Abstract
Background
Patients with malignancy often require urgent surgical consultation for treatment or palliation of disease. The objective of this study is to explore the prognostic determinants affecting care in acute cancer-related surgical presentations and the effect on patient outcomes.
Main body
This is a retrospective review of patients referred to the acute general surgery (ACS) service at a tertiary hospital for management of cancer-related problem from July 2017 to September 2018. Patient demographics, course in hospital, and survival were recorded. Multivariant logistic regression and Kaplan-Meier estimates were performed. One hundred eighty-nine patients were identified (53% female) with a mean age of 65.9 years. Forty-two patients (22%) were newly diagnosed with cancer on presentation, and 94 (50%) patients had metastatic disease. Cancer staging was completed in 84% of patients, and 65% had multidisciplinary team (MDT) assessment during their hospital stay. Surgery was performed on 90 (48%) patients, of which 31.2% was with palliative intent. Overall mortality was 56% with 30- and 60-day mortality of 15% and 22%, respectively. The adjusted odds ratio (OR) for a 60-day mortality was high in patients presenting with new cancer diagnosis (OR 3.18, 95% CI 1.18–9.02, p=0.03), metastatic disease (OR 5.11, 95% CI 2.03–12.85, p=0.001), or systemic therapy on presentation (OR 3.46, 95% CI 1.30–9.22, p=0.013).
Conclusion
Emergency surgical referral is common in patients with malignancy. Surgical decision making can be challenging due to the heterogeneity of this population and their associated comorbidities. Optimizing prognostic determinants such as goal-directed palliative care, MDT discussions, and bridging to systemic therapy can improve patient outcomes.
Similar content being viewed by others
Background
Cancer is the leading cause of death in Canada with an estimated 225,800 new cancer diagnoses and 83,300 cancer-related deaths in 2020 [1]. A significant number of patients with malignancy are presented to the emergency department (ED) with acute symptoms [2]. In the USA, more than 4 million patients annually visit the ED for malignancy-related concerns, with up to 59% requiring admission [3,4,5]. Patients requiring emergency cancer operations have worse outcomes than those undergoing scheduled operations [6,7,8,9,10]. They are more likely to present with advanced stage and suffer higher morbidity and mortality [7,8,9,10]. Preoperative optimization can be challenging in this population due to factors such as poor nutritional status, comorbidities, and lack of cancer staging [5, 11,12,13]. There is limited data in this area as the heterogeneity of patients presenting with acute malignancy-related concerns makes it difficult to analyze outcomes on an individual basis. Therefore, there is a need to identify themes within patient care pathways that can be used to build infrastructure and enhance care for this population [9]. Themes can be categorized into determinants of health such as social, physical, and prognostic determinants. Social and physical determinants that influence outcomes have been described in the literature and include factors like income, ethnicity, or body mass index [14]. Prognostic determinants include assessments and investigations that predict disease burden and assist with developing treatment strategies [15]. The primary objective of this study is to identify prognostic determinants of health that predict outcomes for patients with acute cancer-related surgical presentations and provide guidelines for optimizing care pathways.
Main text
Methods
A retrospective study was performed of patients assessed by the inpatient acute care surgery (ACS) service for the management of any cancer-related surgical problem at Vancouver General Hospital in British Columbia. The study received an approval by the research ethics board. Patients were identified from a REDcap database® of all patients referred to ACS between July 2017 and September 2018 and were included if they were found to have a cancer-related problem at any point during their admission. Time to intervention including surgery, endoscopy, and interventional radiology (IR) was calculated from the day of admission, while time to adjuvant therapy was calculated from the day of discharge. A multidisciplinary team (MDT) was defined as the inclusion of more than one hospital-based team during a patient’s hospital stay. Surgery with palliative intent was defined as a surgical intervention performed in the setting of non-curable disease to provide symptom relief and improve the quality of life. Prognostic determinants of health were defined as factors that predict the outcome of a disease process [15, 16]. A comparison between surgery versus no surgery was made using Student’s t test. Overall, a 30- and 60-day mortality were calculated from the day of admission to hospital and were compared using Student’s t test. Kaplan-Meier analysis was performed comparing overall survival (OS) between patients who underwent surgery and non-surgical patients. A multivariate Cox hazard model was performed using multiple covariates including age, sex, primary cancer site, distant metastasis, date of diagnosis, systemic therapy on presentation, and cancer therapy within 12 weeks of discharge from hospital. Multivariate analysis was performed using logistic regression. A p value of <0.05 was statistically significant.
