Abstract
Research question
Does a frozen-embryo transfer in an artificially-prepared endometrium (FET-HRT) cycle yield similar clinical pregnancy rate with 7 days of oestrogen priming compared to 14 days?
Design
This is a single-centre, randomized, controlled, open-label pilot study. All FET-HRT cycles were performed in a tertiary centre between October 2018 and January 2021. Overall, 160 patients were randomized, with a 1:1 allocation, into two groups of 80 patients each: group A (7 days of E2 prior to P4 supplementation) and group B (14 days of E2 prior to P4 supplementation). Both groups received single blastocyst stage embryos on the 6th day of vaginal P4 administration. The primary outcome was the feasibility of such strategy assessed as clinical pregnancy rate, secondary outcomes were biochemical pregnancy rate, miscarriage rate, live birth rate and serum hormone levels on the day of FET. Chemical pregnancy was assessed by an hCG blood test 12 days after FET and clinical pregnancy was confirmed by transvaginal ultrasound at 7 weeks.
Results
The analysis included 160 patients who were randomly assigned to either group A or group B on the seventh day of their FET-HRT cycle if the measured endometrial thickness was above 6.5 mm. Following screening failures and of drop-outs, 144 patients were finally included both in group A (75 patients) or group B (69 patients). Demographic characteristics for both groups were comparable. The biochemical pregnancy rate was 42.5% and 48.8% for group A and group B, respectively (p 0.526). Regarding the clinical pregnancy rate at 7 weeks, no statistical difference was observed (36.3% vs 46.3% for group A and group B, respectively, p = 0.261). The secondary outcomes of the study (biochemical pregnancy, miscarriage, and live birth rate) were comparable between the two groups for IIT analysis, as well as the P4 values on the day of FET.
Conclusions
In a frozen embryo transfer cycle, performed with artificial preparation of the endometrium, 7 versus 14 days of oestrogen priming are comparable, in terms of clinical pregnancy rate; the advantages of a seven-day protocol include the shorter time to pregnancy, reduced exposure to oestrogens, and more flexibility of scheduling and programming, and less probability to recruit a follicle and have a spontaneous LH surge. It is important to keep in mind that this study was designed as a pilot trial with a limited study population as such it was underpowered to determine the superiority of an intervention over another; larger-scale RCTs are warranted to confirm our preliminary results.
Trial registration
Clinical trial number: NCT03930706.
Similar content being viewed by others
Introduction
Frozen Embryo Transfer (FET) cycles have increased ever since the first pregnancy from IVF using cryopreserved embryos was reported in 1983 [38]. While frozen-thawed embryo transfer was initially developed to perform embryo transfer in oocyte donation cycles [21], it subsequently evolved towards an elective technique for patients with supernumerary embryos and an increased risk of developing ovarian hyperstimulation syndrome [9]. Nowadays, FET cycles are also used in cases with late-follicular progesterone elevation [4, 16, 30], embryo-endometrial asynchrony [34], recurrent implantation failure [24], and pre-implantation genetic diagnosis/screening. This evolution of utility in the FET landscape also reflects itself in the currently available data for FET usage, with a 93% increase of the procedure between 2013 and 2018 [11].
A thorough look at the current FET protocols is important to gain more insight towards an optimal FET strategy. FET can take place in either a natural cycle or in an artificial cycle [23]. According to a recent Cochrane meta-analysis [14] there is no evidence to support the use of one regimen in preference to another. Nonetheless, taking into account the minimal cycle monitoring related to such practice, i.e. hormonal analyses and ultrasound scans of the endometrium, and the applicability to even women without regular bleeding, the protocol of exogenous oestrogen and progesterone administration is widely used for endometrial preparation [42]. However, this approach has some disadvantages such as costs, inconvenience, prolonged treatment (especially in case of pregnancy) and potential side-effects associated with oestrogen supplementation, i.e. increased thrombotic risk and preeclampsia [7, 39]. In fact, several observational studies have already hinted towards an increased risk of pre-eclampsia when using HRT for endometrial preparation [17, 27, 32], and a large systematic review [29] confirmed these findings with statistical significance. A relationship between the duration of oestrogen priming of the endometrium and the increased occurrence of hypertensive disorders was suggested by Roque et al. [29], while Shi et al. [35] found no differences in the occurrence of hypertensive disorders between eFET and fresh ET when eFET was performed in a natural cycle. On the other hand, oestrogen stimulation in FET-HRT activates thrombotic risk markers and a restriction in the use of unnecessary hormone exposure is important, as described recently by Dalsgaard et al. [8]. Moreover, still cycle cancellation due to spontaneous ovulation is an uncontrollable phenomenon that can always occur, especially when the oestrogen preparation takes long time, therefore the rationale for a shorter time to oestrogen exposure could potentially lead to less spontaneous ovulations and easier programming of the FET cycle. Contrasting results do exist, however, further emphasizing the need for additional exploration of this subject [5].
