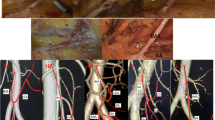
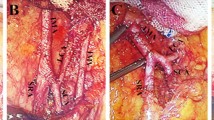
Abstract
Currently, high or low ligation of the inferior mesenteric artery (IMA) is a controversial issue in laparoscopic radical surgery for colorectal cancer. High or low ligation of the IMA has both advantages and disadvantages, and the level of ligation during the left colon and/or rectum resection has been a dilemma for surgeons. One important factor influencing the surgeon’s decision to ligate the IMA in a high or low position is the anatomical type of the IMA and its branches. Some studies confirm that the anatomy of the IMA and its branches is critical to the anastomotic blood supply and, therefore, influences the choice of surgical approach (level of ligation of the IMA). However, many vascular variations in the anatomy of the IMA and its branches exist. Herein, we have summarized the anatomical types of the IMA and its branches, finding that the classification proposed by Yada et al. in 1997 is presently accepted by most scholars. Based on Yada’s classification, we further summarized the characteristics of the IMA’s various anatomical types as a guide for high or low ligation in radical colorectal cancer surgery.
Similar content being viewed by others
Background
The left colic artery (LCA) is the uppermost branch of the inferior mesenteric artery, starting 2–3 cm from the root of the inferior mesenteric artery and traveling leftward to the deep surface of the peritoneum wall. It divides into ascending and descending branches, which nourish the left flexure of the colon and the descending colon, and anastomoses with branches of the middle colic artery and sigmoid artery, respectively. The inferior mesenteric artery (IMA) originates from the anterior surface of the aorta behind the lower border of the duodenum and is located 3–4 cm above the level of the aortic bifurcation L2–L3. The LCA is the first branch of IMA. The IMA and its vessels are among the most important anatomical landmarks in colorectal surgery. Controversy exists on the use of high or low ligation of the IMA in laparoscopic radical surgery for colorectal cancer. One of the important factors influencing the surgeon’s decision to ligate the IMA in a high or low position is the anatomical type of the inferior mesenteric artery and its branches. Some studies [1,2,3,4,5,6,7,8,9,10,11] corroborate that the anatomy of the IMA and its branches is critical to the anastomotic blood supply, therefore influencing the choice of surgical approach (level of IMA ligation). However, owing to the narrow laparoscopic view and lack of palpation, vascular bifurcation variants can easily be misdiagnosed as injuries, causing serious complications such as hemorrhage and intestinal ischemia. Pre-operative knowledge of arterial branching or variants, including the characteristics of each type, is useful for surgeons in developing pre-operative strategies for safe, rapid vascular ligation and lymph node dissection. This review summarizes the anatomical types of IMA and its branches and discusses the significance of the Riolan arterial arch, with the aim of providing guidance for precise ligation of the vessels during laparoscopic radical colorectal cancer surgery.
Material and methods
In this study, we searched the National Institute of Health PubMed database using a combination of subject and free words, including “Cancer,” “Tumor,” “Colon,” “Rectum,” “Colorectal,” “Ligation,” “Anatomical variation,” “Inferior mesenteric artery,” “IMA,” “Left colonic artery,” “LCA,” and “Riolan” as keywords. A total of 303 references were identified during an initial search of the PubMed database, and 11 additional references were identified by a manual search. After excluding duplicate citations and carefully reviewing abstracts, 53 papers were selected for a full-text review. In total, 48 studies were included in the final review.
