Abstract
Background
With the development of laparoscopic techniques and the broad clinical application of various anastomotic types, anal-preserving low anterior rectal resection and ultra-low anterior rectal resection have been popularized. Some patients with rectal cancer have retained their anus and improved their quality of life. Nevertheless, the incidence of postoperative anastomotic stenosis remains high, and anastomotic occlusion is even rarer.
Case presentation
We report a case of anastomotic occlusion in a patient with rectal cancer, which occurred after undergoing laparoscopic low anterior rectal resection + prophylactic terminal ileal fistulation at our department. Under endoscopy, we used a small guidewire to break through the occluded anastomosis, thereby finding the lacuna. After endoscopic balloon dilation, digital anal dilatation, and continuous dilator-assisted dilation, the desired efficacy was achieved, ultimately recovering ileal stoma. Postoperative follow-up condition was generally acceptable, without symptoms like abdominal pain, bloating, or difficulty in defecation.
Conclusion
Numerous factors cause postoperative anastomotic stenosis in patients with rectal cancer. Complete occlusion of anastomosis occurs relatively rare in clinical practice, and is challenging to treat. This case was our first attempt to remove the anastomotic occlusion successfully, which avoided re-operation or pain from the permanent fistula.
Similar content being viewed by others
Background
With the application and popularization of laparoscopic techniques and anastomosis, laparoscopic surgery has become the preferred treatment for rectal cancer. It has the advantages of a clear surgical field, better convenience than open surgery, low invasiveness and fast recovery. Anastomotic fistula and stenosis are major complications following colorectal surgery [1, 2]. The incidence of anastomotic stenosis after colorectal cancer surgery is 3–30% [3]. Despite the rarity, complete occlusion of anastomosis has been reported in some populations [4]. Herein, we report a case of anastomotic occlusion after laparoscopic low anterior rectal resection + prophylactic terminal ileal fistulation, which was diagnosed and treated at our department. After rectal cancer surgery, the causes and treatments of anastomotic stenosis are reviewed with the previous literature reports.
Case presentation
A 53-year-old female patient was hospitalized at our department on August 21, 2020 because of the “rectal mass found 7 days ago.” Digital anal examination findings (chest-knee position): a hard mass of approximately 3 × 3 cm in size was palpable at the 7–9 o’clock orientation 4 cm apart from the anus, with no tenderness and moderate mobility. No blood stain was detected when exiting the finger cot, and the tumor markers were normal. Abdominal CT and pelvic MRI findings: space-occupying lesions in the lower rectum. Preoperative diagnosis: “moderately differentiated adenocarcinoma of the lower rectum(T3N0M0)”. On August 31, 2020, the patient received “laparoscopic low anterior rectal resection + prophylactic terminal ileal fistulation" under general anesthesia. After the surgery, the anastomosis was unobstructed based on digital detection, about 2.8 cm in diameter and 3 cm away from the anus, with circular anastomotic nails palpable. Postoperative pathological diagnosis: moderately differentiated adenocarcinoma of the lower rectum. Good postoperative recovery was achieved without developing complications such as anastomotic leakage, stenosis, or bleeding. The patient refused chemotherapy due to personal reasons. She was told to receive recovery of small intestinal stoma 3 months later, during which regular follow-up visits were required.