Nowadays, most FET-HRT protocols opt for the 14-day period of oestrogen supplementation to mimic the natural proliferative phase of the menstrual cycle [6]. However, scarce evidence has shown that 5 to 7 days is sufficient for endometrial proliferation [3, 26]. Recently, Sekhon et al. [33], Joly et al. [19] and Jiang et al. [18] demonstrated in retrospective cohort studies including more than thousand patients, that the length of E2 supplementation is linked neither to implantation rate, nor live birth rate and cumulative live birth rate; as well as the level of oestradiol on the day of start of progesterone [22].
Besides the very open debate about the ideal length of the E2 supplementation and considering recent results showing that this has no effects on the FET outcome, we should consider another important issue of the FET cycle, which is the delayed time to pregnancy. A recent study on patients’ perspectives regarding elective FET (eFET) revealed that the postponement of embryo transfer is an important deciding factor in the choice of eFET versus fresh embryo transfer [37]. Considering these important results, reducing time-to-pregnancy (TTP) in the FET-HRT protocol would therefore increase patient comfort when choosing eFET over fresh ET. Given the totally arbitrary decision to perform 14 days of oestrogen endometrial preparation in a FET-HRT, and the emerging evidence that the duration of oestrogen exposure does not affect success rates and given the real need to shorten time to pregnancy for patients facing IVF; the main objective of this pilot study is to evaluate the feasibility of a short endometrial preparation in FET-HRT cycles with the administration of 7 consecutive days of oestrogen priming prior to P4 initiation, by comparing clinical pregnancy rates with the standard of care (14 days of oestrogen priming).
Materials and method
Study population and design
This was a single-centre, randomized, controlled, open-label, pilot study. Women who were planning to undergo FET-HRT in our centre were screened and consequently invited to participate in this study. All FET-HRT cycles were performed in a tertiary referral centre (Brussels IVF, Centre for Reproductive Medicine, Universitair Ziekenhuis Brussel, Belgium) between October 2018 and January 2021, and the follow up period was 12 weeks from FET. We included all women between the age of 18 and 40 years with unexplained infertility and showing a normal uterine cavity, undergoing either IVF or ICSI with a GnRH agonist or antagonist protocol (Table 1).
Furthermore, only the first single Day 5 blastocyst transfer with an excellent quality embryo (at least Bl 3BA) was included. Women with a BMI lower than 18 or higher than 29 kg/m2, having a history of recurrent implantation failure/recurrent miscarriage, or showing an abnormal karyotype were excluded. Likewise, women who had a previous diagnosis of PCOS/POI, endometriosis stage 3 or 4, hydrosalpinx, or a systemic disease such as thyroid dysfunction (unless corrected) were omitted. Furthermore, PGT-A/M and oocyte donation cycles were also excluded. Written informed consent obtained from all participants of the study. The study was registered in clinicaltrials.gov with number NCT03930706.
Study outcomes
Our primary outcome was clinical pregnancy at 7 weeks after FET while the secondary outcomes where positive hCG, assessed 12 days after FET, bioquemical pregnancy rate and miscarriage rate assessed during the first 12 weeks of pregnancy, following the definitions of the international glossary in fertility [43].