Advantages and disadvantages of ligating the IMA in high and low positions
In recent years, laparoscopic resection of colorectal cancer has gained widespread clinical acceptance. With continuous improvements in laparoscopic techniques, consensus was reached on many aspects of surgical treatment, including total mesorectal excision, lymph node dissection of the IMA root, and preservation of pelvic autonomic nerves. The 2019 Japanese Society for Colorectal Cancer Research (JSCCR) guidelines recommend that lymph node dissection in progressive rectal cancer should include the root of the inferior mesenteric vessels; however, these guidelines do not specify the site of IMA ligation [12]. Likewise, the National Comprehensive Cancer Network (USA) guidelines do not indicate whether the LCA should be preserved [13]. Therefore, the ligation level (high or low) of the IMA during the left colon and/or rectal resection is unelucidated. Low ligation is the separation and ligation of the branches of the left colonic artery, whereas high ligation is the separation and ligation of the aorta at its origin. Therefore, by definition, low ligation will preserve the left colonic artery, whereas high ligation does not. In laparoscopic radical colorectal cancer surgery, ligation of the IMA at high and low positions has both advantages and disadvantages. Proponents of high ligation believe that it can better clear root lymph nodes, reduce anastomotic tension, provide accurate tumor staging, and preserve the nerves. This enables patients to achieve longer overall survival, minimizing the risk of tumor cell spillage and local recurrence [14,15,16,17,18,19,20], In comparison, low ligation has insufficient lymph node clearance and thus increases the probability of metastatic recurrence. Proponents of low ligation believe that high ligation will reduce the proximal blood supply to the anastomosis because of root ligation, increasing the risk of anastomotic leakage, and damage to autonomic nerve function [21,22,23,24,25,26,27,28,29,30,31,32]. Low ligation after IMA can increase the proximal blood supply to the anastomosis and protect autonomic nerve function because the left colonic artery is preserved. It is also relatively simple to perform. In this case, the anatomy of the IMA and its branches is very important for radical colorectal cancer surgery, and anatomical structures should be operated on differently. Accurate recognition and intra-operative assessment of the anatomy and possible changes in IMA and LCA are crucial when performing radical resection for colorectal tumors.
Anatomical types of IMA and its branches and Riolan arterial arch
Numerous anatomical variants exist of the IMA and its branches, and there is no unified anatomical typing standard. In 1949, Latarjet [33] described two types of anatomical variants of the IMA (Fig. 1): type I: independent origin, the LCA and sigmoid artery (SA) have separate origins and type II: fan-shaped origin, the LCA and SA co-trunk share a common origin. Later, Predesc [34] added to Latarjet’s classification (Fig. 2) and described the following types: type I: identical to Latarjet type I and types IIa, IIb, IIc, and IId: subdivisions of Latarjet type II, defined as follows: IIa: IMA divides into LCA, SA, and superior rectal artery (SRA); IIb: LCA, SA, SRA emanate from the same origin; IIc, on the basis of IIb, LCA divides into the middle left colonic artery (MLCA) or inferior left colonic artery (ILCA); and IId, LCA, and SA co-trunk.
In 1971, Zebroski [35] (Fig. 3) classified IMA anatomic variants into eight types: (A) IMA first emits LCA, two SAs have a common trunk with SRA, respectively; (B) IMA first emits LCA, two SAs share a common trunk (ST), ST co-trunks with SRA; (C) IMA first emits LCA, two SAs share a common trunk ST, ST co-trunks with LCA; (D) IMA first emits LCA and two SAs have a common trunk with LCA, respectively; (E) IMA first emits LCA, two SAs have a common trunk with LCA and SRA, respectively; (F) IMA first emits LCA, three SAs, one of which has a common trunk with LCA and the other two have a common trunk with SRA, respectively; (G) IMA first emits LCA, three SAs, one of which has a common trunk with SRA and the other two have a common trunk with LCA, respectively; and (H) LCA, SA, and SRA emanate from a common starting point. In recent years, Wang [7] (Fig. 4) classified anatomical types into three types: type A: LCA arises independently of the IMA; type B: LCA and SA branches from a common IMA trunk; and type C: LCA, SA, and SRA branches from the IMA at the same point. Miyamoto [2] classified IMA anatomical typology into three types: type A: LCA and SA bifurcate from the same point of the IMA; type B: the common trunk of the LCA and SA separates from the IMA; and type C: the LCA and SA separate from the IMA. Patroni [5] (Fig. 5) divided Latarjet typing into groups N and F (N: < 20 mm, F: ≥ 20 mm) based on the distance between the LCA and IMV at the inferior margin of the pancreas. Many current typing methods have a more limited role in guiding high/low ligation of the IMA during laparoscopic radical surgery for colorectal cancer, creating difficulties in preserving or further identifying and preserving the LCA. Latarjet [33] was the first to propose anatomical variants and typing of IMA and LCA, but its division into two types does not provide a good overview of all types of anatomical variants of IMA and LCA. Predescu [34] further elaborated the typing based on Latarjet’s classification and introduced the concept of the left middle colic artery (MLCA), which nicely complemented Latarjet’s classification. Zebroski [35] classified the anatomical variants of the IMA into eight types, but its overly detailed typing was not conducive to the surgeon’s identification of the target vessels pre-operatively and intra-operatively. Wang’s typing [7] aptly summarized the characteristics of anatomical variants of the IMA and LCA but lacked discussion regarding the absence of LCA. Patroni’s typing method [5] introduced the concept of the submesenteric vein but did not discuss the anatomical variants of the IMA and LCA in detail. These typing methods are not conducive to the surgeon’s precise understanding of their anatomical structures and judgment of the intra-operative ligature location of the vessels, making it difficult to identify and preserve the LCA during laparoscopic radical surgery for colorectal cancer. Therefore, they are not conducive to reducing the incidence of post-operative complications. Yada, a Japanese researcher, was the first to classify IMA in 1997 based on the relationship between LCA, SA, and the root initiation point of SRA [8] (Fig. 6). The four types of typing were accepted by most researchers, and most clinical studies since then have been based on this as it better presents the relationship between IMA, LCA, SA, and SRA. It is helpful for surgeons to precisely recognize the anatomy pre-surgery, thereby they can choose the correct operation and decide whether to preserve the left colon. I: LCA emanates from IMA independently; II: LCA and SA co-trunk; III: LCA, SA, and SRA emanate from the same point; and IV: LCA is absent.
