Abstract
Background and objectives
To evaluate the individual and combined associations of cytokeratin 19 (CK19) and microvascular invasion (MVI) with prognosis of patients with hepatocellular carcinoma (HCC).
Methods
Clinicopathological data on 352 patients with HCC who underwent radical resection at our hospital between January 2013 and December 2015 were retrospectively analyzed. Patients were divided into four groups: CK19(−)/MVI(−), CK19(−)/MVI(+), CK19(+)/MVI(−), and CK19(+)/MVI(+).
Results
Of the 352 HCC patients, 154 (43.8%) were CK19(−)/MVI(−); 116 (33.0%), CK19(−)/MVI(+); 31 (8.8%), CK19(+)/MVI(−); and 51 (14.5%), CK19(+)/MVI(+). The disease-free survival of CK19(−)/MVI(−) patients was significantly higher than that of CK19(−)/MVI(+) patients and CK19(+)/MVI(+) patients. Similar results were observed for overall survival. CK19(+)/MVI(+) patients showed significantly lower overall survival than the other three groups.
Conclusions
CK19 expression and MVI predict poor prognosis after radical resection of HCC, and the two markers jointly contribute to poor OS. Combining CK19 and MVI may predict post-resection prognosis better than using either factor on its own.
Similar content being viewed by others
Hepatocellular carcinoma (HCC) is one of the most common malignancies. Surgical resection is one of the most effective treatments for HCC [1]. However, the high rate of postoperative recurrence seriously affects prognosis [2]. For intermediate and advanced-stage HCC, the 5-year recurrence rate is up to 74% after hepatic resection [3]. The 5-year overall survival rate after hepatic resection is only 30% for those with intermediate disease and only 18% for those with advanced disease [4]. Official guidelines offer few adjuvant therapies to prevent HCC recurrence [5]. Key measures to improve prognosis may be stratification of HCC according to risk factors and effective intervention for patients with those factors. Therefore, it is important to study the risk factors that affect prognosis.
Microvascular invasion (MVI) is defined as the presence of cancer cell nests in the vascular cavity lined by endothelial cells under a microscope, including veins, arteries, and lymphatic vessels [6]. MVI is a marker of aggressive tumor behavior and is considered to be an important risk factor affecting the prognosis of patients with HCC [2, 6,7,8,9,10,11,12]. MVI significantly reduces disease-free survival (DFS) and overall survival (OS) of HCC patients, even after liver resection or transplantation [6].
Biliary cell markers including cytokeratin 19 (CK19) are also associated with poor prognosis after liver resection in HCC [13,14,15,16,17,18,19]. Similarly, CK19 predicts poor prognosis in HCC patients after liver transplantation [20, 21]. The association between CK19 and poor prognosis in HCC may reflect that the protein’s expression is closely related to lymphatic metastasis, which can lead to poor prognosis [18, 22], and to increased risk of portal vein invasion and bile duct cancer thrombosis [17, 23]. The OS of patients with CK19(+) HCC is similar to that of patients with combined HCC and cholangiocarcinoma (cHCC-CC) and higher than that of patients with intrahepatic cholangiocarcinoma (ICC), but lower than that of patients with CK19(−) HCC [24].
Given the association of both CK19 and MVI with poor prognosis in HCC, and given that combinations of biomarkers often predict outcomes better than single biomarkers on their own, we examined whether the two factors may help identify HCC patients at high risk of recurrence or death after hepatic radical resection.
Patients and methods
Patient information
This retrospective study involved patients with HCC who underwent radical resection at Guangxi Medical University Cancer Hospital between January 2013 and December 2015. The study protocol was approved by the Ethics Commission of Guangxi Medical University Cancer Hospital, which waived the requirement for informed consent because at the time of their surgery, all patients had consented for their anonymized medical records to be analyzed and published for research purposes.