Insemination, embryo quality assessment and cryopreservation
Fertilization was assessed 16-18 h after IVF/ICSI by the presence of two pronuclei, and further on, embryo development was evaluated daily until the cryopreservation of either cleavage-stage embryos (Day 3) or blastocysts (Day 5 and 6). Cryopreservation was performed by means of vitrification using a closed vitrification device with high-security straws (CBS-ViT-HS®; Cryobiosystems) using a combination of dimethyl sulfoxide and ethylene glycol as cryoprotectants (Irvine Scientific Freeze Kit®; Irvine Scientific). Day 3 embryos were evaluated based on the number and symmetry of their blastomeres, percentage of fragmentation, vacuolization, granulation and multinucleation. Based on all these parameters, an EQ score was assigned to all normally fertilized embryos using a predefined algorithm, which is divided into four categories: excellent, good, moderate, or poor. These four categories were used as defined by Racca et al. [28]. Fresh transfer of embryos or blastocysts was not included in this study. Blastocysts were scored according to the grading system developed by Gardner and Schoolcraft [13] based on the expansion stage, the number of cells joining compaction or blastulation, and the appearance of the trophectoderm (TE) and inner cell mass (ICM).
The following embryos were considered eligible for cryopreservation: day 3 embryos with ≥ 6 blastomeres and ≤ 50% fragmentation; day 5 and 6, fully expanded or hatching blastocysts with a type A/B/C ICM and type A/B TE. Only transfers of single day 5 embryos of excellent quality were included as part of this study. Vitrified blastocysts were evaluated (Table 1) after warming (with Irvine Scientific Thaw Kit®; Irvine Scientific). FETs of vitrified blastocyst (Days 5 and 6) were performed on the day of warming.
Endometrial preparation, FET timing and patient randomization
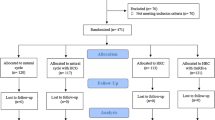
This study included only FETs in an artificially supplemented cycle (FET-HRT). Thus, endometrial preparation consisted of the sequential administration of oestradiol (E2) valerate and micronized vaginal progesterone. Patients with basal hormone values, defined as oestradiol < 80 pg/ml and progesterone < 1,5 ng/ml, and no ovarian cysts on day 1 of their cycle started administering 6 mg oral oestradiol daily. On day 7 of their treatment, serum hormone values and endometrial thickness were evaluated through a blood test and an ultrasound respectively. Patients with an endometrial thickness ≥ 6.5 mm were randomized in 2 groups with a 1:1 allocation: group A, 7 days of E2 priming, and group B, 14 days of E2 intake. Group A started 800 mg intravaginal progesterone daily (divided into 400 mg in the morning and 400 mg in the evening) on Day 8 of treatment and underwent FET on the 6th day of progesterone supplementation. Group B continued 7 more days of oestradiol and started 800 mg intravaginal progesterone daily on Day 15 of E2 treatment and underwent FET on the 6th day of progesterone supplementation. Group B received a total of 20 days of E2 intake before the ET, with an additional evaluation of serum hormone values and endometrial thickness on day 14 of treatment (Fig. 1).
Assessment, data collection and randomization
Pregnancy was assessed by a blood test to evaluate hCG 12 days after FET and ongoing pregnancy was confirmed by the visualization of a fetal heartbeat during a transvaginal ultrasound at 7 weeks [31]. Data were collected in a secure and encrypted eCRF created specifically for the trial using Filemaker Pro® v13 (Filemaker Inc.) and hosted on a dedicated server at our centre (Brussels IVF). The doctors, study nurses, and research assistants collaborating in the trial were responsible for data collection.
Randomization took place on day 7 of endometrial preparation with oestradiol for all patients with endometrial thickness above 6.5 mm. The randomization was performed by means of white sealed opaque envelopes with a 1:1 allocation, and the random list was generated with sequential numbers by using STATA version 15.1 (StataCorp, College Station, Texas, USA). The study nurses’ team together with the senior clinicians involved in the study, were in charge of the enrolment, randomization and allocation.
Sample size and statistical analysis
As there is no evidence supporting only 7 days of oestradiol priming in FET-HRT, no formal sample size calculation was performed. Therefore, we arbitrarily decided to include 160 patients.
