Abstract
Background
The operative results of different approaches for the laparoscopic intersphincteric resection (LAISR) of low rectal cancer vary, and the patient characteristics associated with the best outcomes for each procedure have not been reported. We compared the efficacy of different approaches for LAISR of low rectal cancer and discussed the surgical indications for each approach.
Methods
We retrospectively reviewed data from 235 patients with low rectal cancer treated via LAISR from October 2010 to September 2016. Patients underwent either the transabdominal approach for ISR (TAISR, n = 142), the transabdominal perineal approach for ISR (TPAISR, n = 57), or the transanal pull-through approach for ISR (PAISR, n = 36).
Results
The PAISR and TAISR groups exhibited shorter operation times and less intraoperative blood loss than the TPAISR group. The anastomotic distance was shorter in the PAISR and TPAISR groups than in the TAISR group. No differences in the ability to perform radical resection, overall complications, postoperative recovery, Wexner score recorded 12 months after ostomy closure, 3-year disease-free survival, local recurrence-free survival, distant metastasis-free survival, or overall survival (OS) were observed among the three groups.
Conclusions
TAISR, TPAISR, and PAISR have unique advantages and do not differ in terms of operation safety, patient outcomes, or anal function. TPAISR requires a longer time to complete and is associated with more bleeding and a slower recovery of anal function. PAISR should be considered when TAISR cannot ensure a negative distal margin and the tumor and BMI are relatively small; otherwise, TPAISR is required.
Similar content being viewed by others
Background
Abdominoperineal excision of the rectum (APE) was once the dominant procedure for treating low rectal cancer (5 cm from the inferior margin of the anus); however, APE resulted in extensive trauma and permanent colostomy, thereby markedly affecting the receiving patient’s quality of life [1]. In recent years, the exploration of sphincter-preserving surgery for low rectal cancer is full of promise. Even if the tumor invaded the ipsilateral puborectalis muscle, there has been reported to successful sphincter preservation after hemilevator excision [2]. However, many studies seem to be more interested in transsphincterectomy. Intersphincteric resection (ISR) is proposed as an extreme anal sphincter procedure based on total mesorectal excision (TME). By removing some or all of the internal sphincter, a sufficient distal margin is obtained [3, 4]. Multiple studies have confirmed satisfactory oncological outcomes and quality of life after ISR [5, 6].
Many reports have discussed laparoscopic ISR [5, 7,8,9,10]. Most of these reports have involved dissection of the intersphincteric space based on the laparoscopic TME technique, which is followed by transection of the rectum through the perineum at a safe distance from the distal edge of the tumor under direct vision, and handsewn repair [9, 11, 12]. This operative approach is complicated and technically difficult, particularly because separation of the anterior rectal wall can easily damage the vagina in women (the urethra and prostate may be damaged in men), and many postoperative complications and severe anal functional damage have been noted [3, 4]. Several investigators have postulated that laparoscopic transabdominal partial ISR is feasible and would also achieve the goal of obtaining a negative distal resection margin [13]. However, because determining the inferior margin of the tumor is limited by the pelvic cavity and use of laparoscopic instruments, laparoscopic transabdominal partial ISR is much more likely to lead to a positive distal margin [14, 15]. Other authors have used the laparoscopic ISR technique to separate the intersphincteric space to the distal position of the pelvic cavity, followed by transanal evisceration of the rectum, to clearly reveal the inferior edge of the tumor. Then, the rectum was transected under direct vision, and instrumented anastomosis was completed using the double anastomosis technique [16]. However, pull-through is difficult in patients with mesenteric fat hypertrophy and relatively large tumors [16]. The premise of all three of these laparoscopic ISR approaches is to separate the intersphincteric space based on laparoscope-assisted TME in order to ensure negative circumferential and distal margins, which is also the crucial and most difficult step of laparoscopic ISR.
However, the relative advantages and disadvantages of these three surgical procedures and the most suitable populations for each have not yet been reported. This article reviews the results of three laparoscopic ISR approaches for low rectal cancer and provides an in-depth discussion with the aim of offering individualized sphincter-preserving solutions for patients with low rectal cancer, while ensuring surgical safety and reducing surgical difficulty and complications.
Patients and methods
Patients
Clinical data from 235 consecutive patients with low rectal cancer treated using laparoscopic-assisted ISR radical surgery from October 2010 to September 2016 were retrospectively collected.
