Abstract
Background
With the advance in genome-wide analyses, genetic alternations have been found to play an important role in carcinogenesis and aggressiveness of UC. Through bioinformatic analysis of gene expression profiles of urinary bladder urothelial carcinoma (UBUC) from publicly available GEO dataset (GSE31684), Zinc finger and SCAN domain containing 4 (ZSCAN4) was identified as a significant downregulated gene in muscle-invasive bladder cancer when compared with non-muscle-invasive bladder cancer.
Methods
The expression of ZSCAN4 was evaluated by immunohistochemistry in 340 upper urinary tract urothelial carcinomas (UTUCs) and 295 UBUCs. The expression profiles of ZSCAN4 and potential signaling pathways were analyzed bioinformatically.
Results
In UTUC, low expression of ZSCAN4 was significantly associated with advanced primary pT stage (P = 0.011), increased nodal metastasis (P = 0.002) and increased vascular invasion (P = 0.019). In UBUC, low expression of ZSCAN4 was significantly correlated with advanced primary pT stage (P < 0.001), increased nodal metastasis (P = 0.001), high histological grade (P = 0.003) and increased vascular invasion (P = 0.003). In survival analysis, low expression of ZSCAN4 acted as an independent negative prognostic factor for disease-specific survival and metastasis-free survival both in UTUC and UBUC. Gene ontology analysis showed that ZSCAN4 mRNA and its co-downregulated genes are associated with the mitotic cell cycle.
Conclusions
Low expression of ZSCAN4 predicted worse outcome in urothelial carcinoma and might have potential regulatory role in cell mitosis.
Similar content being viewed by others
Introduction
Urothelial carcinoma (UC) is the most common epithelial malignancy involving the urinary system. Some environmental factors contribute to increasing risk of UC, including tobacco smoking, intake of arsenic-contaminated water, occupational exposure to aromatic amines and polycyclic hydrocarbons, exposure to ionizing radiation and chronic infection of Schistosoma species [1]. Recent genome-wide studies suggested that molecular alternations play an important role in carcinogenesis and aggressiveness of UC. Through analysis of the mRNA expression profiles, multiple molecular subtypes are identified according to their different expression levels of certain key prognostic markers, such as fibroblast growth factor receptor 3 (FGFR3), GATA binding protein 3 (GATA3), forkhead box A1 (FOXA1), uroplakin 3A (UPK3A), and erb-b2 receptor tyrosine kinase 2 (ERBB2) [2,3,4,5]. Molecular stratification may provide better diagnostic, prognostic and/or predictive value than conventional pathologic classification. The diagnostic and prognostic data are often associated with histological grading and classification while the predictive data are linked with the therapeutic response. Moreover, insights into the molecular basis of human cancer provide information of biological functions of neoplasms.
Deletion in chromosome 9 are the earliest genetic events that occurs in the divergent pathways of tumorigenesis in bladder cancer, which leads to two distinct phenotypes: non-muscle-invasive and muscle-invasive urothelial carcinomas. Candidate tumor suppressor genes affected by chromosome 9 deletion includes cyclin-dependent kinase inhibitor 2A (CDKN2A) and cyclin-dependent kinase inhibitor 2B (CDKN2B) at 9p21 [6, 7], patched 1 (PTCH1) at 9q22 [8, 9], deleted in bladder cancer 1 (DBC1) at 9q32–33 [10], and tuberous sclerosis 1 (TSC1) at 9q34 [11]. The main genetic alterations in non-muscle-invasive urothelial carcinoma involves three receptor tyrosine kinase genes, FGFR3, v‑Ha-ras Harvey rat sarcoma viral oncogene homolog (HRAS), and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) [12,13,14]. By contrast, alterations involved in P53 and RB transcriptional corepressor 1 (RB1) lead to progression to non-invasive high-grade and muscle-invasive urothelial carcinomas [15,16,17].