Classification of Patroni. Type I and type II represent diffuse or fan-shaped IMA branching patterns, respectively (Laterjet typing). Subgroup N represents IMV-LCA distances greater or less than 20 mm at the inferior margin of the pancreas, respectively; subgroup F represents IMV-LCA distances greater than 20 mm at the inferior margin of the pancreas, respectively
Under the Yada classification, the percentage occurrence of each type in the nine papers was counted, as shown in Table 1. According to the statistical results, the probability of occurrence of type I ranged from 32.1 to 59.4%, type II from 10.3 to 53.6%, type III from 8.5 to 44.7%, and type IV from 0 to 2.8%.
The significance of IMA branch classifications
Type I: Luo et al. [37] concluded that in patients with colorectal cancer with type I IMA, low ligation can be performed when the LCA origin is revealed, preserving the blood supply and reducing unnecessary operative time. According to Patroni [5], a single LCA origin was observed in 71% of cases (types I, II), and since the LCA is more easily preserved at the origin, this is considered a good outcome of low ligation (preserved LCA). This study concluded that in more than two-thirds of cases, the preservation of the LCA at its origin is highly feasible because of its different starting points. In addition, several studies [1, 4, 6, 7, 36] have found that the average distance of LCA initiation from IMA initiation was closer in type I patients than in other IMA types, which could impact the extent of intraoperative lymph node dissection, a crucial point to consider during the procedure.
Type II: Huang et al. [10] concluded that the LCA originates far from the root of the IMA and co-stems with the SA, which is easier to locate during laparoscopic surgery and can be considered for radical colorectal cancer surgery with preservation of the LCA. Luo et al. [37] concluded that for type II IMA, LCA preservation will likely lead to difficulty in proximal bowel pull-down, increase the anastomotic tension, and increase the possibility of anastomotic leakage.
Type III: According to Huang et al. [10], type III is easier to detect and separate because LCA, SA, and SRA are co-interstitial. In type III, the LCA divides from the SA and SRA at the same point low in the IMA and moves towards the descending colon. The blood supply to the splenic flexure of the colon and descending colon is supplied only by the marginal arch between the left branch of the middle colonic artery and the LCA. Moreover, the descending colon has a longer segment of the intestinal canal and lacks a direct blood supply from the tertiary arteries. Therefore, patients with colorectal cancer with type III vascular dissection should undergo radical colorectal cancer surgery with preservation of the LCA because high ligation of the IMA during radical resection will block LCA blood flow and may lead to inadequate blood supply to the descending colon and distal anastomosis, thus increasing the risk of anastomotic leakage. However, according to Luo et al. [37], for patients with type III IMA, it is imperative to reveal the SRA, SA, and LCA common trunk, including dissociating the sigmoid and superior colorectal arteries. This dissociation should be performed with extra care because incorrect surgery can easily lead to LCA bleeding and necessitate abandoning low ligation, reducing the anastomotic blood supply.