Results
One hundred eighty-nine patients were referred to ACS for malignancy-related surgical problems, comprising of 11% of all general surgery consults (n=1725). The mean age was 65.9 ± 14.5 years with 53% female. Forty-two patients (22.2%) received their first diagnosis of cancer during this presentation, and 33.4% of these patients (14/42) had metastatic disease. Of the patients with an established malignant diagnosis (77.8%), 54.4% (80/147) had metastatic disease at the time of referral to ACS. The most frequent primary sites of malignancy were lower gastrointestinal (LGI) (colon and rectum) (51.3%), non-visceral (skin, breast, sarcoma, and lymphoma) (15.3%), and upper gastrointestinal (UGI) (esophageal, gastric, small bowel, and pancreas) (13.8%). The most common reasons for referral were bowel obstruction, post-operative complications (from a separate hospital admission), and admission for urgent staging investigations or interventions. Fifty-five patients (29.1%) were on systemic therapy on presentation (i.e., chemotherapy, immunotherapy). Table 1 shows the demographics for cancer related referrals.
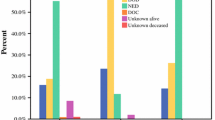
Of the 189 referred patients, 99/189 (52.4%) received non-operative management, 28/189 (14.8%) underwent surgery with palliative intent, 49/189 (25.9%) underwent surgery with curative intent, and 13 underwent surgery for diagnostic purposes. Thirty-five patients had an endoscopic procedure (e.g., endoscopy, endoscopic ultrasound, stent placement), and 25 had a procedure performed by IR (e.g., percutaneous biopsy, drain placement, aspiration). Complete staging with computed tomography (CT) of the chest, abdomen, and pelvis was performed in 84.4% (76/90) of the patients who underwent surgery. Figure 1 shows the sites of the primary malignancy and the goals of care with intervention performed.
The goals of care and the management approach based on the primary cancer site. Curative approach was defined as undergoing surgery with a goal of cure. Palliative approach was defined as surgical or conservative management with the goal of symptom control with a non curative intention. Supportive management indicated that patients underwent non-operative management of a cancer related problem or complication. Diagnostic approach mean that patients were admitted for cancer diagnosis or staging
The mean length of hospital stay was 12.2 ± 15.4 day. The MDT approach was performed in 65% of the patients. The average waiting time to surgery was 3.2± 4.4 days from admission, while time to endoscopy and IR was 2.8± 6.7 days and 3.3± 3.8 days, respectively. Most patients were discharged home (81.2%) (Table 2).
Overall mortality was 55.6% with 30-day mortality and 60-day mortality rates of 14.8% and 21.7%, respectively. Compared to patients managed non-surgically, patients who underwent surgery had lower 30-day (6.7% vs. 22.2%, p<0.05) and 60-day mortality (15.6 vs. 27.3%, p=0.051). The median survival was 15.4 months for the entire population with a clinical trend towards a longer survival in patients who underwent surgery compared to non-surgical management (surgery vs. no surgery, 22.4 vs. 8.6 months, p=0.84). Patients with early MDT involvement during their presentation were found to have lower 30-day mortality (OR 0.61, 95% CI 0.50–0.73, p=0.001). Sub-analysis was performed comparing patients undergoing palliative surgery compared to non-operative management. The 30-day mortality was lower in patients undergoing surgery with palliative intent (10.7 vs. 22.2%, p<0.05); however, there was no difference in the 60-day mortality (21.4% vs. 27.3%, p=0.19) or overall survival (palliative surgery vs. no surgery, 9.7 vs. 11.6 months, p=0.39). Figure 2 illustrates a Kaplan-Meier curve of the overall survival (OS) between the surgery and non-surgery groups. Table 3 shows the adjusted hazard ratios (HR) for the overall mortality of the population. The odds ratio (OR) for a 30- and 60-day mortality in patients undergoing surgery was 0.29 (95% CI 0.10–0.81, p=0.019) and 0.63 (95% CI 0.28–1.43, p=0.270), respectively. New cancer diagnosis (OR 3.18, 95% CI 1.12–9.02, p=0.030), systemic therapy (OR 3.46, 95% CI 1.3–9.22, p=0.013), and metastatic disease on presentation (OR 5.11, 95% CI 2.03–12.85, p=0.001) were associated with higher odds of a 60-day mortality. Table 4 shows the adjusted OR for a 30- and 60-day mortality.
