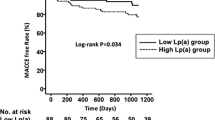
Significantly increased risks of MACCE, all-cause death, and cardiac death occurred in the high Lp(a) group compared with the low Lp(a) group (all p < 0.05, Table 2). Of note, the significantly higher risk of MACCE in the higher Lp(a) group was mainly driven by all-cause death, because there were no significant differences among groups in the risk for MI, stroke and revascularization (all p > 0.05). Cumulative free survival of the whole cohort according to Lp(a) subgroups was estimated by Kaplan–Meier curves and revealed similar results (Fig. 3. log-rank p < 0.001 for MACCE, all-cause death and cardiac death). Univariable Cox analysis showed that high Lp(a) level was associated with higher risks of MACCE(crude HR 1.239, 95% CI 1.072–1.433, p = 0.004), all-cause death (crude HR 1.597, 95% CI 1.146–2.225, p = 0.006) and cardiac death (crude HR 1.939, 95%CI 1.269–2.962, p = 0.002), but not MI, stroke, or unplanned revascularization (Table 2). After adjustment for covariates, high Lp(a) level remained an independent risk factor for MACCE (adjusted HR 1.240, 95% CI 1.065–1.443, p = 0.006), all-cause death (adjusted HR 1.445, 95% CI 1.023–2.042, p = 0.037) and cardiac death (adjusted HR 1.724, 95% CI 1.108–2.681, p = 0.016). When modeled as a continuous variable per unit increase in log-transformed Lp(a) concentration, multivariate Cox regression analysis showed an independent association that high Lp(a) group had 1.140-fold risk of MACCE (95%CI 1.006–1.292, p = 0.040), 1.386-fold risk of all-cause death (95%CI 1.026–1.872, p = 0.033) and 1.591-fold risk of cardiac death (95%CI 1.087–2.330, p = 0.017) after adjustment. RCS showed a nonlinear relation between Lp(a) on continuous scales and the risk of MACCE and all-cause death (Fig. 4).
The relationship of higher Lp(a) level (< 30 or ≥ 30 mg/dL) with MACCE risk was relatively consistent across the subgroups of age, sex, diabetes, hypertension, presentation, baseline statins use, LDL-C levels and hsCRP levels(Fig. 5). There were no significant interactions between high Lp(a) level and these covariates (interaction p > 0.05 for all subgroups).
In a sensitivity analysis by excluding population with Lp(a) levels in the top or the bottom 5%(n = 447), revealed that high Lp(a) was considered to be a statistically risk factor of MACCE (adjusted HR 1.253, 95%CI 1.062–1.479, p = 0.007). In addition, another sensitivity analysis which excluding subjects with CABG, stroke and peripheral arterial disease (n = 1686), showed that high Lp(a) remains an independent predictor of MACCE in this population (adjusted HR 1.238, 95%CI 1.016–1.509, p = 0.035) after adjusting for potential confounding factors (shown in Supplementary Table 2).
Discussion
This study is conducted on a sizable high-risk population with prior CVEs to investigate an association between higher baseline Lp(a) levels and recurrent CVEs. Our study demonstrated that high plasma Lp(a) level is an independent risk factor for 5-year MACCE, all-cause death, and cardiac death and is relatively consistent across subgroups. This finding was confirmed again in two sensitivity analyses. The present study illustrates that Lp(a) level might be a valuable biomarker for risk stratification and prognostic assessment in prior CVEs population.
Cardiovascular disease(CVD) have been widely established as the leading cause of premature morbidity and mortality in China [13]. Results of subanalysis of the NAGOYA HEART Study(NHS) reported that subsequent composite CVE incidence in patients with previous CVD was approximately 3.5 greater than patients without CVD [14]. Consequently, the substantial clinical burden of prior CVE patients urgently need a novel risk factors that mediate residual risk for early diagnosis, integrated treatment and targeted intervention.
Circulating Lp(a) level is a genetically determined risk factor and remain relatively constant throughout a person’s lifetime [15]. Evidence from observational and genetic studies support a causal role of Lp(a) in the development of CAD, including CHD and peripheral arterial disease, as well as ischemic stroke [3, 16]. Effect sizes are most pronounced for MI and peripheral arterial disease where Lp(a) concentrations predict 2- to threefold increases in risk. Moreover, several evidences from secondary prevention studies also presented that elevated Lp(a) level is significantly and independently associated with subsequent CVEs during follow-up after occurrence of CVEs including individual history of PCI, CABG, MI, stroke and peripheral arterial disease [17,18,19,20,21]. However inconsistent findings from other studies have been reported that elevated Lp(a) have no impact on atherothrombotic events in population with established CVD [22, 23]. Thus, further exploration of the association between Lp(a) and future cardiovascular outcomes is warranted to provide more evidence-based information, especially for high-risk populations such as history of composite CVEs.