The Riolan arterial arch, which is the anastomotic branch between the ascending branch of the LCA and left branch of the middle colonic artery, has been described in several studies. In a study by Huang et al. [10], 60.3% (70/116) of the Riolan arterial arches were absent, with 49.3% (33/67), 83.3% (10/12), 72.2% (26/36), and 100% (1/1) of the IMA types absent, respectively. However, these differences were not statistically significant (P = 0.125). This study concluded that type III and Riolan arterial arch defects are independent risk factors for the development of anastomotic leakage. In this study, no anastomotic leakage occurred after high ligation in type III patients with a Riolan arch, whereas all patients with a type III anastomotic leakage had a combined Riolan arch defect. In patients with an absent Riolan arch, left hemicolectomy is dependent on the IMA for blood supply, and the high-ligation technique can possibly cause ischemic changes in the anastomosis. Therefore, a low-ligation technique that preserves the LCA in order to maintain blood supply is recommended. Low ligation with highly selective lymph node dissection may be considered for patients with type III and Riolan artery arch agenesis. Wang et al. [7] concluded that Riolan artery arch agenesis is an independent risk factor for anastomotic leakage after laparoscopic radical colorectal cancer surgery. Since left hemicolectomy relies on the IMA for blood supply in cases of Riolan artery arch deficiency, the use of high ligation leads to ischemic changes in the anastomosis, thereby increasing the risk of postoperative anastomotic leakage.
Discussion
In the laparoscopic resection of colorectal cancer, high/low ligation of the IMA remains controversial. Tumor staging is the basis of colorectal cancer treatment and determines the choice of treatment strategy as well as the ability to preserve LCA. Thorough lymph node dissection is the key to radical resection and accurate tumor staging of colorectal cancer. A study [38] showed that patients with stage T1 rectal cancer had no metastasis in group 253 lymph nodes, and the metastasis rate was 0.95% (1/105) in stage T2, 5.22% (6/115) in stage T3, and 6.12% (12/196) in stage T4, suggesting that metastasis in group 253 lymph nodes might theoretically exist in all rectal cancers above stage T2. Therefore, whether the group 253 lymph nodes can be completely cleared is the key to the preservation of the LCA procedure and the main point of controversy regarding the preservation of the LCA. High ligation of the IMA provides effective, complete, and intact clearance of group 253 lymph nodes, but it is not the only method for complete clearance of this group of lymph nodes. Studies have shown no statistically significant difference in the clearance of 253 lymph nodes in patients with and without retained LCA [21, 23, 25, 26, 28,29,30,31,32]. Both retrospective and prospective studies have shown that high and low ligation in rectal cancer surgery has comparable effects on overall and recurrence-free survival, with no statistically significant differences in survival even in patients with lymph node metastases [11, 39, 40]. The oncological safety of the preserved LCA procedure was confirmed. However, it is worth noting that in patients with excessive metastases and fusion of group 253 lymph nodes into clusters, dissection to reveal the LCA and preservation of the LCA can pose technical challenges and increase the probability of intra-operative bleeding. Therefore, for patients found to be in this category on intra-operative exploration, preservation of the LCA is not recommended, and high IMA ligation is feasible to reduce the surgical difficulty and ensure oncological safety [41]. According to a recent expert consensus published in 2021 [41], the decision to preserve the LCA depends on various factors, including rectal and partial colon cancer, older adults, combined metabolic diseases, neoadjuvant therapy, risk of multiple primary colorectal cancers, and persistent descending mesocolon (PDM). There are numerous reasons for this. Advanced age and diabetes mellitus are recognized as high-risk factors for anastomotic leakage [42, 43]. In resected colorectal cancer specimens after radiotherapy, the peri-cancerous tissue mucosa, submucosa, and surrounding adipose tissue show varying degrees of inflammatory changes and fibrosis and the colorectal mucosa and mesocolon tissue show microvascular damage and increased fragility. This in turn affects the healing ability of the anastomosis, and neoadjuvant radiotherapy is a high-risk factor for postoperative anastomotic leak in colorectal cancer [44]. For patients at risk of multiple primary colorectal cancers, the preservation of the LCA during the first operation can reserve vascular reserve for later surgery. Patients with PDM mostly have abnormalities of the vascular arch, including the LCA, branches of the SA, and SA in a radial pattern, forming a bear claw-like structure. In addition, the LCA in this group of patients is often short and even, directly forming part of the marginal vascular arch [45, 46]; therefore, not preserving the LCA may lead to extensive intestinal ischemia. In contrast, patients with a high risk of IMA root lymph node metastasis [47] and patients with surgical findings of high anastomotic tension [48] are not recommended for LCA preservation.