Inclusion criteria were as follows: (1) histologically proven rectal adenocarcinoma; (2) tumors located 3–5 cm above the anal edge; (3) mobile tumors detected in a digital rectal examination; (4) tumors that did not involve the external anal sphincter and peripheral structures, as shown by endorectal ultrasound (ERUS), and magnetic resonance imaging (MRI); (5) no evidence of distant metastasis in the preoperative evaluation; (6) patients younger than 70 years of age; and (7) patients with normal anal sphincter function (Wexner fecal continence score < 4).
The exclusion criteria were as follows: (1) patients with preoperative synchronous cancers; (2) lateral lymph node metastases or invasion of the external sphincter, levator ani, or other adjacent organs; and (3) patients who underwent emergency surgery, palliative resection, or lateral lymph node dissection.
The evaluation included a physical examination, colonoscopy with biopsy, endorectal ultrasonography, MRI, and chest CT. Patients with cT3 or node-positive disease received a long course of preoperative chemoradiotherapy (CRT) (5-fluorouracil-based) with a radiation protocol of 45 Gy/25 fractions followed by a 5.4-Gy boost, for a total of 50.4 Gy. However, their right to refuse preoperative chemoradiotherapy was respected. The operation was performed 8–12 weeks after the radiation treatment was complete. All patients underwent ostomy closure at 3 months after the radical operation. The Wexner incontinence score was used to evaluate anal function from the third month onward after ostomy closure. Procedures were performed by the same surgical team. Our team has experience with more than 2000 cases of laparoscopic colorectal surgery. The surgical team selected TAISR, TPAISR, or PAISR based on the patient’s body conformation, pelvic condition, tumor size, tumor site, distance from the inferior margin of the tumor to the anal margin, and the experience of the surgeon. All patients provided written informed consent.
Surgical procedures
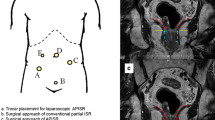
The intraoperative images of the three different LAISR approaches are shown in Fig. 1.
Intraoperative images of the three different LAISR approaches. a–c Laparoscopic transabdominal approach for intersphincteric resection (TAISR). a Inferior mesenteric artery was transected at the root. b Hiatal ligament was dissected and transected at the posterior side. c Tissue was separated along the puborectalis and internal sphincter to the deep and superficial parts of the external sphincter, and a coloanal anastomosis was performed. J: Puborectalis muscle k: Deep external sphincter muscle l: Superficial external sphincter muscle. m: Rectal stump. d–f Laparoscopic transabdominal perineal approach for intersphincteric resection (TPAISR). d Circumferential incision of the rectal mucosa into the intersphincteric space. e Prolapse of the rectum after complete rectal dissociation along the sphincter space. f Colonic anal canal or rectal anal canal anastomosis was performed through the perineum. g–i Laparoscopic transanal pull-through approach for intersphincteric resection (PAISR). g Pull-through of the distal rectum with two rubber tubes. h Transection of the distal intestine under direct vision. i Tumor specimen was intact, and the distal margin was clearly visible
Transabdominal approach for ISR (TAISR) (Fig. 1a–c)
Surgery included high ligation of the inferior mesenteric artery and full mobilization of the left colon and the splenic flexure. A standard laparoscopic TME procedure was completed to the levator ani plane. The rectum and its mesentery were separated to the levator ani plane, and the hiatal ligament was exposed with a standard laparoscopic TME procedure. The lower edge of the tumor was determined by digital examination of the anus. The “U” puborectalis muscle was exposed after the hiatal ligament was transected on the posterior side. The line between the puborectalis muscle and the internal anal sphincter was extended, and the tissue was separated to the deep and superficial components of the external sphincter until the level of the dentate line was reached. The anal examination again confirmed that the inferior margin was ≥ 2 cm. The cancer’s lower edge was transected with the stapling device, and the distal margin was observed visually. If unsatisfactory, a specimen of the distal resection margin was sent for frozen sectioning and pathological examination. If the resection margin was clean, anastomosis of the colon and anus was performed with a circular stapler.
Transabdominal perineal approach for ISR (TPAISR) (Fig 1d–f)
Abdominal surgery was performed by the transabdominal team. The perineal team exposed the anus, dilated the anal canal up to three horizontal finger widths, and exposed the anal canal with a Lone Star hook to reveal the inferior margin of the tumor. The rectum and anal canal were irrigated with povidone iodine to prevent scatter of cancer cells. Following closure of the anal orifice at least 1 cm below the tumor, the internal sphincter was circumferentially incised and the intersphincteric plane was dissected. The dissection was then carried cephalad to connect with the abdominal team. After the distal rectum was pulled out of the anus, the sigmoid colon was transected 15 cm above the superior tumor margin and specimen was removed. Next, a 5% iodophor solution was used to wash the surgical wound. Ultimately, coloanal anastomosis was performed via a handsewn procedure.