To identify potential candidate genes associated with aggressiveness of UC, we analyzed gene expression profiles of urinary bladder urothelial carcinomas (UBUCs) from publicly available Gene Expression Omnibus (GEO) dataset with the accession number of GSE31684 [18]. The analytic data suggested that Zinc finger and SCAN domain containing 4 (ZSCAN4) was found to be significantly associated with tumor invasion depth, characterized by significant downregulation in muscle-invasive UBUCs (T2–T4) when compared with non-muscle-invasive UBUCs (Ta-T1). Its strong statistically significance (P < 0.0001) draws our attention to select ZSCAN4 for further study. In this study, we tried to validate the prognostic significance of ZSCAN4 in UC patients and to investigate its potential regulatory signaling pathways.
Materials and methods
Data mining of publicly available transcriptome
We performed data mining of publicly available transcriptome of urinary bladder urothelial carcinoma with the accession number of GSE31684 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31684) in Gene Expression Omnibus (GEO) database, which includes 93 UBUCs. The raw CEL files were analyzed on Affymetrix Human Genome U133 Plus 2.0 Array platform by using the Nexus Expression 3 software (BioDiscovery, EI Segundo, CA, USA). All probes were included in the analysis. Supervised comparative analyses were performed to identify potential genes that were differentially expressed between muscle-invasive (T2–T4) and non-muscle-invasive (Ta-T1) bladder cancer. Genes were selected based on the condition that a P value is less than 0.01 and log2 fold gene expression change more than ± 0.1. Further survival analysis was performed to evaluate the prognostic significance of this gene.
Patients and tumor samples
Tumor tissues with available paraffin-embedded tissue blocks were obtained from the archives of Chi-Mei medical center for tissue microarray construction, including 340 upper urinary tract urothelial carcinomas (UTUCs) and 295 UBUCs. Those with squamous, glandular or neuroendocrine component were excluded. The acquisition of clinical samples was approved by the Institutional Review Board (IRB10302015) of Chi-Mei medical center. Patients’ characteristics were described previously [19]. Criteria of histopathological diagnosis and assessment for various histopathological parameters were based on the updated 4th edition of WHO classification of the Urinary System and Male Genital Organs.
Immunohistochemistry and scoring
The immunohistochemical staining was performed on 4-μm-thick sections from formalin fixed paraffin embedded tissue blocks according to the manufacturer’s recommendations. After antigen retrieval, the slides were incubated with a primary antibody against ZSCAN4 (Abcam, ab106646, 1:50). The assessment of ZSCAN4 staining was based on H-score method. The H-score was calculated according to the following formula: 3 × strongly positive tumor cells (%) + 2 × moderately positive tumor cells (%) + 1 × weakly positive tumor cells (%). Tumors with high and low expression of ZSCAN4 are defined by their H-scores that are higher and lower than the median, respectively.
Functional annotation of The Cancer Genome Atlas (TCGA) data
To correlate ZSCAN4 with unrealized functions in UC, the associations between the levels of ZSCAN4 mRNA and its co-expressed genes in the bladder urothelial carcinoma dataset (n = 411) from The Cancer Genome Atlas (TCGA) database were analyzed using the cBioPortal online platform (http://cbioportal.org). The top 200 transcripts with either positive associations or negative associations with ZSCAN4 were further explored using the Gene Ontology (GO) classification system (http://geneontology.org/) according to cellular components, molecular functions, or biological processes and were graded by fold enrichment for functional annotation. An R script with ggplot2 package was used to present representative GO terms.
Statistical analyses
All analyses were performed using SPSS Version 20.0 software (Armonk, NY: IBM Corp., USA). For associations between immunohistochemical expression of ZSCAN4 and clinicopathological parameters, we used Pearson’s chi-squared test to identify significant differences between variables. Kaplan–Meier plots were applied to evaluate survival data, including disease-specific survival (DSS) and metastasis-free survival (MeFS). The prognostic significances of each parameter with suitable cut-offs were determined by the log-rank test. The Cox proportional hazards regression model was used to measure the effects of variables on survival rates. The level of significance was determined according to two-sided tests with a cut-off P value of 0.05.
Results
ZSCAN4 is identified as a significant downregulated gene in muscle-invasive UBUCs (T2-T4) when compared with non-muscle-invasive UBUCs (Ta-T1)
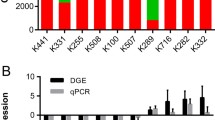
Though analysis of publicly available transcriptome of UBUC (GSE31684), ZSCAN4 was found to be the most significantly downregulated in muscle-invasive UBUCs (T2–T4) when compared with non-muscle-invasive UBUCs (Ta–T1), displaying significant downregulated fold change (Log2 ratio at − 0.9972 and − 0.7781, both P < 0.0001, Fig. 1 and Table 1).