Another important factor is the anatomical variation of arteries. Because of a large variation in the anatomical structure of the LCA, a definite theoretical foundation and operational skills are required for surgeons to accurately locate the root of the LCA under laparoscopic guidance and to avoid accidental bleeding when dealing with the vessels. Preoperative mastery of IMA subtyping; accurate determination of the relationship between the LCA, SA, and SRA; and measurement of the distance between the root of the IMA and the beginning of the LCA are essential for successful implementation of LCA-preserving surgery. Therefore, in addition to the routine examination, preoperative patients with colorectal cancer need accurately determined IMA staging by CT angiography and different methods of LCA preservation adopted intra-operatively according to their staging. In this study, we summarized the clinical classifications. Based on the Yada classification and the perspective of the presence or absence of the Riolan artery arch, we conclude that type III is an independent risk factor for the occurrence of anastomotic leakage, and that it is easier to detect free type III cases, which tend to preserve the LCA. For patients without a Riolan artery arch, radical colorectal cancer surgery with preservation of the LCA should be performed. For patients with type III and Riolan artery agenesis, radical colorectal cancer resection with preservation of the LCA should be highly recommended as it offers more advantages for patients’ survival post-surgery. For patients with types I and II, low ligation may be performed for anatomic convenience, but the choice should be made in the context of the patient’s own condition and the intra-operative situation. To better define the surgical plan for the operator pre-surgery, we believe that “IMA-accurate staging” should be incorporated into the standard of care for colorectal cancer surgery, which still requires evidence-based studies with large multicenter samples.
Conclusion
After fully assessing factors such as patient condition and tumor characteristics, anatomical variation of the IMA and its branches is of great significance for the preservation of the LCA during radical surgery for colorectal cancer. In summary, type III IMA is an independent risk factor for the development of anastomotic leakage and is easier to detect and separate, favoring the preservation of the LCA. In patients with Riolan artery arch agenesis, radical colorectal cancer resection with preservation of the LCA should be performed. For patients with type III IMA and Riolan artery agenesis, radical colorectal cancer resection with preservation of the LCA should be performed, which provides a survival benefit post-surgery. For patients with type I and II IMA, low ligation can be performed for anatomical convenience; however, this is in the context of the patient’s own choices and the prevailing intra-operative conditions.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- AA:
-
Abdominal aorta
- C:
-
Common artery
- ILCA:
-
Left inferior colonic artery
- IM:
-
Inferior mesenteric artery
- IMA:
-
Inferior mesenteric artery
- IMV:
-
Inferior mesenteric vein
- JSCCR:
-
Japanese Society for Colorectal Cancer Research
- LC:
-
Left colonic artery
- LCa:
-
Left colic artery
- LCA:
-
Left colic artery
- LC acc.a:
-
Left middle colonic artery or the left inferior colonic artery
- MLCA:
-
Left middle colonic artery
- PDM:
-
Persistent descending mesocolon
- RST:
-
Rectosigmoid trunk
- S:
-
Sigmoid artery
- SA:
-
Sigmoid artery (SA)
- SR:
-
Superior rectal artery
- SRa:
-
Superior rectal artery
- SRA:
-
Superior rectal artery
- ST:
-
Sigmoid trunk
- S trunk:
-
Sigmoid artery trunk
References
Ke J, Cai J, Wen X, Wu X, He Z, Zou Y, et al. Anatomic variations of inferior mesenteric artery and left colic artery evaluated by 3-dimensional CT angiography: insights into rectal cancer surgery - A retrospective observational study. Int J Surg. 2017;41:106–11. https://doi.org/10.1016/j.ijsu.2017.03.012.
Miyamoto R, Nagai K, Kemmochi A, Inagawa S, Yamamoto M. Three-dimensional reconstruction of the vascular arrangement including the inferior mesenteric artery and left colic artery in laparoscope-assisted colorectal surgery. Surg Endosc. 2016;30(10):4400–4. https://doi.org/10.1007/s00464-016-4758-4.
Zhang W, Yuan WT, Wang GX, Song JM. Anatomical study of the left colic artery in laparoscopic-assisted colorectal surgery. Surg Endosc. 2020;34(12):5320–6. https://doi.org/10.1007/s00464-019-07320-w.
Bertrand MM, Delmond L, Mazars R, Ripoche J, Macri F, Prudhomme M. Is low tie ligation truly reproducible in colorectal cancer surgery? Anatomical study of the inferior mesenteric artery division branches. Surg Radiol Anat. 2014;36(10):1057–62. https://doi.org/10.1007/s00276-014-1281-y.
Patroni A, Bonnet S, Bourillon C, Bruzzi M, Zinzindohoué F, Chevallier JM, et al. Technical difficulties of left colic artery preservation during left colectomy for colon cancer. Surg Radiol Anat. 2016;38(4):477–84. https://doi.org/10.1007/s00276-015-1583-8.