To be included in the study, patients (1) had to be diagnosed with HCC that was confirmed by postoperative pathology; (2) had to be in Barcelona Clinic Liver Cancer (BCLC) stage 0, A or B; (3) had to have undergone radical resection; and (4) had to have complete follow-up information available. Radical resection of liver cancer was defined as surgery conducted without gross tumor thrombus in large vessels such as the hepatic or portal vein; without invasion of nearby organs, hilar lymph nodes or distant metastasis; with a resection margin lying more than 1 cm from the tumor boundary, or a resection margin ≤ 1 cm but without residual tumor cells at the margin; and with no detection of tumors by ultrasonography, computed tomography or magnetic resonance imaging at 1–2 months after surgery.
Patients were excluded if they had received other antitumor treatments before surgery, had a history of other tumors, or did not have complete pathology data available.
Clinicopathological data
The following clinicopathological data were collected: age, sex, Barcelona Clinic liver cancer stage (BCLC stage), tumor diameter, tumor number, tumor envelope, ascites, hepatitis B surface antigen (HBsAg), hepatitis B virus DNA (HBV-DNA), antibodies against hepatitis C virus (Anti-HCV), white blood cell (WBC) count, hemoglobin (HB) level, neutrophil percentage (N%), lymphocyte percentage (L%), blood platelet (PLT) count, alpha fetoprotein (AFP) level, prothrombin time (PT), international normalized ratio (INR), fasting plasma glucose (FPG), total bilirubin (TBiL), albumin (Alb), Prealbumin (PA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptadase (GGT), alkaline phosphatase (ALP), CK19 expression status, and MVI presence or absence.
CK19 status was determined by immunohistochemistry. CK19 positivity was defined as membranous and/or cytoplasmic expression in ≥ 5% of tumor cells with moderate or strong intensity. MVI status was determined by histopathology. MVI was defined as the presence of cancer cell nests in the vascular cavity lined by endothelial cells under a microscope. CK19 and MVI findings were retrieved retrospectively from pathological reports.
Enrolled patients were divided into four groups based on expression of CK19 and on the presence of MVI: CK19 (−)/MVI (−), CK19 (−)/MVI (+), CK19 (+)/MVI (−), and CK19 (+)/MVI (+).
Follow-up
All patients were followed up until December 2019 or death. Tumor recurrence was diagnosed based on at least two imaging methods [25]. DFS was defined as the interval between the date of surgery and the date of diagnosis of tumor recurrence. OS was defined as the interval between the date of surgery and the date of death.
Statistical analysis
Statistical analysis was performed using SPSS 23.0 (IBM, Chicago, IL, USA). Differences in categorical variables were assessed for significance using the chi-squared test. Differences in continuous variables were assessed using the t test, ANOVA, Mann-Whitney U test or Kruskal-Wallis H test, as appropriate, after determining whether data were normally distributed using the Shapiro-Wilk test and Q-Q plots.
DFS and OS were calculated using the Kaplan-Meier method, and differences in survival rate were assessed for significance using the log-rank test. Univariable analysis was conducted to identify factors significantly associated with DFS and OS, and variables that emerged as significant were entered in multivariable Cox proportional hazard modeling with forward stepwise selection. Differences associated with P < 0.05 were considered significant.
Results
Clinicopathological features of the study population
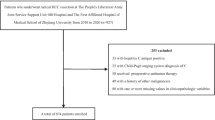
From 2013 to 2015, 830 patients with HCC underwent surgery at our hospital, of whom 352 were finally included in the analysis (Fig. 1). The median follow-up period was 51 months, during which 54 (15.3%) patients were lost to follow-up and 95 (27.0%) died. The average age was 49.2 years, and 83.5% of patients were male. CK19 expression was detected in 23.3% (82/352) patients; MVI was detected in 47.4% (167/352). The distribution of patients across the four groups was as follows: CK19(−)/MVI(−), 154 of 352 (43.8%); CK19(−)/MVI(+), 116 (33.0%); CK19(+)/MVI(−), 31 (8.8%); and CK19(+)/MVI(+), 51 (14.5%). The clinicopathological parameters of each group are described in Table 1.