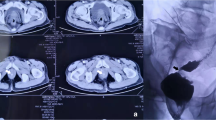
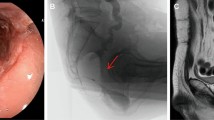
After hospital discharge, the patient did not return for re-examination. On December 1, 2020, she visited our department and requested a small intestinal stoma recovery. Digital anal examination revealed a blind cavity at the rectal end, with undetectable anastomosis and palpable anastomotic nails. Colonoscopy and abdominal CT showed occluded anastomosis (Fig. 1A, B). We attempted to puncture the occluded anastomosis with a small guide wire under the endoscope. A small gap was observed after passing the guidewire (Fig. 2A). Furthermore, balloon dilation was performed with a guidewire (Fig. 2B), and the anastomosis was dilated to 0.3 cm in size (Fig. 2C). Later, endoscopic balloon dilation was performed multiple times, and the anastomosis was dilated to 0.8 cm (Fig. 2D). Whenever balloon dilation was performed, the patient would experience severe and unbearable pain in the anastomosis, and pain symptoms were not relieved after pain killer injection. Thus, the endoscopic dilation was terminated, and rectal imaging was performed (Fig. 3). Later, the right index fingertip was used to dilate the anastomosis, and the patient could tolerate pain. The dilation was performed 2–3 times per day for 2 consecutive weeks. The stoma was dilated to about 1 cm, which was far from satisfying the requirements of stoma recovery and defecation. Next, we used different models of anal dilators (diameters 10–25 mm) (Fig. 4A, B) for anal dilation. The patient presented with pain symptoms, which though could be tolerated. Initially, a 10-mm-diameter dilator was used to dilate the anus for 2–3 h once every 2–3 days, which lasted for 2 weeks. Next, the dilator was gradually replaced with larger diameter ones. The size of the anal dilator could be gradually enlarged according to the diameter of anastomosis. Three months later, a 25-mm-diameter dilator could easily enter the anastomosis, yielding satisfactory results. Upon enteroscopy reexamination, the anastomotic diameter was 25 mm (Fig. 5). The recovery of a small intestinal stoma was completed in this patient on April 2, 2021. We have followed this patient for 1 year after the surgery. During this period, the patient’s general condition was fine, without symptoms like abdominal pain, bloating, or difficulty in defecation, and she did not require a stool softener or any laxative medication. We will continue to follow up the patient for a long-time period.
A A small anastomotic space was visible after puncturing the occluded anastomosis with a thin guide wire under endoscopy. B Using a guidewire, anastomosis was dilated with a balloon under endoscopy. C After initial dilation, the diameter of the anastomosis was approximately 0.3 cm. D After multiple times of endoscopic balloon dilation, the anastomosis was dilated to 0.8 cm in size
Discussion and conclusions
Anastomotic stenosis (AS) is one of the late complications following rectal cancer surgery. Very few patients develop anastomotic occlusion. Symptoms of AS usually include difficulty in defecation, bloating, and anal pain [5]. Causes of AS: (1) Surgeon’s experience: factors like the excessively small size of anastomotic, rectal division by multiple staplers, thick anastomotic intestine and high anastomotic tension can cause contraction of internal and external rectal sphincters, resulting in excessive proliferation of anastomotic fibrous tissues to form stenosis. (2) Anastomotic leakage and infection: they directly lead to the anastomotic scar healing and result in AS. (3) Anastomotic tissue ischemia: tissue ischemia is considered a possible risk factor for AS [6]. (4) Distance between lower tumor edge and anal edge: at low tumor positions, there is excessive tension and spasm of the anal sphincter, as well as edema and thickening of the intestinal wall, leading to AS. (5) Protective stoma: the anastomosis is in a disused state, lacking physical stimulation from feces, leading to AS. (6) Neoadjuvant chemo-radiotherapy: it has always been identified as a risk factor for anastomotic leakage and maybe eventually leads to AS. Using indocyanine green may reduce the rate of anastomotic leakage in colorectal surgery, even in particularly risky anastomoses [7]. (7) Recurrence of an anastomotic tumor. (8) Patient factors: general condition, gender, age, smoking, etc. For patients who smoke frequently, their blood vessels tend to contract due to arteriosclerosis, causing reduced tissue blood flow and cell hypoxia. The anastomotic healing fails because of ischemia and hypoxia, leading to anastomotic fistula or AS [8]. (9) Other factors: poor intestinal preparation, electric thermal injury, and the use of immunosuppressors. Anastomotic occlusion occurred in this patient is extremely rare. Reviewing the entire treatment process, we concluded that anastomotic ischemia caused by the low position of the rectal tumor and rectal division by 4 staplers was the primary cause of anastomotic occlusion. Another cause was the disused state of the anastomosis.