Kobayashi M, Morishita S, Okabayashi T, Miyatake K, Okamoto K, Namikawa T, et al. Preoperative assessment of vascular anatomy of inferior mesenteric artery by volume-rendered 3D-CT for laparoscopic lymph node dissection with left colic artery preservation in lower sigmoid and rectal cancer. World J Gastroenterol. 2006;12(4):553–5. https://doi.org/10.3748/wjg.v12.i4.553.
Wang KX, Cheng ZQ, Liu Z, Wang XY, Bi DS. Vascular anatomy of inferior mesenteric artery in laparoscopic radical resection with the preservation of left colic artery for rectal cancer. World J Gastroenterol. 2018;24(32):3671–6. https://doi.org/10.3748/wjg.v24.i32.3671.
Yada H, Sawai K, Taniguchi H, Hoshima M, Katoh M, Takahashi T. Analysis of vascular anatomy and lymph node metastases warrants radical segmental bowel resection for colon cancer. World J Surg. 1997;21(1):109–15. https://doi.org/10.1007/s002689900202.
Zhong M, Luo Y, Yu MH. Laparoscopic radical resection of rectal cancer with preservation of the left colic artery: anatomical basis and surgical experience. Zhonghua Wai Ke Za Zhi. 2020;58(8):600–3. https://doi.org/10.3760/cma.j.cn112139-20200325-00252.
Huang J, Zhou J, Wan Y, Lin Y, Deng Y, Zhou Z, et al. Influences of inferior mesenteric artery types and Riolan artery arcade absence on the incidence of anastomotic leakage after laparoscopic resection of rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19(10):1113–8. https://doi.org/10.3760/cma.j.issn.1671-0274.2016.10.008.
Zhou J, Zhang S, Huang J, Huang P, Peng S, Lin J, et al. Accurate low ligation of inferior mesenteric artery and root lymph node dissection according to different vascular typing in laparoscopic radical resection of rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21(1):46–52. https://doi.org/10.3760/cma.j.issn.1671-0274.2018.01.009.
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. https://doi.org/10.1007/s10147-019-01485-z.
Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, et al. Willett C NCCN Clinical Practice Guidelines in Oncology: rectal cancer. J Natl Compr Canc Netw. 2009;7(8):838–81. https://doi.org/10.6004/jnccn.2009.0057.
Alici A, Kement M, Gezen C, Akin T, Vural S, Okkabaz N, et al. Apical lymph nodes at the root of the inferior mesenteric artery in distal colorectal cancer: an analysis of the risk of tumor involvement and the impact of high ligation on anastomotic integrity. Tech Coloproctol. 2010;14(1):1–8. https://doi.org/10.1007/s10151-009-0547-6.
Charan I, Kapoor A, Singhal MK, Jagawat N, Bhavsar D, Jain V, et al. High ligation of inferior mesenteric artery in left colonic and rectal cancers: lymph node yield and survival benefit. Indian J Surg. 2015;77(Suppl 3):1103–8. https://doi.org/10.1007/s12262-014-1179-2.
Chin CC, Yeh CY, Tang R, Changchien CR, Huang WS, Wang JY. The oncologic benefit of high ligation of the inferior mesenteric artery in the surgical treatment of rectal or sigmoid colon cancer. Int J Colorectal Dis. 2008;23(8):783–8. https://doi.org/10.1007/s00384-008-0465-5.
Kanemitsu Y, Hirai T, Komori K, Kato T. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery. Br J Surg. 2006;93(5):609–15. https://doi.org/10.1002/bjs.5327.
Kessler H, Hohenberger W. Extended lymphadenectomy in colon cancer is crucial. World J Surg. 2013;37(8):1789–98. https://doi.org/10.1007/s00268-013-2130-6.
Mc EJ, Bacon HE, Trimpi HD. Lymph node metastases; experience with aortic ligation of inferior mesentery artery in cancer of the rectum. Surgery. 1954;35(4):513–31.
West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, et al. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol. 2012;30(15):1763–9. https://doi.org/10.1200/jco.2011.38.3992.
Chen JN, Liu Z, Wang ZJ, Zhao FQ, Wei FZ, Mei SW, et al. Low ligation has a lower anastomotic leakage rate after rectal cancer surgery. World J Gastrointest Oncol. 2020;12(6):632–41. https://doi.org/10.4251/wjgo.v12.i6.632.