Low mRNA transcript level of ZSCAN4 predicts worse outcome in the UBUC transcriptome (GSE31684)
To further investigate the prognostic significance of ZSCAN4 in UBUC, we performed survival analysis for the UBUC transcriptome (GSE31684), consisting of 93 cases. Among them, 8 cases had high mRNA expression levels of ZSCAN4 while the other 85 cases had low expression. Of note, low expression of ZSCAN4 was significantly associated with worse overall survival (Fig. 2).
Low protein expression of ZSCAN4 is associated with advanced disease status in UTUC and UBUC
The immunoexpression of ZSCAN4 was successfully evaluated with H-score method in all UC tissue samples (Fig. 3). As shown in Table 2, the association between ZSCAN4 expression levels and various clinicopathological parameters were statistically analyzed. In UTUC and UBUC, there was no significant difference in gender, age, perineural invasion or mitotic rate according to the expression status of ZSCAN4. In UTUC, low expression of ZSCAN4 was significantly associated with advanced primary pT stage (P = 0.011), increased nodal metastasis (P = 0.002) and increased vascular invasion (P = 0.019). In UBUC, low expression of ZSCAN4 was significantly correlated with advanced primary pT stage (P < 0.001), increased nodal metastasis (P = 0.001), high histological grade (P = 0.003) and increased vascular invasion (P = 0.003). These findings indicated that there is a close correlation between low ZSCAN4 expression and aggressive tumor behavior in patients with UTUC or UBUC.
Immunohistochemical staining of ZSCAN4 in representative cases. The expression intensities of ZSCAN4 immunostains were strong in normal urothelium (A) and non-invasive urothelial carcinoma (B), weak in superficially invasive urothelial carcinoma (C), and faint or absent in muscle-invasive urothelial carcinoma (D)
Low protein expression of ZSCAN4 predicts worse outcome in UTUC and UBUC
The results of univariate log-rank analyses and multivariate analyses that investigate the impact of ZSCAN4 expression and various clinicopathological variables on survival in patients with UTUC and UBUC are shown in Tables 3 and 4, respectively. In patients with UTUC (Table 3), low expression of ZSCAN4 predicted worse DSS (P < 0.0001) (Fig. 4A) and MeFS (P < 0.0001) (Fig. 4B). In addition, tumor location, multifocality, advanced primary pT stage, presence of nodal metastasis, high histologic grade, increased vascular invasion, and increased perineural invasion was significantly associated with worse DSS and/or MeFS. At multivariate analyses, low expression of ZSCAN4 remain acted as an independent negative prognostic factor for DSS (95% CI 1.572–5.667, P = 0.001) and MeFS (95% CI 1.498–4.548, P = 0.001), along with multifocality (P = 0.010 in DSS; P = 0.010 in MeFS), advanced pT stage (P = 0.043 in DSS), presence of nodal metastasis (P < 0.001 in DSS; P = 0.009 in MeFS), high histologic grade (P = 0.007 in DSS; P = 0.007 in MeFS), increased vascular invasion (P = 0.004 in MeFS), and increased perineural invasion (P < 0.001 in DSS; P = 0.003 in MeFS). In UBUC patients (Table 4), low expression of ZSCAN4 was also significantly associated with worse DSS (P = 0.0001) (Fig. 4C) and MeFS (P < 0.0001) (Fig. 4D). Moreover, advanced primary pT stage, presence of nodal metastasis, high histologic grade, increased vascular invasion, increased perineural invasion, and high mitotic rate were significantly predicted worse DSS and/or MeFS. At multivariate analyses, low expression of ZSCAN4 still emerged as an independent negative prognostic factor for DSS (95% CI 1.382–5.123, P = 0.003) and MeFS (95% CI 1.010–2.759, P = 0.046), along with advanced pT stage (P < 0.001 in DSS; P = 0.002 in MeFS), increased perineural invasion (P = 0.023 in DSS), and increased mitotic rate (P = 0.003 in DSS; P = 0.006 in MeFS). These data suggested that low ZSCAN4 expression significantly predicted worse clinical outcome in patients with UTUC or UBUC.