Transanal pull-through approach for ISR (PAISR) (Fig 1h–i)
Abdominal surgery was performed by the same transabdominal team. To excise the rectum, two rubber tubes were inserted through the anus. At 10 cm from the proximal end of the tumor, the two rubber tubes and the rectum were cut together using a linear stapler. Then, the anal side of the two rubber tubes was grasped by hand, and the rectal mucosa was gently pulled out in the anal direction via a laparoscopic operation. This procedure allowed the surgeon to easily pull the rectal stump through. Next, the intestinal cavity was rinsed several times with 0.5% iodophor and distilled water. The tumor was clearly detected, and then the intestine was transected 1–2 cm from distal margin of tumor with a CONTOUR cutter under direct vision. Finally, the rectal stump was returned to the pelvic cavity through the anus, and anastomosis of the intestine was performed using a tubular stapler.
Statistical analysis
Quantitative data are presented as mean and standard deviation or median and percentile (25th–75th). Qualitative data are presented as absolute numbers and percentages. Categorical data were compared using the Chi2 test or Fisher’s exact test. Continuous variable was compared using the Kruskal-Wallis test. Kaplan-Meier estimates were performed for survival analysis, and the difference in survival between groups was tested using the log-rank method. All statistical analyses were performed using SPSS 20.0 software. Differences were considered significant at P values < 0.05.
Results
Patient and tumor characteristics
A comparison of the characteristics of the 235 patients is provided in Table 1. In total, 142 patients underwent TAISR, 57 underwent TPAISR, and 36 underwent PAISR. Figure 2 shows the patient selection flow chart. Significant differences in gender, age, ASA classification, previous abdominal surgery, tumor size, pre-neoadjuvant therapy, tumor markers (CEA and CA199), neoadjuvant chemoradiation, preoperative T stage, or preoperative baseline Wexner scores were not observed among the three groups (P > 0.05). BMI was lower in the PAISR group than in the TPAISR group (21.8 kg/m2 vs. 23.2 kg/m2, P = 0.032). The distance from the inferior margin to the anal margin was smaller in both the PAISR group and the TPAISR group (3.8 cm and 4.0 cm, respectively) than in the TAISR group (5.0 cm) (P < 0.001). The proportion of patients with a luminal circumference of the tumor (LCIT) > 50% was significantly smaller in the PAISR group (27.8%) than in the TPAISR group (63.2%) (P = 0.001).
Comparison of intraoperative and postoperative complications
Preventive ileostomy was performed in all patients without conversion to laparotomy or death. The operation duration was shorter in the PAISR group (214 ± 40 min) and TAISR group (212 ± 51 min) than in the TPAISR group (263 ± 68 min) (P < 0.001) (Fig. 3a). The PAISR group (62.8 ± 33.5 ml) and TAISR group (62.6 ± 56.7 ml) experienced less bleeding than the TPAISR group (86.9 ± 65.3 ml) (P < 0.05) (Fig. 3b). The PAISR group (1.8 ± 0.5 cm) was equivalent to the TPAISR group in terms of the anastomotic distance (from the anastomosis to the anal verge) (1.7 ± 0.5 cm) (P = 0.360), although the anastomotic distance was greater in the TAISR group (2.6 ± 0.5 cm) (P < 0.001) (Fig. 3c).
Intraoperative and postoperative indicators. The operation time, intraoperative blood loss, anastomotic distance from the anal margin, length of the distal resection margin, postoperative exhaust time, and postoperative hospital stay were compared. a ***P < 0.001 (TPAISR group compared with the TAISR group); (TPAISR group compared with the PAISR group). b **P = 0.006 (TPAISR group compared with the TAISR group), *P = 0.045 (TPAISR group compared with the PAISR group). c ***P < 0.001 (TPAISR group compared with the PAISR group) and (TAISR group compared with the PAISR group)
Comparison of surgical safety and oncological outcomes
The overall complication rate after ISR was 25.1% (59 cases), including 6 cases (2.6%) of anastomotic leakage (all grade A or B leakage [17]), 6 cases of intestinal obstruction (2.6%), which were cured after non-surgical treatment. No differences in anastomotic leakage, intestinal obstruction, and other surgical related complications were observed among the three groups. The proportions of grade I complications in TPAISR, TAISR, and PAISR groups were 15.8%, 9.2%, and 8.3%, respectively. The rates of grade II complications were 14.0%, 8.5%, 2.8%. Only 1 case of grade III-a complications was observed in the TAISR group. There was no statistical difference in the incidence of grade I-III-a in the three groups. No grade III-b-IV complications occurred in the three groups. No significant differences in safety and radical outcomes were observed among the three groups (P < 0.05) (Table 2).