Cirocchi R, Farinella E, Trastulli S, Desiderio J, Di Rocco G, Covarelli P, et al. and Boselli C High tie versus low tie of the inferior mesenteric artery: a protocol for a systematic review. World J Surg Oncol. 2011;9:147. https://doi.org/10.1186/1477-7819-9-147.
Crocetti D, Cavallaro G, Tarallo MR, Chiappini A, Polistena A, Sapienza P, et al. and De Toma G Preservation of left colic artery with lymph node dissection of IMA root during laparoscopic surgery for rectosigmoid cancer. Results of a retrospective analysis. Clin Ter. 2019;170(2):e124–8. https://doi.org/10.7417/ct.2019.2121.
Fan D, Zhang C, Li X, Yao C, Yao T. Evaluation of the clinical efficacy of preserving the left colic artery in laparoscopic resection for rectal cancer: a meta-analysis. Mol Clin Oncol. 2018;9(5):553–60. https://doi.org/10.3892/mco.2018.1714.
Fan YC, Ning FL, Zhang CD, Dai DQ. Preservation versus non-preservation of left colic artery in sigmoid and rectal cancer surgery: a meta-analysis. Int J Surg. 2018;52:269–77. https://doi.org/10.1016/j.ijsu.2018.02.054.
Guo Y, Wang D, He L, Zhang Y, Zhao S, Zhang L, et al. Marginal artery stump pressure in left colic artery-preserving rectal cancer surgery: a clinical trial. ANZ J Surg. 2017;87(7-8):576–81. https://doi.org/10.1111/ans.13032.
Hinoi T, Okajima M, Shimomura M, Egi H, Ohdan H, Konishi F, et al. Effect of left colonic artery preservation on anastomotic leakage in laparoscopic anterior resection for middle and low rectal cancer. World J Surg. 2013;37(12):2935–43. https://doi.org/10.1007/s00268-013-2194-3.
Kim CS, Kim S. Oncologic and anastomotic safety of low ligation of the inferior mesenteric artery with additional lymph node retrieval: a case-control study. Ann Coloproctol. 2019;35(4):167–73. https://doi.org/10.3393/ac.2018.10.09.
Liu J, Gong Y, He M, Zeng X, Liu Y. Clinical effect of preservation or nonpreservation of left colic artery in total mesorectal excision under laparoscopy: a meta-analysis. Gastroenterol Res Pract. 2020;5(21):1958573. https://doi.org/10.1155/2020/1958573.
Si MB, Yan PJ, Du ZY, Li LY, Tian HW, Jiang WJ, et al. Lymph node yield, survival benefit, and safety of high and low ligation of the inferior mesenteric artery in colorectal cancer surgery: a systematic review and meta-analysis. Int J Colorectal Dis. 2019;34(6):947–62. https://doi.org/10.1007/s00384-019-03291-5.
Yang X, Ma P, Zhang X, Wei M, He Y, Gu C, et al. and Wang Z Preservation versus non-preservation of left colic artery in colorectal cancer surgery: an updated systematic review and meta-analysis. Medicine (Baltimore). 2019;98(5):e13720. https://doi.org/10.1097/md.0000000000013720.
Zeng J, Su G. High ligation of the inferior mesenteric artery during sigmoid colon and rectal cancer surgery increases the risk of anastomotic leakage: a meta-analysis. World J Surg Oncol. 2018;16(1):157. https://doi.org/10.1186/s12957-018-1458-7.
Latarjet A. Traite d’anatomie humaine. Tome quatrieme: Appareil de la Digestion; 1949.
Predescu D, Popa B, Gheorghe M, Predescu I, Jinescu G, Boeriu M, et al. The vascularization pattern of the colon and surgical decision in esophageal reconstruction with colon. A selective SMA and IMA arteriographic study. Chirurgia (Bucur). 2013;108(2):161–71.
Zebrowski W, Augustyniak E, Zajac S. Variations of origin and branching of the interior mesenteric artery and its anastomoses. Folia Morphol (Warsz). 1971;30(4):575–83.
Murono K, Kawai K, Kazama S, Ishihara S, Yamaguchi H, Sunami E, et al. Anatomy of the inferior mesenteric artery evaluated using 3-dimensional CT angiography. Dis Colon Rectum. 2015;58(2):214–9. https://doi.org/10.1097/dcr.0000000000000285.
Luo Y, Zhong M. The clinical value of inferior mesenteric arterial classification for preserving left colon artery in laparoscopic radical resection of rectal cancer. Chin J Colorec Dis (Electronic Edition). 2020;9(5):502–6. https://doi.org/10.3877/cma.j.issn.2095-3224.2020.05.012.