Discussion
Establishing prognostic determinants
Patients with acute oncologic surgical presentations are a vulnerable population that require thoughtful management strategies to optimize the risk benefit ratio of treatment. The determinants that influence their treatments are complex and multifaceted, so by enhancing our understanding of these factors, there is a potential to improve outcomes. Prognostic determinants are modifiable factors that contribute to the understanding of an individual’s burden of disease and when identified can contribute to the selection of effective management strategies [14,15,16,17]. This study highlights several factors including new cancer diagnosis, the presence of metastatic disease, and systemic therapy that aid in establishing patient prognosis. By identifying prognostic factors related to disease, theme-based prognostic determinants can be developed that can enhance patient experience and outcomes. The themes of prognostic determinants identified in this paper include completeness of work up and accurate staging, multidisciplinary assessment, goal-directed palliative care involvement, and bridging to systemic therapy (Fig. 3).
Completeness of workup and accurate staging
An important component of oncologic care is staging of disease, including tissue diagnosis and appropriate imaging. This can be challenging in the emergency setting because of the acuity of presentation. Although the initial goal in an urgent setting is to ensure patient stability, the type of cancer and the extent of malignancy are essential in determining prognosis and guiding medical and surgical management [10, 18]. One example depending on histologic diagnosis would be treatment of bleeding tumors with radiation rather than surgical resection to avoid wounding healing complications and incomplete resection [19]. Studies show that up to 30% of patients with emergency cancer presentations have an unknown stage on presentation, and around 18% of patients with colorectal cancer undergo surgery without complete staging [20, 21]. In our study, over one third of patients with a new diagnosis of cancer had metastatic disease. As metastatic disease is a predictor of overall mortality (HR 5.59, p<0.001), it is important to establish this early in a patients’ care pathway to guide treatment appropriately. For example, in a patient with known lung metastases from colon cancer presenting with a malignant bowel obstruction, a diverting ostomy may be favored over an extensive bowel resection. Therefore, the completeness of workup is an important prognostic determinant in the setting of surgical care as it can influence operative and non-operative planning [10, 22].
Multidisciplinary assessment
Patients with malignancy have diverse care needs that require multidisciplinary assessment to ensure a comprehensive care plan is developed. Assessments can include, but are not limited to, surgical and medical consultants, symptom management specialists, nursing, and allied health teams. The involvement of these members is important for all patients but becomes essential for patients with emergency presentations. Bosscher et al. showed that MDT evaluation of patients with acute cancer presentations improved accurate assessment of physical status and prevented overtreatment in advanced stages [9, 19]. Early advanced practice nursing involvement in the care of elderly patients with cancer who received surgical intervention improved survival in this group by an average of seven months. As well, the need for emergency care was lower in patients receiving MDT treatment within a year of cancer diagnosis (OR=0.87) [2324]. In our study, 65% of the patients had MDT assessment during their stay comprised largely by gastroenterology and interventional radiology. Patients with MDT involvement were found to have lower 30-day mortality (OR 0.61, 95% CI 0.50–0.73, p=0.001). However, attributing this improvement in survival with MDT assessment can be difficult due to the heterogeneity of the population. This finding, in combination with previous studies noting a survival advantage with MDT care, supports the inclusion of the MDT assessment as a prognostic determinant. Input from appropriate specialists on nuances of care outside the surgical realm of practice such as interventional techniques, available systemic options, and health optimization is essential for enhancing prognosis.