Survival analysis
In univariable analyses, CK19 expression, presence of MVI, BCLC stage B, HBsAg positive, HBV-DNA ≥ 5 × 102 IU/ml, large tumor diameter, tumor number ≥ 2, AFP ≥400 ng/ml, high N%, low L% , low Alb, low PA, high GGT, and high ALP were significantly associated with worse DFS after radical resection. In addition, CK19 expression, presence of MVI, HBV-DNA ≥ 5 × 102 IU/ml, larger tumor diameter, incomplete envelope, presence of ascites, low PA, high GGT, and high ALP were significantly associated with worse OS (Tables 2 and 3).
In multivariable analysis, CK19 expression, HBsAg positive and larger tumor diameter, but not presence of MVI, were independent predictors of DFS (Table 2). Presence of MVI, HBV-DNA ≥ 5 × 102 IU/ml, larger tumor diameter, incomplete envelope, high GGT and high ALP, but not CK19 expression, were independent predictors of OS (Table 3).
On its own, CK19 expression was associated with significantly lower DFS (Fig. 2a) and OS (Fig. 2b) after radical resection. The same was observed for MVI on its own (Fig. 3a, b).
The combination of the two markers also showed a significant association with worse survival. DFS rate was significantly lower for CK19(+)/MVI(+) patients than for CK19(−)/MVI(−) patients, and it was significantly lower for CK19(−)/MVI(+) patients than for CK19(−)/MVI(−) patients (Fig. 4a). No other pairs of the four groups differed significantly in DFS rate. Similarly, OS rate was significantly lower for CK19(+)/MVI(+) patients than for the other three groups, while it was significantly higher for CK19(−)/MVI(−) patients than for CK19(−)/MVI(+) or CK19(+)/MVI(+) patients (Fig. 4b).
Discussion
Here, we provide evidence that combining CK19 expression and MVI, each of which on its own predicts poor prognosis in HCC patients, may better predict the survival of such patients after potentially curative hepatic resection. DFS was significantly worse for CK19(+)/MVI(+) patients than for CK19(−)/MVI(−) patients, and OS was significantly worse for CK19(+)/MVI(+) patients than for patients who were negative for one or both of these markers. Our results are consistent with other studies showing that combinations of biomarkers often predict prognosis better than the individual biomarkers on their own [16, 18]. Our findings may help personalize the management of HCC patients, improving their long-term outcomes.
CK19, a marker of biliary/progenitor cells, is expressed in 10-20% of patients with HCC [13, 14, 19, 26], and the prevalence in our cohort was 23%. Our CK19(+) patients were younger and had higher levels of AFP and more MVI than CK19(−) patients. CK19(+) HCC seems to be more aggressive than CK19(−) disease and to involve higher risk of relapse and worse postoperative prognosis, which we observed in the present cohort. This is consistent with previous studies [27, 28].
CK19 expression may be associated with worse prognosis because tumor cells expressing that protein show stem cell characteristics of self-renewal [21, 29, 30]. In HBV-related HCC, cadherin 17 (CDH17) is significantly correlated with CK19 in primary tumor tissue. Epidermal growth factor can induce the expression of both CK19 and CDH17, and CDH17 in turn can enhance the expression of CK19 in HCC. Thus, expression of CDH17 may be associated with the early recurrence and poor prognosis of CK19(+) HCC [18]. One study of 237 cases of HCC found that CK19 was significantly associated with expression of EMT-related proteins, leading the investigators to propose that CK19 up-regulates EMT-related genes to make the cancer more invasive [19]. Other studies have suggested that the invasiveness of CK19(+) HCC may be related to expression of genes related to invasion and metastasis, to genes characteristic of biliary or hepatic progenitor cells and to microRNA 200 family members [13].