ZSCAN4 downregulation may be linked to high mitotic activity
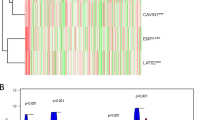
A gene co-expression assessment was performed to correlate ZSCAN4 with unrealized functions in UC. Using the BLCA dataset (n = 411) from the TCGA database, we examined the top 200 transcripts that were positively associated (Supplementary Table S1) or negatively associated (Supplementary Table S2) with ZSCAN4. Next, these genes were functionally annotated by means of the GO classification system. In the context of biological processes (Fig. 5A), the top terms negatively associated with ZSCAN4 comprised spindle assembly involved in female meiosis I (GO 0,007,057, fold enrichment 72.86), positive regulation of chromosome condensation (GO 1,905,821, fold enrichment 58.29), and mitotic spindle elongation (GO 0,000,022, fold enrichment 48.57). In terms of molecular functions (Fig. 5B) and cellular components (Fig. 5C), the most significant terms negatively associated with ZSCAN4 were anaphase-promoting complex binding (GO 0,010,997, fold enrichment: 32.38) and centralspindlin complex (GO: 0,097,149, fold enrichment 97.15), respectively. As the mitotic rate has been used to measure how fast cancer cells are dividing (proliferating) and growing, tumors mostly have higher mitotic activity than normal tissues. Accordingly, our observations disclosed that the levels of ZSCAN4 mRNA and its co-downregulated genes are greatly associated with the mitotic cell cycle, suggesting that ZSCAN4 is more likely to play a role in the suppression of UC progression.
The significant GO terms enriched in ZSCAN4 upregulation. The top 200 transcripts with negative associations with ZSCAN4 were explored using the GO classification system according to A biological processes, B molecular functions, or C cellular components and were graded by fold enrichment for functional annotation. An R script with ggplot2 package was used to present representative GO terms
Discussion
In this study, we found that low expression of ZSCAN4 was significantly associated with advanced disease status and key pathological parameters of aggressive behavior, such as high histological grade and vascular invasion. More importantly, low expression of ZSCAN4 was found to be an independent negative prognostic factor for DSS and MeFS in patients with UTUC or UBUC. In line with the finding from our initial expression profiling analysis of UBUC transcriptome (GSE31684), ZSCAN4 was identified as a tumor suppressor in UC. Previous studies mainly focused on the mechanism of telomere elongation of ZSCAN4 in embryonic stem cells [20]. The functional role and prognostic significance of ZSCAN4 in cancer have never been elucidated. This was the first study that investigates the prognostic significance of ZSCAN4 in a well-defined cohort of cancer patients. Assessment of ZSCAN4 expression in patients with UC could provide information for risk stratification and aid in treating patients in a personalized manner.
ZSCAN4 is a newly identified embryonic stem cell marker and is highly expressed exclusively in late 2-cell embryonic stem cells [21]. ZSCAN4 was responsible for attenuating the DNA damage response, improving genomic stability and promoting telomere elongation during reprogramming [20, 22, 23]. ZSCAN4, in combination with the Yamanaka factors (Oct3/4, Sox2, Klf4, and c-Myc), significantly promoted the efficiency of induced pluripotent stem (iPS) cell generation. During iPS cell formation, ZSCAN4 reduced DNA double-strand break (DSB) signals, characterized by decreased total phosphorylated histone H2AX (γ-H2AX) level during reprogramming [22]. γ-H2AX is formed rapidly after DSBs, and critical lesions can cause genomic instability and tumorigenesis [24].