Epidemiological studies and guidelines proposed that CVD risk is associated with increasing Lp(a) levels > 30 mg/dl (> 75 nmol/l) in primary care populations in a dose dependent fashion [24], thereby we further divided the population into three groups based on Lp(a) levels of 10 and 30 mg/dL. Ultimately, in the present study with large sample size and long duration of follow-up, we discovered that elevated Lp(a) either as a categorical or a continuous variable remained an independent risk factor for MACCE. Our findings are consistent with those of a previous study [12], which recruited 2,284 diabetes patients with prior CVEs, showed that Lp(a) was an independent predictor for recurrent CVEs in diabetes patients with prior CVEs. Additionally, the association between Lp(a) and incidences of MACCE were further confirmed in two sensitivity analyses. Moreover, because Lp(a) levels differed between individuals of genders and age, and the prognostic value of Lp(a) may affected by LDL-C level, hsCRP levels and comorbidities states. The subgroup analyses were performed and turned out to be consistent across the different subgroups. Concurrently, we also found that Lp(a) ≥ 30 mg/dL was significantly associated with greater risk of all-cause mortality and cardiac death. A similar trend was found in a large cohort study, which studied individuals from two prospective studies of the Danish general population [25], of which 6,976 had information on Lp(a) concentrations. Observationally, Lp(a) > 93 mg/dL group were associated with a hazard ratio of 1.50 (95% CI1.28–1.76) for cardiovascular mortality and of 1.20 (1.10–1.30) for all-cause mortality, not for non-cardiovascular mortality compared with Lp(a) < 10 mg/dL. However, Given the data mainly conducted in healthy participant of the general population rather than patients with prior CVE. Future secondary prevention studies are warranted to clarify these results.
Plasma Lp(a) levels contributes to the poor prognosis in prior CVEs patients may through proatherogenic, prothrombotic and proinflammatory mechanisms [26,27,28]. The true role of elevated Lp(a) levels in the setting of secondary prevention is a profoundly important issue, given that this patient population is at high risk of recurrent CVEs and has higher Lp(a) levels than general population. The phase 3 clinical trial is on the horizon, which assess the impact of Lp (a) lowering with antisense oligonucleotide therapies directed against apolipoprotein(a) expression on CVEs in patients with CVD, may address this issue [29]. In conclusion, our present study further extended the association of Lp(a) with long-term cardiovascular outcomes in the setting of established CVEs with heavy atherosclerotic burden, and Lp(a) measurement may help to further risk stratification for this high-risk population.
Limitations
There were several strengths of our study, including the large sample size, extended follow-up to 5-year duration, the high follow‐up rate, adequate adjustment for potential confounders and performed analyses on subgroups. However, several limitations that should be noticed in the present study. First, single measurement of Lp(a) was only tested at admission, the level of Lp(a) may change over follow-up was not available. Second, Lp(a) was measured by immunoturbidimetry method in the current study, its accuracy might be influenced by the apo(a) size isoform-dependent bias, Lp(a) assay standardization is needed [30]. Meanwhile, effect of sample handling and storage on Lp(a) measurement should be considered. Last, this was an observational study that may be subject to potential selection biases. More high-quality researches are required to address the impact of Lp(a)-lowing on poor prognosis of high-risk population in secondary prevention.
Availability of data and materials
Due to ethical restrictions related to the consent given by subjects at the time of study commencement, our datasets are available from the corresponding author upon reasonable request after permission of the Institutional Review Board of Fuwai Hospital.
References
Vaccaro O, Vitale M, Costanzo S, Masulli M, Riccardi G, De Curtis A, et al. Cardiovascular risk factors control according to diabetes status and prior cardiovascular events in patients managed in different settings. Diabetes Res Clin Pract. 2020;168:108370.
Hallberg S, Gandra SR, Fox KM, Mesterton J, Banefelt J, Johansson G, et al. Healthcare costs associated with cardiovascular events in patients with hyperlipidemia or prior cardiovascular events: estimates from Swedish population-based register data. Eur J Health Econ. 2016;17(5):591–601.
Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017;69(6):692–711.
Laschkolnig A, Kollerits B, Lamina C, Meisinger C, Rantner B, Stadler M, et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res. 2014;103(1):28–36.
Paré G, Çaku A, McQueen M, Anand SS, Enas E, Clarke R, et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation. 2019;139(12):1472–82.
Langsted A, Nordestgaard BG, Kamstrup PR. Elevated Lipoprotein(a) and Risk of Ischemic Stroke. J Am Coll Cardiol. 2019;74(1):54–66.
Xu N, Tang XF, Yao Y, Jia SD, Liu Y, Zhao XY, et al. Lipoprotein(a) levels are associated with coronary severity but not with outcomes in Chinese patients underwent percutaneous coronary intervention. Nutr Metab Cardiovasc Dis. 2020;30(2):265–73.
Xu N, Tang XF, Yao Y, Zhao XY, Chen J, Gao Z, et al. Association of Plasma Lipoprotein(a) With Long-Term Adverse Events in Patients With Chronic Kidney Disease Who Underwent Percutaneous Coronary Intervention. Am J Cardiol. 2018;122(12):2043–8.