MVI is a mark of aggressive biological behavior and is associated with worse DFS and OS after liver resection or transplantation [6]. Patients with recurrent liver cancer also obtained similar results [31]. Some scholars even believe that the impact of MVI on prognosis is the same as that of gross vascular invasion confined to a segmental/sectional branch [32]. Our MVI(+) patients had larger tumors, lower prevalence of an intact tumor envelope, higher APF levels and worse DFS and OS than MVI(−) patients, consistent with previous results [7, 33]. Studies had shown that elderly patients with HCC were more prone to vascular invasion, which was not consistent with our findings [34]. Gross vascular invasion is usually a consequence of MVI progression. The poor prognosis of HCC with gross vascular invasion has been clarified, but it has a relatively large impact on the prognosis, which is not conducive to the accurate classification of the prognosis [35]. Therefore, we advocate combining CK19 and MVI to analyze the prognosis.
In fact, CK19(+) HCC seems to be associated with MVI. In our study, the prevalence of MVI was significantly higher among CK19(+) patients than CK19(−) patients (62.2% vs 43%). In a previous study, 73.5% of CK19(+) HCC patients had MVI, significantly more than the 56.8% of CK19(−) HCC patients with MVI [19]. In another study, MVI was more frequent among HCC patients expressing CK19, both in the surgical specimen cohort (100.0% vs 52.0%) and needle biopsy specimen cohort (66.7% vs 21.7%) [13]. Univariable analysis found that CK19 and MVI were significantly associated with worse DFS and OS. However, in multivariable analysis, MVI did not independently predict DFS, while CK19 did not independently predict OS. Thus, using CK19 or MVI on their own to predict prognosis has limitations, arguing for using the combination of the two.
Using the combination of CK19 and MVI, we found that OS was significantly lower for CK19(+)/MVI(+) patients than for CK19(+)/MVI(−) and CK19(−)/MVI(+) patients, suggesting an additive effect. In contrast, we did not find evidence that CK19 and MVI exert an additive effect on DFS, since the DFS rate of CK19(+)/MVI(+) patients did not differ significantly from those of CK19(+)/MVI(−) or CK19(−)/MVI(+) patients. This may reflect that CK19(+)/MVI(+) HCC patients progress faster after tumor recurrence, leading to shorter survival. Therefore, research on recurrent HCC is key to improving OS of patients.
We also found that the deleterious effects of CK19 on prognosis did not fully manifest unless tumor cells had invaded microvessels. Once they invade, tumor cells expressing CK19 may behave as stem cells and migrate efficiently, eventually leading to a decline in survival. This may explain why the prognosis of HCC patients with CK19(+) and MVI(+) is worse than the prognosis of the other three groups.
The 5-year OS rate in our cohort was 72.5%, even higher than the 62.9% reported in another study that included patients from 2000 to 2017 [36]. This may be due to recent improvements in comprehensive treatment, as well as to selection bias that favored higher survival rates. Therefore, our results need to be verified in a larger, more diverse sample from multiple centers. In addition, data on CK19 and MVI in our study was provided by post-resectional histopathology, which had certain limitations in preoperative risk stratification assessment. However, there are abundant and reliable methods for preoperative prediction of CK19 and MVI, which can make up for this limitation to a certain extent [37,38,39,40].
Pathological features can help predict the prognosis of liver cancer. This study may have higher predictive power if we add more pathological information such as fibrolamellar HCC [41]. At the same time, how to improve the accuracy of CK19 and the detection rate of MVI in pathology is worth exploring [42].
In conclusion, our study suggests that CK19 expression and presence of MVI predict poor prognosis after radical resection of HCC, and the two markers jointly contribute to poor OS. Thus, combining CK19 and MVI may predict post-resection prognosis better than either factor on its own.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fan ST, Lo CM, Poon RTP, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253(4):745–58.
Lang H, Sotiropoulos GC, Brokalaki EI, et al. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg. 2007;205(1):27–36.
Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–40.
Zhong JH, Ke Y, Wang YY, Li LQ. Liver resection for patients with hepatocellular carcinoma and macrovascular invasion, multiple tumours, or portal hypertension. Gut. 2015;64(3):520–1.