To determine, with 80% power, superiority of one strategy over the other, considering a difference in clinical pregnancy of 10%, with a formal sample size calculation (alfa 0.05 and beta 0.2) we would have required 421 patients in each group, with a total of 842 patients.
Continuous variables were presented using mean and standard deviation while categorical characteristics as well as all primary and secondary outcomes were reported using absolute and relative values within their respective groups. Continuous variables were analysed using the Mann–Whitney U test while dichotomous variables were analysed using Fisher’s Exact test. The outcomes were reported with p values and difference of proportion. A p-value was considered significant whenever < 0.05. The study was conducted with respect to the Pilot study consort 2010 [10].
All statistical analyses were performed with STATA version 15.1 (StataCorp, College Station, Texas, USA).
Results
The analysis included 160 patients who were randomly assigned to either group A or group B on the 7th day of oestradiol intake of their FET-HRT cycle. After the exclusion of drop-outs and screening failures, 144 patients were included either in group A (75 patients) or group B (69 patients) (Fig. 2).
Patients’ demographic characteristics such as age, BMI, AMH, as well as smoking habits, parity, and indication for ART, are summarised in Table 2, while cycle characteristics such as endometrial thickness on the day of P4 start as well as E2 and P4 on day of ET are summarised in Table 3.
Demographic characteristics for both groups were comparable. Notably, there was no significant difference in the parity of both groups. Most patients had undergone one or no previous ART cycles and had either one or no previous live births. Additionally, the indications for ART were evenly distributed between both groups. In the entire study population, the most frequent indications for ART were the idiopathic cause and male factor. There was no significant difference between E2 and P4 levels, as well as endometrial thickness measured on the day of ET.
Primary outcome
Outcomes of the analysis are reported, following the ITT principle to avoid possible bias due to the excluded patients, (Table 4). Our primary outcome was clinical pregnancy at 7 weeks after FET, no statistical difference was found between the intervention and control group, (36.3% vs 46.3%, for group A and group B, respectively, p = 0.261, difference of proportions 10%, 95% CI -0.05 – 0.25).
Secondary outcomes
Positive pregnancy rate was 42.5% and 48.8% for group A and group B, respectively (p 0.526, difference of proportions 0.6, 95% CI -0.09 – 0.21). Biochemical pregnancy rate was 14.7 versus 5.1 for group A and B, respectively (p = 0.443, difference of proportions -0.096, 95% CI -0.23 – 0.04) and miscarriage rate was 13.8 and 8.1 for group A and B, respectively (p = 0.69, difference of proportions – 0.06, 95%CI -0.21 – 0–096). Live birth rate was 31.3 for group A and 42.5 for group B (p = 0.19, difference of proportions 0.11, 95% CI -0.03 – 0.26).
Serum hormonal levels on day of ET
The oestradiol levels on the day of ET were 225.4 and 228.5, respectively (p = 0.835) while the levels of P4 were comparable between the two groups (12.8 ng/ml mean for both arms, p value 0.318). LH levels also were similar with 5.9 and 7.1 for groups A and B, respectively (p = 0.259).
Discussion
To the best of our knowledge, this is the first randomized controlled study investigating a shorter endometrial exposure to oestrogens (only 7 days) before starting P4 supplementation in a FET with HRT. In fact, the current results show similar clinical pregnancy rates between 7 versus 14 days of oestrogens priming in a FET HRT, opening the floor for future larger scale RCTs with the aim to confirm these exploratory results.
The wider implications of such a study are the TTP reduction which could reduce the costs but also increased patient comfort and acceptance when choosing FET over fresh ET [37], and the safety. In fact, even in terms of safety, a shorter duration of E2 supplementation could provide an advantage relating to the possible reduced risk of thrombotic and hypertensive disorders associated with the FET-HRT protocol [8].
The results of the present study are in line with a retrospective cohort study that was published in 2022, [18], in which 4142 FET-HRT cycles were divided according to 7 vs 14 days of oestrogen exposure and found no difference in cumulative live birth rate.