Xu N, Jiang L, Xu L, Tian J, Zhang C, Zhao X, et al. Impact of Lipoprotein(a) on Long-Term (Mean 6.2 Years) Outcomes in Patients With Three-Vessel Coronary Artery Disease. Am J Cardiol. 2020;125(4):528–33.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third Universal Definition of Myocardial Infarction. Eur Heart J. 2012;33(20):2551–67.
Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–89.
Zhang Y, Jin JL, Cao YX, Zhang HW, Guo YL, Wu NQ, et al. Lipoprotein (a) predicts recurrent worse outcomes in type 2 diabetes mellitus patients with prior cardiovascular events: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19(1):111.
Du X, Patel A, Anderson CS, Dong J, Ma C. Epidemiology of Cardiovascular Disease in China and Opportunities for Improvement: JACC International. J Am Coll Cardiol. 2019;73(24):3135–47.
Yamashita K, Kondo T, Muramatsu T, Matsushita K, Nagahiro T, Maeda K, et al. Effects of valsartan versus amlodipine in diabetic hypertensive patients with or without previous cardiovascular disease. Am J Cardiol. 2013;112(11):1750–6.
Kronenberg F. Human genetics and the causal role of lipoprotein(a) for various diseases. Cardiovasc Drugs Ther. 2016;30(1):87–100.
Kamstrup PR. Lipoprotein(a) and Cardiovascular Disease. Clin Chem. 2021;67(1):154–66.
Ezhov MV, Tmoyan NA, Afanasieva OI, Afanasieva MI, Pokrovsky SN. Lipoprotein(a) and Cardiovascular Outcomes after Revascularization of Carotid and Lower Limbs Arteries. Biomolecules. 2021;11(2):257.
Lange KS, Nave AH, Liman TG, Grittner U, Endres M, Ebinger M. Lipoprotein(a) Levels and Recurrent Vascular Events After First Ischemic Stroke. Stroke. 2017;48(1):36–42.
Cao YX, Zhang HW, Jin JL, Liu HH, Zhang Y, Zhang M, et al. Lipoprotein(a) and Cardiovascular Outcomes in Patients with Previous Myocardial Infarction: A Prospective Cohort Study. Thromb Haemost. 2021;121(9):1161–8.
Ezhov MV, Safarova MS, Afanasieva OI, Kukharchuk VV, Pokrovsky SN. Lipoprotein(a) level and apolipoprotein(a) phenotype as predictors of long-term cardiovascular outcomes after coronary artery bypass grafting. Atheroscler. 2014;235(2):477–82.
Yoon YH, Ahn JM, Kang DY, Lee PH, Kang SJ, Park DW, et al. Association of Lipoprotein(a) With Recurrent Ischemic Events Following Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2021;14(18):2059–68.
Schwartz GG, Ballantyne CM, Barter PJ, Kallend D, Leiter LA, Leitersdorf E, et al. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: Analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol. 2018;3(2):164–8.
Zewinger S, Kleber ME, Tragante V, McCubrey RO, Schmidt AF, Direk K, et al. Relations between lipoprotein(a) concentrations, LPA genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 2017;5(7):534–43.
Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can J Cardiol. 2021;37(8):1129–50.
Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J. 2019;40(33):2760–70.
Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, et al. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374–92.
Kiechl S, Willeit J. The mysteries of lipoprotein(a) and cardiovascular disease revisited. J Am Coll Cardiol. 2010;55(19):2168–70.
Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16(5):305–18.
Tsimikas S, Moriarty PM, Stroes ES. Emerging RNA Therapeutics to Lower Blood Levels of Lp(a): JACC Focus Seminar 2/4. J Am Coll Cardiol. 2021;77(12):1576–89.
Boffa MB, Stranges S, Klar N, Moriarty PM, Watts GF, Koschinsky ML. Lipoprotein(a) and secondary prevention of atherothrombotic events: A critical appraisal. J Clin Lipidol. 2018;12(6):1358–66.
Acknowledgements
We are grateful to all staff members for their contribution to the study.
Funding
This work was supported by The National Key Research and Development Program of China (No. 2016YFC1301300 and 2016YFC1301301); National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences (No. NCRC2020013), CAMS Innovation Fund for Medical Sciences (CIFMS)(No.2020-I2M-C&T-B-049) and the National Natural Science Foundation for Young Scholars of China (No.81900323).
Author information
Authors and Affiliations
Contributions
XN, YYJ, XB, GRL and YJQ contributed to the conception and design of the work. YY, JL, XJJ, WHH and SY contributed to in data collection and analysis. XN drafted the manuscript. YJQ and GRL critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Ethical Review Board of Fuwai Hospital approved the study protocol in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants.
Consent for publication
The manuscript was approved by all authors for publication.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Baseline clinical, angiographic and medication of the study patients with and without MACCE at 5 yeas.
Additional file 2:
Supplementary Table 2. Relation of Lp(a) levels with prior CVEs patients at 5-year MACCE in Sensitivity Analyses.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, N., Yao, Y., Jiang, L. et al. Lipoprotein(a) predicts recurrent cardiovascular events in patients with prior cardiovascular events post-PCI: five-year findings from a large single center cohort study. Thrombosis J 20, 69 (2022). https://doi.org/10.1186/s12959-022-00424-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-022-00424-9