Mammalian telomeres are composed of repetitive TTAGGG sequences that are responsible for formation of the capping structures, which are bound by telomere-binding factors called shelterin [25, 26]. The shelterin complex consists of a six subunit complex, including directly binding proteins telomeric repeat-binding factor 1 (TRF1), telomeric repeat-binding factor 2 (TRF2), and protection of telomeres 1 (POT1) and their associated proteins repressor/activator protein 1 (RAP1), TPP1 (Adrenocortical dysplasia protein homolog), and TRF1-interacting nuclear factor 2 (TIN2) [27, 28]. Overexpression of ZSCAN4 could trigger rapid telomere extension and inhibit TRF2, POT1b and RAP1 and which, in turn, suppresses spontaneous telomere sister chromatid exchange [22]. In breast cancer cells (MCF7) and osteosarcoma cells (SaOS2), ZSCAN4 has been found to be directly bound to RAP1 in the nucleus, possibly regulating shelterin complex-controlled telomere elongation in both telomerase positive and alternative lengthening of telomere pathways [29]. Interestingly, in these two types of cancer cells, the protein expression of ZSCAN4 was also dependent on RAP1. However, as mentioned before, the mRNA transcript level of RAP1 could be repressed by ZSCAN4 in embryonic stem cells [22]. Although direct binding between ZSCAN4 and RAP1 was evident, definite functional interaction between ZSCAN4 and RAP1 remains obscure.
Though the role of ZSCAN4 in embryonic stem cells became increasingly clear in recent years, little is known with respect to the biological function of ZSCAN4 in cancer cells. The expression of ZSCAN4 has been demonstrated in a small proportion of cancer cells, including cervical cancer cells (HeLa), breast cancer cells (MCF7) and osteosarcoma cells (SaOS2 and U2OS) [29]. Additionally, in head and neck squamous cell carcinoma (HNSCC), ZSCAN4 played an important role in facilitating chromatin remodeling and activating cancer stem cell factor expression, including OCT3/4, NANOG, KLF4, and SOX2. Depletion of ZSCAN4 was found to have inhibitory effect on tumor growth in HNSCC [30]. Moreover, Zhang et al. found that ZSCAN4 expression is increased in DNA-damaged stromal cells that leads to a senescence-associated secretory phenotype (SASP), mediated by the ATM/TRAF6/TAK1/p65 signaling axis [31]. They also disclosed that targeting TAK1 in vivo increases chemosensitization and promotes tumor regression. These aforementioned findings suggested that ZSCAN4 have oncogenic role in some cancer types, other than UC. Currently, there is no data available regarding the expression and biological function of ZSCAN4 in UC cells. In cancer cells, telomere maintenance is an important mechanism to keep immortality. Accordingly, in terms of the known biological function of telomere elongation of ZSCAN4, ZSCAN4 expression in cancer cells may aid in telomere elongation, prevent cellular senescence and maintain normal karyotype for many cell divisions, and which, subsequently, result in cell immortalization [20]. In addition, during reprogramming in iPS cells, ZSCAN4 has been found to indirectly downregulate p53, a key tumor suppressor [22]. However, more studies are needed to clarify mechanisms about the tumor suppressor role of ZSCAN4 in UC.
High mitotic activity has been associated with progression and recurrence of non-muscle-invasive bladder cancer and could be a useful prognostic marker beyond tumor grades [32]. Impressively, many genes co-downregulated with ZSCAN4 were implicated in the mitotic cell cycle (Fig. 5A–C). Initially, as cells transit from interphase to mitosis, diverse events occur to prepare for chromosome separation, including chromosome condensation (GO 1,905,821, fold enrichment 58.29), nuclear envelope breakdown, spindle assembly (GO 0,007,057, fold enrichment 72.86), and segregation and movement of duplicated centrosomes to opposite poles of the cell [33]. Subsequently, the mitotic spindle attaches to and lines up chromosomes at its center, known as the metaphase plate [34]. The representative shape of metaphase spindle is featured by mirror symmetry of sister chromatids alongside this equator. Afterwards, during anaphase (GO 0,010,997, fold enrichment 32.38), the mitotic spindle elongates (GO 0,000,022, fold enrichment 48.57) and the central spindle (GO 0,097,149, fold enrichment 97.15) emerges in the middle of the spindle [35]. Despite the similar organization of spindle and central spindle, they assemble at different times during the cell cycle. The central spindle generates as cells exit mitosis and modulates cleavage furrow formation and completion of daughter cell separation. Accordingly, the association among the level of ZSCAN4 mRNA, its co-downregulated genes and mitosis regulation, as well as their roles in the suppression of UC progression warrant further analysis.