European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–39.
Han J, Li ZL, Xing H, et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford). 2019;21(8):962–71.
Wang CC, Iyer SG, Low JK, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2009;16(7):1832–42.
Yang P, Qiu J, Li J, et al. Nomograms for pre- and postoperative prediction of long-term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg. 2016;263(4):778–86.
Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108–13.
Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49(3):344–54.
Ye JZ, Chen JZ, Li ZH, et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol. 2017;23(41):7415–24.
Govaere O, Komuta M, Berkers J, Spee B, Janssen C. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63(4):674–85.
Fatourou E, Koskinas J, Karandrea D, et al. Keratin 19 protein expression is an independent predictor of survival in human hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27(9):1094–102.
Jin Y, Liang ZY, Zhou WX, Zhou L. Combination with CK19 might increase the prognostic power of Hep Par 1 in hepatocellular carcinoma after curative resection. J Invest Surg. 2018;31(5):412–9.
Yuan RH, Jeng YM, Hu RH, et al. Role of p53 and β-catenin mutations in conjunction with CK19 expression on early tumor recurrence and prognosis of hepatocellular carcinoma. J Gastrointest Surg. 2011;15(2):321–9.
Yoneda N, Sato Y, Kitao A, et al. Epidermal growth factor induces cytokeratin 19 expression accompanied by increased growth abilities in human hepatocellular carcinoma. Lab Invest. 2011;91(2):262–72.
Lee CW, Lin SE, Tsai HI, et al. Cadherin 17 is related to recurrence and poor prognosis of cytokeratin 19-positive hepatocellular carcinoma. Oncol Lett. 2018;15(1):559–67.
Kim H, Choi GH, Na DC, Ahn EY, Kim GI. Human hepatocellular carcinomas with “stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54(5):1707–17.
Lee SH, Lee JS, Na GH, You YK, Kim DG. Immunohistochemical markers for hepatocellular carcinoma prognosis after liver resection and liver transplantation. Clin Transplant. 2017;31(1):10.1111/ctr.12852. https://doi.org/10.1111/ctr.12852.
Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49(2):138–51.
Xiang ZL, Zeng ZC, Tang ZY, Fan J, Sun HC, Tan YS. Expression of cytokeratin 19 and matrix metalloproteinase 2 predicts lymph node metastasis in hepatocellular carcinoma. Mol Biol Rep. 2011;38(5):3531–9.
Pang YB, Zhong JH, Luo XL, et al. Clinicopathological characteristics and liver stem cell marker expression in hepatocellular carcinoma involving bile duct tumor thrombi. Tumour Biol. 2016;37(5):5879–84.
Lee JI, Lee JW, Kim JM, Kim JK, Chung HJ, Kim YS. Prognosis of hepatocellular carcinoma expressing cytokeratin 19: comparison with other liver cancers. World J Gastroenterol. 2012;18(34):4751–7.
Tobe T, Kameda H, Okudaira M, et al. Primary liver cancer in Japan: Springer Japan; 1992. p. 111–60.
Cai X, Feng L, Liu H, et al. Cytokeratin19 positive hepatocellular carcinoma is associated with increased peritumoral ductular reaction. Ann Hepatol. 2016;15(3):386–93.
Seino S, Tsuchiya A, Watanabe Y, et al. Clinical outcome of hepatocellular carcinoma can be predicted by the expression of hepatic progenitor cell markers and serum tumour markers. Oncotarget. 2018;9(31):21844–60.
Lu XY, Xi T, Lau WY, et al. Hepatocellular carcinoma expressing cholangiocyte phenotype is a novel subtype with highly aggressive behavior. Ann Surg Oncol. 2011;18(8):2210–7.
Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44.
Andersen JB, Loi R, Perra A, et al. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology. 2010;51(4):1401–9.
Huang ZY, Liang BY, Xiong M, et al. Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: a single-center experience in China. Ann Surg Oncol. 2012;19(8):2515–25.