At our institution, an ACS model was developed to address the acute general surgery demands of a tertiary referral center. By recognizing the unique needs of oncology patients within this larger population, infrastructure for care has been developed as part of an integrated practice unit (IPU). An emergency surgical oncology IPU was initiated by mapping care pathways to identify rate limiting steps or areas for improvement (diagnostic, interventional, or consultant based). An example of MDT implementation based on IPU mapping involves an oncology care provider attending daily surgical handover rounds to provide recommendation and feedback for patients presenting with acute oncologic concerns. MDT involvement allows for more accurate prognostication which may circumvent unnecessary invasive procedures or facilitate access to appropriate systemic therapy. Hence, it becomes a prognostic determinant with the potential to improve patient outcomes and should be considered for all emergency surgical oncology presentations.
Goal-directed palliative care
Involvement of palliative care teams and focus on symptom management can prolong life while improving quality [25,25,26,28]. Major surgical facilities report consultations for surgical palliation between 18 and 40% [9, 29]. In some centres, surgery with palliative intent accounts for up to one third of emergency surgeries in cancer patients [12, 29,29,31]. One approach to mitigating a surgical risk is through the “palliative triangle” as described by Miner et al. [25]. The palliative triangle is a communication strategy through which the potential for reaching treatment goals, durability of the procedure, and postoperative complications are discussed with the patients and their families preoperatively to create realistic expectations and guide the decision-making process. Using this method, Miner et al. noted that only 47% of the patients for which palliative surgery was discussed underwent a procedure. This suggests that with appropriate insight into the proposed value and purpose of surgery, patients may choose alternative strategies to achieve personal goals. The early involvement of a goal-directed palliative care teams allow patients to explore options for optimizing symptom control and quality of life which potentially provide a survival advantage [32, 33]. The ENABLE III trial in 2015 showed that patients with early palliative care involvement (within 30–60 days of diagnosis) were shown to have an improved 1-year survival (63%) compared to the delayed group (48%, 3 months after diagnosis) [34]. As an example, in our population, 31.2% of surgeries were performed with palliative intent. Most patients who underwent palliative surgery were discharged home (93%), and many went on to have systemic therapy (25%) postoperatively. As reception of adjuvant therapy is associated with improved overall survival, factors that facilitate adjuvant therapy would be considered a prognostic determinant. Moreover, patients whom underwent goal-directed palliative surgical therapy had lower 30-day mortality compared to nonsurgical patients (10.7 vs. 22.2%, p<0.05). As such, goal-directed palliative care is an important prognostic determinant with prior literature suggesting that early involvement may provide maximum benefit to patients.
Facilitating systemic therapy
Timely initiation of adjuvant chemotherapy is a positive predictor of outcomes in patients with cancer [30]. In patients with emergency cancer presentation, the majority are potential candidates for systemic therapy [7,8,9,10]. The need for urgent surgical intervention may lead to an interruption of systemic therapy or delay in initiation, both of which can impact overall and cancer specific survival [35]. Treatment delay can also lead to negative effect on local control rates, functional outcomes, complications from disease progression, and quality of life [35]. In our population, the ability to receive adjuvant systemic therapy was associated with a reduced risk of mortality (HR 0.40, 95% CI 0.25–0.65, p<0.001)). A study by Heyler et al. in 2007 examined the role of palliative surgery in patients with malignant bowel obstruction and showed a survival benefit in patients undergoing surgery as a bridge to systemic therapy compared to surgery alone or no surgery (10.3 versus 0.3 versus 2.7 months, respectively) [36]. This highlights the importance of reception systemic therapy as a prognostic determinant and encourages surgeons to explore options for facilitating this through individual care plans.
This study is limited by its retrospective nature for which comprehensive patient demographics and treatment course were difficult to capture. For example, post-operative complications were not available for collection, which represent an important aspect on evaluating surgical management. The heterogeneity of the study population presents a challenge when applying generalized conclusions to an individual patients’ specific cancer diagnosis. Larger sample sizes and prospective follow-up are necessary to further clarify the influence of MDT dynamics and oncology-specific pathways on clinical and patient-reported outcomes.