There are many treatments strategies for AS, including mechanical dilation, endoscopic therapy and transabdominal surgery [9,10,11]. These treatments are a gradual process rather than isolated from each other. (1) Mechanical dilation is the foremost method, the first-choice treatment for lower-position AS. Sloane et al. [9] reported that low rectal AS could be relieved or even removed by mechanical dilation. Finger anal dilatation is the simplest and most common method, although long-term persistence is required for alleviation. (2) Endoscopic therapy has been gradually applied to clinics in recent years, mainly balloon dilatation, self-expanding metal stenting and anastomotic radial incision. The advantages of balloon dilatation are minimal invasiveness and repeated applicability. It is suitable for patients with unreachable stenosis by digital rectal examination or with poor efficacy by finger dilation. If a single dilation is not satisfactorily effective, repeated colonoscopy operations are required, which do not have the effect of continuous dilation or relief [12]. For patients with failed balloon dilation, stenting can be considered. Stents can continuously support the stenosis section compared to balloon dilation, yielding a higher success rate. Nevertheless, it also has the risks and defects of high cost, stent displacement, and partial perforation [13, 14]. There have been reports on treating colorectal anastomotic stenosis with biodegradable stents. Further observation is required to confirm its long-term efficacy despite good preliminary efficacy [15]. For refractory AS with failed multiple balloon dilations or stent implantation, endoscopic electrocautery dilation is found effective, despite stenosis recurrence risk. (3) For patients with severe AS or non-surgical failure, surgical treatment is recommended, such as proximal neostomy, resection plus anastomosis and transanal radial incision. However, given their reoperation nature, there are probable risks of postoperative anastomotic leakage, bleeding, and restenosis, so careful choice is required.
The occurrence of AS is associated with numerous factors, where prevention should be emphasized. Once discovered, early treatment should be given. Complete occlusion is a rare condition that is difficult to manage. Improper management may exacerbate the patients' condition and economic burden. From simple to difficult and from non-invasive to invasive, the therapeutic strategy should depend on individual conditions [16,17,18,19]. Where necessary, multiple treatments can be used in combination. When conditions permit, novel therapeutic protocols can also be attempted upon patients’ consent. Our ultimate goal is to remove the occlusion and attain good efficacy.
Availability of data and materials
All data during the study are included within the article.
Abbreviations
- AS:
-
Anastomotic stenosis
References
Tuson JR, Everett WG. A retrospective study of colostomies, leaks and strictures after colorectal anastomosis. Int J Colorectal Dis. 1990;5:44–8. https://doi.org/10.1007/BF00496150.
Bokey EL, Chapuis PH, Fung C, Hughes WJ, Koorey SG, Brewer D, et al. Postoperative morbidity and mortality following resection of the colon and rectum for cancer. Dis Colon Rectum. 1995;38(480-86):486–7. https://doi.org/10.1007/BF02148847.
Mckee R, Pricolo VE. Stapled revision of complete colorectal anastomotic obstruction. Am J Surg. 2008;195:526–7. https://doi.org/10.1016/j.amjsurg.2007.02.030.
Lefevre JH, Bretagnol F, Maggiori L, Ferron M, Alves A, Panis Y. Redo surgery for failed colorectal or coloanal anastomosis: a valuable surgical challenge. Surgery. 2011;149:65–71. https://doi.org/10.1016/j.surg.2010.03.017.
Lee SY, Kim CH, Kim YJ, Kim HR. Anastomotic stricture after ultralow anterior resection or intersphincteric resection for very low-lying rectal cancer. Surg Endosc. 2018;32:660–6. https://doi.org/10.1007/s00464-017-5718-3.
Polese L, Vecchiato M, Frigo AC, Sarzo G, Cadrobbi R, Rizzato R, et al. Risk factors for colorectal anastomotic stenoses and their impact on quality of life: what are the lessons to learn? Colorectal Dis. 2012;14:e124–8. https://doi.org/10.1111/j.1463-1318.2011.02819.x.
Brescia A, Muttillo EM, Angelicone I, Madaffari I, Maggi F, Sperduti I, et al. The role of indocyanine green in laparoscopic low anterior resections for rectal cancer previously treated with chemo-radiotherapy: a single-center retrospective analysis. Anticancer Res. 2022;42:211–6. https://doi.org/10.21873/anticanres.15475.
Kim MJ, Shin R, Oh HK, Park JW, Jeong SY, Park JG. The impact of heavy smoking on anastomotic leakage and stricture after low anterior resection in rectal cancer patients. World J Surg. 2011;35:2806–10. https://doi.org/10.1007/s00268-011-1286-1.