The basis of our hypothesis to shorten the TTP was inspired by Navot et al. [25] reporting that it is biologically feasible to simulate the essential hormonal and endometrial milieu of a fertile menstrual cycle and early gestation solely by the administration of oestrogen and progesterone. Since then, the length of oestrogen supplementation has been empirically chosen as 14 days to mimic the follicular phase of a physiological menstrual cycle. However, it is generally acceptable that P levels are the driving force behind endometrial receptivity [12, 20, 36]. Thus, we did not expect different cycle outcomes when using good quality blastocyst and the ET was done with a protocol in which solely the duration of E2 priming varied.
The duration of oestrogen administration before frozen embryo transfer did not impact implantation nor clinical pregnancy and early pregnancy loss or live birth rate from a statistical point of view, as shown by Sekhon et al. [33] and Joly et al. [19]. However, based on their results, the mean length of oestrogen supplementation was 17 and 20 days, respectively. The results of the present study are in line with Sekhon and Joly and co-authors, demonstrating no difference in outcomes between the two groups, however, it is important to acknowledge that we compared shorter time of exposure to oestrogens. Furthermore, we should also point out that, although statistically irrelevant, the difference in clinical pregnancy between to two groups was about 10% in favour of the 14 days (group B), therefore we warrant caution in opting for such a short protocol, until larger scale studies will confirm, or not, our results.
Our primary and secondary outcomes were similar to the results of the FET-HRT population in most studies comparing eFET with fresh ET [1, 2, 40]. The oestradiol values on the day of ET were comparable between the two groups, although a significant difference in the time of exposure. The hormonal results on the day of ET were in line with a previous study from our group where it was reported by Mackens et al. [22] that oestradiol levels do not influence the outcome of the FET-HRT cycle.
Limitations and strengths
Considering the lack of evidence related to a shorter exposure to oestrogen in an HRT cycle, we did not have enough knowledge and evidence to directly perform a powered RCT. Therefore, a major limitation of the present study is the design as a pilot trial. As a result of its limited study population, it was underpowered to determine the superiority of one intervention over another. Instead, the purposes of the present study were to explore trends in pregnancy rates for each HRT strategy and to provide us with enough knowledge for the sample size calculation of further definitive RCTs in which a non-inferiority of the 7 days E2 priming approach could be confirmed. Although the pilot design, these results allow us to safely design a larger confirmatory RCT, exploring the safety and efficacy of a FET-HRT with the aim to reduce TTP.
A vital strength of this trial is its strict inclusion criteria, such as the choice to include only single transfer of good quality blastocysts [15, 41]. This allowed us to truthfully compare the influence of reduced time of oestrogen priming on cycle outcomes. Furthermore, the study was conducted in a rigorous way with respect to the Pilot study consort 2010 [10].
Lastly, we performed a hormonal assessment on the day of ET to understand the possible influence of the different protocols on hormonal trends and cycle outcomes, to have a fully comprehensible understanding of the HRT protocol.
Conclusions
In a frozen embryo transfer cycle, performed with artificial preparation of the endometrium, 7 versus 14 days of oestrogen priming are comparable, in terms of clinical pregnancy rate; the advantages of a seven-day protocol include the shorter time to pregnancy, reduced exposure to oestrogens, and more flexibility of scheduling and programming, as we could program with 7–8-9 days of oestrogens intake and yield similar results, even with less probability to recruit a follicle and have a spontaneous LH surge. These are the main results of the present pilot-controlled trial, which needs to be confirmed firstly with endometrial outcomes measures, such as molecular expression and, secondly, with future larger-scale RCTs.
Availability of data and materials
Full data set is available from the corresponding author, upon reasonable request.
References
Aflatoonian A, Mansoori-Torshizi M, Farid Mojtahedi M, Aflatoonian B, Khalili MA, Amir-Arjmand MH, Soleimani M, Aflatoonian N, Oskouian H, Tabibnejad N, et al. Fresh versus frozen embryo transfer after gonadotropin-releasing hormone agonist trigger in gonadotropin-releasing hormone antagonist cycles among high responder women: A randomized, multi-center study. Int J Reprod Biomed. 2018;16:9–18.
Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet. 2010;27:357–63.
Borini A, Dal Prato L, Bianchi L, Violini F, Cattoli M, Flamigni C. Effect of duration of estradiol replacement on the outcome of oocyte donation. J Assist Reprod Genet. 2001;18:185–90.
Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, Pellicer A. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100.
Chen Z-J, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N, et al. Fresh versus frozen embryos for infertility in the Polycystic Ovary Syndrome. N Engl J Med. 2016;375:523–33.
Conrad KP, Rabaglino MB, Post Uiterweer ED. Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta. 2017;60:119–29.
Conrad KP, von Versen-Höynck F, Baker VL. Potential role of the corpus luteum in maternal cardiovascular adaptation to pregnancy and preeclampsia risk. Am J Obstet Gynecol. 2022;226:683–99.
Dalsgaard TH, Hvas AM, Kirkegaard KS, Jensen MV, Knudsen UB. Impact of frozen thawed embryo transfer in hormone substituted cycles on thrombotic risk markers. Thromb Res. 2022;209:23–32. https://doi.org/10.1016/j.thromres.2021.11.016. (Epub 2021 Nov 22 PMID: 34847404).
Devroey P, Polyzos NP, Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26:2593–7.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA, PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239.
Fertility treatment 2018: trends and figures | HFEAAvailable from: https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2018-trends-and-figures/.
Franasiak JM, Ruiz-Alonso M, Scott RT, Simón C. Both slowly developing embryos and a variable pace of luteal endometrial progression may conspire to prevent normal birth in spite of a capable embryo. Fertil Steril. 2016;105:861–6.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–11.
Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2017;7:CD003414.
Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;CD002118.
Healy MW, Patounakis G, Connell MT, Devine K, DeCherney AH, Levy MJ, Hill MJ. Does a frozen embryo transfer ameliorate the effect of elevated progesterone seen in fresh transfer cycles? Fertil Steril. 2016;105:93-99.e1.
Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101:128–33.
Jiang WJ, Song JY, Sun ZG. Short (seven days) versus standard (fourteen days) oestrogen administration in a programmed frozen embryo transfer cycle: a retrospective cohort study. J Ovarian Res. 2022;15(1):36. https://doi.org/10.1186/s13048-022-00967-5. (PMID:35313944;PMCID:PMC8939227).
Joly J, Goronflot T, Reignier A, Rosselot M, Leperlier F, Barrière P, et al. Impact of the duration of oestradiol treatment on live birth rate in Hormonal Replacement Therapy cycle before frozen blastocyst transfer. Hum Fertil (Camb). 2023:1–8. https://doi.org/10.1080/14647273.2022.2163467.
Lawrenz B, Fatemi HM. Effect of progesterone elevation in follicular phase of IVF-cycles on the endometrial receptivity. Reprod Biomed Online. 2017;34:422–8.
Legro RS, Ary BA, Paulson RJ, Stanczyk FZ, Sauer MV. Premature luteinization as detected by elevated serum progesterone is associated with a higher pregnancy rate in donor oocyte in-vitro fertilization. Hum Reprod. 1993;8:1506–11.
Mackens S, Santos-Ribeiro S, Orinx E, De Munck N, Racca A, Roelens C, Popovic-Todorovic B, De Vos M, Tournaye H, Blockeel C. Impact of serum oestradiol levels prior to progesterone administration in artificially prepared frozen embryo transfer cycles. Front Endocrinol (Lausanne). 2020;11:255.
Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, Blockeel C. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. 2017;32:2234–42.
Magdi Y, El-Damen A, Fathi AM, Abdelaziz AM, Abd-Elfatah Youssef M, Abd-Allah AA-E, Ahmed Elawady M, Ahmed Ibrahim M, Edris Y. Revisiting the management of recurrent implantation failure through freeze-all policy. Fertil Steril. 2017;108:72–77.
Navot D, Laufer N, Kopolovic J, Rabinowitz R, Birkenfeld A, Lewin A, Granat M, Margalioth EJ, Schenker JG. Artificially induced endometrial cycles and establishment of pregnancies in the absence of ovaries. N Engl J Med. 1986;314:806–11.