Conclusion
In this study, we firstly identified that ZSCAN4 acts as a tumor suppressor in UC. In patients with UTUC or UBUC, low expression of ZSCAN4 was significantly associated with some aggressive clinicopathological parameters. Moreover, low ZSCAN4 expression served as an adverse prognostic factor for disease-specific survival and metastasis-free survival.
Availability of data and materials
The data generated or analyzed in the current study were available from the corresponding author on reasonable request.
Abbreviations
- ZSCAN4:
-
Zinc finger and SCAN domain containing 4
- UC:
-
Urothelial carcinoma
- UBUC:
-
Urinary bladder urothelial carcinoma
- UTUC:
-
Upper urinary tract urothelial carcinomas
- TCGA:
-
The Cancer Genome Atlas (TCGA)
- DSS:
-
Disease-specific survival (DSS)
- MeFS:
-
Metastasis-free survival
- GO:
-
Gene ontology
- iPS:
-
Induced pluripotent stem
- DSB:
-
Double-strand break
- HNSCC:
-
Head and neck squamous cell carcinoma
- SASP:
-
Senescence-associated secretory phenotype
References
Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15:25–41.
Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22.
Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–65.
Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, Yeh JJ, Milowsky MI, Iyer G, Parker JS, Kim WY. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111:3110–5.
Sjodahl G, Lauss M, Lovgren K, Chebil G, Gudjonsson S, Veerla S, Patschan O, Aine M, Ferno M, Ringner M, et al. A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 2012;18:3377–86.
Cairns P, Mao L, Merlo A, Lee DJ, Schwab D, Eby Y, Tokino K, van der Riet P, Blaugrund JE, Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994;265:415–7.
Williamson MP, Elder PA, Shaw ME, Devlin J, Knowles MA. p16 (CDKN2) is a major deletion target at 9p21 in bladder cancer. Hum Mol Genet. 1995;4:1569–77.
Aboulkassim TO, LaRue H, Lemieux P, Rousseau F, Fradet Y. Alteration of the PATCHED locus in superficial bladder cancer. Oncogene. 2003;22:2967–71.
McGarvey TW, Maruta Y, Tomaszewski JE, Linnenbach AJ, Malkowicz SB. PTCH gene mutations in invasive transitional cell carcinoma of the bladder. Oncogene. 1998;17:1167–72.
Nishiyama H, Hornigold N, Davies AM, Knowles MA. A sequence-ready 840-kb PAC contig spanning the candidate tumor suppressor locus DBC1 on human chromosome 9q32-q33. Genomics. 1999;59:335–8.
Sjodahl G, Lauss M, Gudjonsson S, Liedberg F, Hallden C, Chebil G, Mansson W, Hoglund M, Lindgren D. A systematic study of gene mutations in urothelial carcinoma; inactivating mutations in TSC2 and PIK3R1. PLoS ONE. 2011;6:e18583.
Kompier LC, Lurkin I, van der Aa MN, van Rhijn BW, van der Kwast TH, Zwarthoff EC. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS ONE. 2010;5:e13821.
Lopez-Knowles E, Hernandez S, Malats N, Kogevinas M, Lloreta J, Carrato A, Tardon A, Serra C, Real FX. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 2006;66:7401–4.
Mitra AP, Cote RJ. Molecular pathogenesis and diagnostics of bladder cancer. Annu Rev Pathol. 2009;4:251–85.
Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol. 2006;24:5552–64.
Sanchez-Carbayo M, Socci ND, Charytonowicz E, Lu M, Prystowsky M, Childs G, Cordon-Cardo C. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002;62:6973–80.
Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25.
Riester M, Taylor JM, Feifer A, Koppie T, Rosenberg JE, Downey RJ, Bochner BH, Michor F. Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clin Cancer Res. 2012;18:1323–33.
Lee HY, Yeh BW, Chan TC, Yang KF, Li WM, Huang CN, Ke HL, Li CC, Yeh HC, Liang PI, et al. Sulfatase-1 overexpression indicates poor prognosis in urothelial carcinoma of the urinary bladder and upper tract. Oncotarget. 2017;8:47216–29.