Sloane JA, Zahid A, Young CJ. Rhomboid-shaped advancement flap anoplasty to treat anal stenosis. Tech Coloproctol. 2017;21:159–61. https://doi.org/10.1007/s10151-016-1560-1.
Kawaguti FS, Martins BC, Nahas CS, Marques CF, Ribeiro U, Nahas SC, et al. Endoscopic radial incision and cutting procedure for a colorectal anastomotic stricture. Gastrointest Endosc. 2015;82:408–9. https://doi.org/10.1016/j.gie.2015.03.1975.
Osera S, Ikematsu H, Odagaki T, Oono Y, Yano T, Kobayashi A, et al. Efficacy and safety of endoscopic radial incision and cutting for benign severe anastomotic stricture after surgery for lower rectal cancer (with video). Gastrointest Endosc. 2015;81:770–3. https://doi.org/10.1016/j.gie.2014.11.011.
Acar T, Aslan F, Acar N, Kamer E, Unsal B, Haciyanli M. Role of endoscopic interventions and electroincision in benign anastomotic strictures following colorectal surgery. Turk J Gastroenterol. 2019;30:673–9. https://doi.org/10.5152/tjg.2019.18673.
Lamazza A, Fiori E, Schillaci A, Sterpetti AV, Lezoche E. Treatment of anastomotic stenosis and leakage after colorectal resection for cancer with self-expandable metal stents. Am J Surg. 2014;208:465–9. https://doi.org/10.1016/j.amjsurg.2013.09.032.
Lamazza A, Fiori E, Sterpetti AV, Schillaci A, Scoglio D, Lezoche E. Self-expandable metal stents in the treatment of benign anastomotic stricture after rectal resection for cancer. Colorectal Dis. 2014;16:O150–3. https://doi.org/10.1111/codi.12488.
Janik V, Horak L, Hnanicek J, Malek J, Laasch HU. Biodegradable polydioxanone stents: a new option for therapy-resistant anastomotic strictures of the colon. Eur Radiol. 2011;21:1956–61. https://doi.org/10.1007/s00330-011-2131-5.
Mukai M, Kishima K, Iizuka S, Fukumitsu H, Fukasawa M, Yazawa N, et al. Endoscopic hook knife cutting before balloon dilatation of a severe anastomotic stricture after rectal cancer resection. Endoscopy. 2009;41(Suppl 2):E193–4. https://doi.org/10.1055/s-0029-1214776.
Curcio G, Spada M, di Francesco F, Tarantino I, Barresi L, Burgio G, et al. Completely obstructed colorectal anastomosis: a new non-electrosurgical endoscopic approach before balloon dilatation. World J Gastroenterol. 2010;16:4751–4. https://doi.org/10.3748/wjg.v16.i37.4751.
De Lusong MA, Shah JN, Soetikno R, Binmoeller KF. Treatment of a completely obstructed colonic anastomotic stricture by using a prototype forward-array echoendoscope and facilitated by SpyGlass (with videos). Gastrointest Endosc. 2008;68:988–92. https://doi.org/10.1016/j.gie.2008.05.028.
Kaushik N, Rubin J, Mcgrath K. Treatment of benign complete colonic anastomotic obstruction by using an endoscopic rendezvous technique. Gastrointest Endosc. 2006;63:727–30. https://doi.org/10.1016/j.gie.2005.10.022.
Acknowledgements
We thank the Department of Hepatobiliary and Pancreatic Surgery of The Affiliated Hospital of Yunnan University for technical assistance.
Funding
None.
Author information
Authors and Affiliations
Contributions
CHH, HZ, and LPY designed the study and drafted the manuscript. JZ, QC, and LJ performed the surgery. LM, ZW, ZRW, and YHW provide academic advice. ZYY edited the paper. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient prior to submitting the case report and approved by the Affiliated Hospital of Yunnan University Ethics Committee. Additional informed consent was obtained from the patient for whom identifying information is included in this article.
Consent for publication
The patient has given her consent for the case report to be published. The written informed consent to publish this information was obtained from this patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, C., Zhang, H., Yang, L. et al. Anastomotic occlusion after laparoscopic low anterior rectal resection: a rare case study and literature review. World J Surg Onc 20, 145 (2022). https://doi.org/10.1186/s12957-022-02610-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02610-5