Li X, Li Q. Significance of the preservation of left colic artery in laparoscopic resection of rectal cancer. Chin J Gastrointest Surg. 2018;21(3):272–5. https://doi.org/10.3760/cma.j.issn.1671-0274.2018.03.006.
Liang JT, Huang KC, Lai HS, Lee PH, Sun CT. Oncologic results of laparoscopic D3 lymphadenectomy for male sigmoid and upper rectal cancer with clinically positive lymph nodes. Ann Surg Oncol. 2007;14(7):1980–90. https://doi.org/10.1245/s10434-007-9368-x.
Akagi T, Inomata M, Hara T, Mizusawa J, Katayama H, Shida D, et al. Clinical impact of D3 lymph node dissection with left colic artery (LCA) preservation compared to D3 without LCA preservation: exploratory subgroup analysis of data from JCOG0404. Ann Gastroenterol Surg. 2020;4(2):163–9. https://doi.org/10.1002/ags3.12318.
Lan P. Chinese expert consensus on radical resection of rectal cancer with preservation of left colonic artery (2021 edition). Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24(11):950–5. https://doi.org/10.3760/cma.j.cn441530-20210927-00389.
Jung SH, Yu CS, Choi PW, Kim DD, Park IJ, Kim HC, et al. Risk factors and oncologic impact of anastomotic leakage after rectal cancer surgery. Dis Colon Rectum. 2008;51(6):902–8. https://doi.org/10.1007/s10350-008-9272-x.
Zheng H, Wu Z, Wu Y, Mo S, Dai W, Liu F, et al. Laparoscopic surgery may decrease the risk of clinical anastomotic leakage and a nomogram to predict anastomotic leakage after anterior resection for rectal cancer. Int J Colorectal Dis. 2019;34(2):319–28. https://doi.org/10.1007/s00384-018-3199-z.
Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, et al. Impact of preoperative radiotherapy on anastomotic leakage and stenosis after rectal cancer resection: post hoc analysis of a randomized controlled trial. Dis Colon Rectum. 2016;59(10):934–42. https://doi.org/10.1097/dcr.0000000000000665.
Furuichi Y, Kumamoto K, Asano E, Kondo A, Uemura J, Suto H, et al. and Suzuki Y Four cases of laparoscopic colectomy for sigmoid colon and rectal cancer with persistent descending mesocolon. Surg Case Rep. 2020;6(1):255. https://doi.org/10.1186/s40792-020-00988-6.
Morgenstern L. Persistent descending mesocolon. Surg Gynecol Obstet. 1960;110:197–202.
Taflampas P, Christodoulakis M, DeBree E. Prognostic impact of inferior mesenteric artery lymph node metastasis in colorectal cancer. Ann Surg Oncol. 2011;18(Suppl 3):S235 author reply S236; doi: 10.1245/s10434-010-1497-y.
Girard E, Trilling B, Rabattu PY, Sage PY, Taton N, Robert Y, et al. Level of inferior mesenteric artery ligation in low rectal cancer surgery: high tie preferred over low tie. Tech Coloproctol. 2019;23(3):267–71. https://doi.org/10.1007/s10151-019-01931-0.
Acknowledgements
Not applicable.
Funding
National Natural Science Foundation of China (Grant number:81973646; 82104596), Special Scientific Research Project of New Coronary Pneumonia Epidemic Prevention and Control of Guangdong Provincial Department of Education (Grant number:2020KZDZX1170), Shenzhen Key Medical Discipline Construction Fund and Sanming Project of Medicine in Shenzhen (SZSM202111002), China Postdoctoral Science Foundation (Grant number:2020M682887), Guangdong Basic and Applied Basic Research Fund (Guangdong Natural Science Fund; Grant number:2020A1515110083), and Shenzhen Science and Technology Innovation Commission (Grant number: RCBS20200714114958333).
Author information
Authors and Affiliations
Contributions
P.G. and X. Z designed the study; T.Q. and S.Z. collected the data; W.W., and S.Z. wrote the manuscript. All of the authors listed have revised and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zeng, S., Wu, W., Zhang, X. et al. The significance of anatomical variation of the inferior mesenteric artery and its branches for laparoscopic radical resection of colorectal cancer: a review. World J Surg Onc 20, 290 (2022). https://doi.org/10.1186/s12957-022-02744-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02744-6