Conclusion
Surgical decision making for patients presenting malignancy-related emergencies is challenging. There are many factors that determine patient outcomes including social, physical, and prognostic determinants. By identifying prognostic determinants of health such as disease staging, early MDT involvement, and palliative care assessment, patient care pathways can be initiated that have the potential to improve outcomes. The development of an emergency surgical oncology care pathway may help to establish appropriate treatment goals, minimize waiting times, and improve patient experience.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ED:
-
Emergency department
- ACS:
-
Acute care surgery
- IR:
-
Interventional radiology
- MDT:
-
Multidisciplinary team
- OS:
-
Overall survival
- LGI:
-
Lower gastrointestinal
- UGI:
-
Upper gastrointestinal
- CT:
-
Computed tomography
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- IPU:
-
Integrated practice unit
References
Canadian Cancer Society (2020) Canadian Cancer Society’s Advisory Committee on Cancer Statistics: cancer statistics at a glance. Can Cancer Soc Available from: https://www.cancer.ca/en/cancer-information/cancer-101/cancer-statistics-at-a-glance/?region=on.
Suhail A, Crocker CE, Das B, et al. Initial presentation of lung cancer in the emergency department: a descriptive analysis. CMAJ Open. 2019;7(1):E117–23.
Rivera DR, Gallicchio L, Brown J, et al. Trends in adult cancer-related emergency department utilization: an analysis of data from the nationwide emergency department sample. JAMA Oncol. 2017;3(10):e172450.
Ng HJ, Yule M, Twoon M, et al. Current outcomes of emergency large bowel surgery. Ann R Coll Surg Engl. 2015;97(2):151–6.
Sjo OH, Larsen S, Lunde OC, et al. Short term outcome after emergency and elective surgery for colon cancer. Colorectal Dis. 2009;11(7):733–9.
Solsky I, Friedmann P, Muscarella P, et al. Poor outcomes of gastric cancer surgery after admission through the emergency department. Ann Surg Oncol. 2017;24(5):1180–7.
Lee CHA, Kong JCH, Heriot AG, et al. Short-term outcome of emergency colorectal cancer surgery: results from Bi-National Colorectal Cancer Audit. Int J Colorectal Dis. 2019;34(1):63–9.
Amri R, Bordeianou LG, Sylla P, et al. Association of radial margin positivity with colon cancer. JAMA Surg. 2015;150(9):890–8.
Bosscher MR, van Leeuwen BL, Hoekstra HJ. Mortality in emergency surgical oncology. Ann Surg Oncol. 2015;22(5):1577–84.
Hoshino N, Endo H, Hida K, et al. Emergency surgery for gastrointestinal cancer: a nationwide study in Japan based on the National Clinical Database. Ann Gastroenterol Surg. 2020;4(5):549–61.
Bayar B, Yilmaz KB, Akinci M, et al. Acil ve elektif şartlarda ameliyat edilen kolorektal kanserli hastalarin tedavi sonuçlarinin deʇerlendirilmesi. Turkish J Surg. 2016;32(11):17.
Amri R, Bordeianou LG, Sylla P, et al. Colon cancer surgery following emergency presentation: effects on admission and stage-adjusted outcomes. Am J Surg. 2015;209(2):246–53.
Hwang H. Emergency presentation of colorectal cancer at a regional hospital: an alarming trend? BCMJ. 2012;54(2):83–7.
Heidarnia M, Mohseny M, Amanpour F, et al. Application of cox and parametric survival models to assessment social determinants of health affecting the three-year survival of breast cancer patients. APJCP. 2017;18(4):11–21.
Paesmans M. Prognostic and predictive factors for lung cancer. BRE. 2012;9(2):112–21.
Ruffini E, Weder W, Filosso PL, et al. Thymic tumors. IASLC Thoracic. Oncology. 2018;2(56):569–89.
Gospodarowicz M, O’Sullivan B. Prognostic factors in cancer. Semin Surg Oncol. 2003;21(1):13–8.
Grossmann I, Klaase JM, Avenarius JK, et al. The strengths and limitations of routine staging before treatment with abdominal CT in colorectal cancer. BMC Cancer. 2011;11:433.