Zalzman M, Falco G, Sharova LV, Nishiyama A, Thomas M, Lee SL, Stagg CA, Hoang HG, Yang HT, Indig FE, et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature. 2010;464:858–63.
Falco G, Lee SL, Stanghellini I, Bassey UC, Hamatani T, Ko MS. Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev Biol. 2007;307:539–50.
Jiang J, Lv W, Ye X, Wang L, Zhang M, Yang H, Okuka M, Zhou C, Zhang X, Liu L, Li J. Zscan4 promotes genomic stability during reprogramming and dramatically improves the quality of iPS cells as demonstrated by tetraploid complementation. Cell Res. 2013;23:92–106.
Thool M, Sundaravadivelu PK, Sudhagar S, Thummer RP. A comprehensive review on the role of ZSCAN4 in embryonic development, stem cells, and cancer. Stem Cell Reviews and Reports. 2022;18:2740–56.
McKinnon PJ, Caldecott KW. DNA strand break repair and human genetic disease. Annu Rev Genomics Hum Genet. 2007;8:37–55.
Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85:6622–6.
Thanasoula M, Escandell JM, Martinez P, Badie S, Munoz P, Blasco MA, Tarsounas M. p53 prevents entry into mitosis with uncapped telomeres. Curr Biol. 2010;20:521–6.
Diotti R, Loayza D. Shelterin complex and associated factors at human telomeres. Nucleus. 2011;2:119–35.
Hockemeyer D, Daniels JP, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126:63–77.
Lee K, Gollahon LS. Zscan4 interacts directly with human Rap1 in cancer cells regardless of telomerase status. Cancer Biol Ther. 2014;15:1094–105.
Portney BA, Arad M, Gupta A, Brown RA, Khatri R, Lin PN, Hebert AM, Angster KH, Silipino LE, Meltzer WA, et al. ZSCAN4 facilitates chromatin remodeling and promotes the cancer stem cell phenotype. Oncogene. 2020;39:4970–82.
Zhang B, Fu D, Xu Q, Cong X, Wu C, Zhong X, Ma Y, Lv Z, Chen F, Han L, et al. The senescence-associated secretory phenotype is potentiated by feedforward regulatory mechanisms involving Zscan4 and TAK1. Nat Commun. 2018;9:1723.
Zaleski M, Gogoj A, Walter V, Raman JD, Kaag M, Merrill SB, Drabick J, Joshi M, Holder S, DeGraff DJ, Warrick JI. Mitotic activity in noninvasive papillary urothelial carcinoma: its value in predicting tumor recurrence and comparison with the contemporary 2-tier grading system. Hum Pathol. 2019;84:275–82.
Petry S. Mechanisms of Mitotic Spindle Assembly. Annu Rev Biochem. 2016;85:659–83.
Prosser SL, Pelletier L. Mitotic spindle assembly in animal cells: a fine balancing act. Nat Rev Mol Cell Biol. 2017;18:187–201.
Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009;10:9–20.
Acknowledgements
Thanks for the biobank at ChiMei Medical Center for providing tumor samples.
Funding
This work was supported by grants from the Ministry of Science and Technology of Taiwan (109–2628-B-384–003, 109–2314-B-037–110-MY3, and 110–2628-B-384–001) and in part by grants from the National Health Research Institutes of Taiwan (CA-110-PP-11) and Chi Mei Medical Center.
Author information
Authors and Affiliations
Contributions
All authors contributed to conception and design of this study and approved the final manuscript. HL He, HY Lai and TC Chan performed material preparation, data collection and interpretation. HL He wrote the main manuscript text. CH Hsing, SK Huang, KL Hsieh, TJ Chen, WS Li and YH Kuo gave valuable comments on the study design. YL Shiue and CF Li participated in supervision and data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study is reviewed and approved by the Institutional Review Board of Chi-Mei Medical Center (IRB10302015).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table 1.
The top 200 genes positively correlated with ZSCAN4. Table 2. The top 200 genes negatively correlated with ZSCAN4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, HL., Lai, HY., Chan, TC. et al. Low expression of ZSCAN4 predicts unfavorable outcome in urothelial carcinoma of upper urinary tract and urinary bladder. World J Surg Onc 21, 62 (2023). https://doi.org/10.1186/s12957-023-02948-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-02948-4