Park YK, Song SK, Kim BW, Park SK, Chung CW, Wang HJ. Prognostic significance of microvascular invasion in tumor stage for hepatocellular carcinoma. World J Surg Oncol. 2017;15(1):225.
Lin S, Ye F, Rong W, et al. Nomogram to assist in surgical plan for hepatocellular carcinoma: a prediction model for microvascular invasion. J Gastrointest Surg. 2019;23(12):2372–82.
Shimada S, Kamiyama T, Orimo T, et al. Prognoses, outcomes, and clinicopathological characteristics of very elderly patients with hepatocellular carcinoma who underwent hepatectomy. World J Surg Oncol. 2020;18(1):122.
Bae B, Song SK, Choi E, Chung CW, Park Y. Secondarily estimated cure fraction and five-year recurrence-free conditional survival probabilities among patients undergoing surgical resection for hepatocellular carcinoma presenting with minor gross vascular invasion. World J Surg Oncol. 2021;19(1):222.
Tsilimigras DI, Moris D, Hyer JM, et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br J Surg. 2020;107(7):854–64.
Choi SY, Kim SH, Park CK, et al. Imaging features of gadoxetic acid-enhanced and diffusion-weighted MR imaging for identifying cytokeratin 19-positive hepatocellular carcinoma: a retrospective observational study. Radiology. 2018;286(3):897–908.
Wang W, Gu D, Wei J, et al. A radiomics-based biomarker for cytokeratin 19 status of hepatocellular carcinoma with gadoxetic acid-enhanced MRI. Eur Radiol. 2020;30(5):3004–14.
Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus–related hepatocellular carcinoma within the milan criteria. JAMA Surg. 2016;151(4):356–63.
Lee CW, Yu MC, Lin G, et al. Serum metabolites may be useful markers to assess vascular invasion and identify normal alpha-fetoprotein in hepatocellular carcinoma undergoing liver resection: a pilot study. World J Surg Oncol. 2020;18(1):121.
Lemekhova A, Hornuss D, Polychronidis G, et al. Clinical features and surgical outcomes of fibrolamellar hepatocellular carcinoma: retrospective analysis of a single-center experience. World J Surg Oncol. 2020;18(1):93.
Hu HT, Wang Z, Kuang M, Wang W. Need for normalization: the non-standard reference standard for microvascular invasion diagnosis in hepatocellular carcinoma. World J Surg Oncol. 2018;16(1):50.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81960450), National Major Special Science and Technology Project (2017ZX10203207), High-level innovation team and outstanding scholar program in Guangxi Colleges and Universities, “139” projects for training of high-level medical science talents from Guangxi, The Key Research and Development Project of Guangxi (AA18221001, AB18050020), The Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education/Guangxi, Independent Research Project (GKE2017-ZZ0, GKE2018-KF02, GKE2019-ZZ07), Development and application of medical and health appropriate technology in Guangxi (S2019039), the China Postdoctoral Science Foundation (2019M663876XB) and the Specific Research Project of Guangxi for Research Bases and Talents (GuiKe AD22035057) and Bagui Scholars Programs of Guangxi Zhuang Autonomous Region (2019AQ20).
Author information
Authors and Affiliations
Contributions
Shang-Dong Qin collected most of the clinicopathological data, and was a major contributor in writing the manuscript. Jie Zhang collected some clinicopathological data, followed up most of patients and participated in the revision of the manuscript. Ya-Peng Qi followed up some patients, made statistical analysis of the data and participated in the revision of the manuscript. Bang-De Xiang and Jian-Hong Zhong instructed research design and thesis writing. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Guangxi Medical University Cancer Hospital, China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qin, SD., Zhang, J., Qi, YP. et al. Individual and joint influence of cytokeratin 19 and microvascular invasion on the prognosis of patients with hepatocellular carcinoma after hepatectomy. World J Surg Onc 20, 209 (2022). https://doi.org/10.1186/s12957-022-02632-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02632-z