Navot D, Anderson TL, Droesch K, Scott RT, Kreiner D, Rosenwaks Z. Hormonal manipulation of endometrial maturation. J Clin Endocrinol Metab. 1989;68:801–7.
Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, Wennerholm UB, Gissler M, Skjærven R, Romundstad LB. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: a cohort study from the CoNARTaS group. Hum Reprod. 2015;30:1724–31.
Racca A, De Munck N, Santos-Ribeiro S, Drakopoulos P, Errazuriz J, Galvao A, Popovic-Todorovic B, Mackens S, De Vos M, Verheyen G, et al. Do we need to measure progesterone in oocyte donation cycles? A retrospective analysis evaluating cumulative live birth rates and embryo quality. Hum Reprod. 2020;35:167–74.
Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 2019;25:2–14.
Roque M, Valle M, Guimarães F, Sampaio M, Geber S. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. 2015;103:1190–3.
Santos-Ribeiro S, Polyzos NP, Haentjens P, Smitz J, Camus M, Tournaye H, Blockeel C. Live birth rates after IVF are reduced by both low and high progesterone levels on the day of human chorionic gonadotrophin administration. Hum Reprod. 2014;29:1698–705.
Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm U-B, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod. 2012;27:1343–50.
Sekhon L, Feuerstein J, Pan S, Overbey J, Lee JA, Briton-Jones C, Flisser E, Stein DE, Mukherjee T, Grunfeld L, et al. Endometrial preparation before the transfer of single, vitrified-warmed, euploid blastocysts: does the duration of oestradiol treatment influence clinical outcome? Fertil Steril. 2019;111:1177-1185.e3.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect differences in embryo-endometrium synchrony. Fertil Steril. 2008;89:20–6.
Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378:126–36.
Simón C, Martín JC, Pellicer A. Paracrine regulators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:815–26.
Stormlund S, Schmidt L, Bogstad J, Løssl K, Prætorius L, Zedeler A, Pinborg A. Patients’ attitudes and preferences towards a freeze-all strategy in ART treatment. Hum Reprod. 2019;34:679–88.
Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305:707–9.
von Versen-Höynck F, Narasimhan P, Selamet Tierney ES, Martinez N, Conrad KP, Baker VL, Winn VD. Absent or excessive corpus luteum number is associated with altered maternal vascular health in early pregnancy. Hypertension. 2019;73:680–90.
Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, Nguyen LK, Norman RJ, Mol BW. IVF Transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med. 2018;378:137–47.
Yang L, Cai S, Zhang S, Kong X, Gu Y, Lu C, Dai J, Gong F, Lu G, Lin G. Single embryo transfer by Day 3 time-lapse selection versus Day 5 conventional morphological selection: a randomized, open-label, non-inferiority trial. Hum Reprod. 2018;33:869–76.
Younis JS, Simon A, Laufer N. Endometrial preparation: lessons from oocyte donation. Fertil Steril. 1996;66:873–84.
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, et al. The International Glossary on Infertility and Fertility Care, 2017{\dag}{\ddag}{\textsection}. Fertil Steril. 2017;32:1786–801.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AR and CB conceived the original idea and the overall design of the study. AR and YD performed the statistical analysis. AR, SSR, PD, YD, LVL, and CB made substantial contributions to the acquisition and interpretation of data and critically appraised the results in the context of scientific literature. AR wrote the article. HT and CB revised the final draft of the manuscript. All the authors substantially revised the manuscript, have approved the submitted version, and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by UZ Brussel Ethical committee on July 11th 2018, approval Number 2018/228. The UZ Brussel Medical Ethics Committee is operating and organized according to the ICH-GCP guidelines.
All participating patients were explained the trial and asked to signed an informed consent before entering in the study.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Racca, A., Santos-Ribeiro, S., Drakopoulos, P. et al. Clinical pregnancy rate for frozen embryo transfer with HRT: a randomized controlled pilot study comparing 1 week versus 2 weeks of oestradiol priming. Reprod Biol Endocrinol 21, 62 (2023). https://doi.org/10.1186/s12958-023-01111-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-023-01111-8