Bosscher MR, Van Leeuwen BL, Hoekstra HJ. Surgical emergencies in oncology. Cancer Treat Rev. 2014;40(8):1028–36.
McPhail S, Elliss-Brookes L, Shelton J, et al. Emergency presentation of cancer and short-term mortality. Br J Cancer. 2013;109(8):2027–34.
Comber H, Sharp L, de Camargo CM, et al. Causes and outcomes of emergency presentation of rectal cancer: Emergency presentation of rectal cancer. Int J Cancer. 2016;139(5):1031–9.
Zhou Y, Abel GA, Hamilton W, et al. Diagnosis of cancer as an emergency: a critical review of current evidence. Nat Rev Clin Oncol. 2017;14(1):45–56.
Liao CM, Kung PT, Wang YH, Tsai WC. Effects of multidisciplinary team on emergency care for colorectal cancer patients: a nationwide-matched cohort study. Medicine (Baltimore). 2017;96(23):e7092. https://doi.org/10.1097/MD.0000000000007092 PMID: 28591052; PMCID: PMC5466230.
Cunningham RS. Advanced practice nursing outcomes: a review of selected empirical literature. Oncology Nursing Forum. 2004;31(2):219–32.
Miner TJ, Cohen J, Charpentier K, et al. The palliative triangle: improved patient selection and outcomes associated with palliative operations. Arch Surg. 2011;146(5):517–22.
Putera I. Redefining health: implication for value-based healthcare reform. Cureus. 2017;9(3):e1067.
Porter ME, Lee TH. The strategy that will fix health care. Harv Bus. 2013;91(10):50–70.
Hui D, Hannon BL, Zimmermann C, Bruera E. Improving patient and caregiver outcomes in oncology: team-based, timely, and targeted palliative care: timely palliative care for cancer patients. CA Cancer J Clin. 2018;68(5):356–76.
Kim BJ, Aloia TA. Cost-effectiveness of palliative surgery versus nonsurgical procedures in gastrointestinal cancer patients. J Surg Oncol. 2016;114(3):316–22.
Kim IY, Kim BR, Kim YW. Factors affecting use and delay (≥8 weeks) of adjuvant chemotherapy after colorectal cancer surgery and the impact of chemotherapy-use and delay on oncologic outcomes. PLoS ONE. 2015;10(9):e0138720.
Schweitzer N, Weber T, Kirstein MM, et al. The effect of adjuvant chemotherapy in patients with intrahepatic cholangiocarcinoma: a matched pair analysis. J Cancer Res Clin Oncol. 2017;143(7):1347–55.
Goodyear SJ, Leung E, Menon A, et al. The effects of population-based faecal occult blood test screening upon emergency colorectal cancer admissions in Coventry and north Warwickshire. Gut. 2008;57(2):218–22.
Ellis PM. The importance of multidisciplinary team management of patients with non-small-cell lung cancer. Curr Oncol. 2012;19(1):S7–S15.
Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1438–45.
Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systemic review and meta-analysis. BMJ. 2020;371:m4087.
Helyer LK, Law CHL, Butler M, Last LD, Smith AJ, Wright FC. Surgery as a bridge to palliative chemotherapy in patients with malignant bowel obstruction from colorectal cancer. Ann Surg Oncol. 2007;14(4):1264–71.
Acknowledgements
None.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
Kadhim Taqi: Corresponding author, design, analysis, and interpretation of the data. Diane Kim: Design of the work and data collection. Lily Yip: Data collection. Charlotte Laane: Creation of new software used in the work. Zeeshan Rana: Creation of new software used in the work. Morad Hameed: Drafted the work and substantively revised it. Trevor Hamilton: Data interpretation, drafted the work and substantively revised it. Heather Stuart: Substantial contributions to the conception and design of the work, drafted the work and substantively revised it. The authors read and approved the final submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received approval from the University of British Columbia research ethics board.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Taqi, K., Kim, D., Yip, L. et al. Emergency surgical consultation for cancer patients: identifying the prognostic determinants of health. World J Surg Onc 20, 232 (2022). https://doi.org/10.1186/s12957-022-02694-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02694-z