Evaluation of postoperative anal function
The distribution of anal function in the three groups is listed below (Fig. 4). Ninety-one percent of all patients after ISR underwent ostomy closure. In addition, 47 patients, 119 patients, and 30 patients in the TPAISR, TAISR, and PAISR groups were followed up respectively. In the 3rd month after ostomy closure, the Wexner score of the TAISR group (14.5 ± 5.6) was significantly lower than the TPAISR group (16.7 ± 3.9) (P = 0.008), but was not different from the PAISR group (16.2 ± 1.1) (P > 0.05). In the 6th month after ostomy closure, the Wexner score of the TAISR group (10.4 ± 4.7) was still significantly lower than the TPAISR group (12.2 ± 3.9) (P = 0.013), but similar to the PAISR group (10.7 ± 1.2) (P > 0.05). In the 12th month after ostomy closure, the Wexner scores of the three groups were 8.0 ± 4.0, 7.0 ± 4.1, and 7.2 ± 0.9, respectively. The anal function scores of the three groups were similar (P > 0.05). Seven patients (3.0%) (5 treated with TPAISR) were unable to tolerate poor anal function and underwent a permanent colostomy.
Comparison of intermediate-term oncological results
The median duration of follow-up was 32 months (range 10–77 months), and 96% (226 cases) of the patients underwent follow-up. Ten patients in the TAISR group died within 3 years after surgery: 6 died of distant metastasis, 3 died of unknown causes, and 1 died of local recurrence. One patient in the TPAISR group died due to distant metastasis, and no deaths occurred in the PAISR group. Among the TPAISR, TAISR, and PAISR groups, the 3-year disease-free survival (DFS) (79%, 83.4%, and 81.8%, respectively) (Fig. 5a), local recurrence-free survival (LRFS) (93.7%, 97.7%, and 90.0%, respectively) (Fig. 5b), distant metastasis-free survival (DMFS) (84.3%, 86.6%, and 90.9%, respectively) (Fig. 5c), and overall survival (OS) (97.1%, 89.4%, and 100%, respectively) (Fig. 5d) were not significantly different (P > 0.05).
Discussion
Laparoscopic ISR preserves the anus due to the technique’s ability to dissect the tissue to the rectum, a lower point than that allowed by traditional open ISR, and thus protects both urinary and sexual functions [11]. Despite controversy, many studies have confirmed the feasibility of this technique and have reported satisfactory oncological results as well as acceptable anal function [5, 12, 18]. Based on current evidence, most scholars prefer laparoscopic ISR combined with transperineal tumor resection and manual anastomosis [19, 20], which is called TPAISR. Several scholars have postulated that laparoscopic ISR combined with a double stapler can achieve TAISR without manual anastomosis, is simple to perform, and reduces complications [13]. Several scholars have reported that laparoscopic ISR combined with the pull-through approach removes the difficulty of judging the distal margin, resulting in anal function similar to that observed after low anterior resection (LAR) [16]. Choosing between these three approaches is a challenge for the surgeon.
A method for choosing the most appropriate LAISR surgical approach based on the individual patient characteristics has not been published, and the indications for different approaches for LAISR remain controversial. Fukunaga [21] considered the pull-through technique more suitable for early-stage tumors, although pull-through anal procedures are difficult in patients with lymph node metastasis. In the PAISR group, some of the tumors presented with more than 50% LCIT and T3 stage. This difference in characteristics may be related to the selection bias of this retrospective study and the exploration phase of the PAISR procedure. Furthermore, height from anastomosis level to anal verge was similar in both the PAISR and TPAISR groups, whereas the distance from the lower margin of the tumor to the anal verge in the PAISR group was significantly shorter than that in the TAISR group, but equivalent to that in the TPAISR group. Compared with TAISR, PAISR is considered to perform lower level anastomosis. Therefore, when the TAISR surgeon suspects that the distal margin is insufficient, PAISR may be considered instead. Kwok and Andreola et al. [22, 23] reported that only 4–10% of patients presented with cancer cells spread more than 1 cm to the distal rectum, and only 2% with distal rectal cancer spread 2 cm to the distal end. According to Bujko [24], a distal margin of < 1 cm or > 1 cm does not significantly affect the local recurrence and OS rates. In this study, the average length of the distal margin in the three groups was greater than 1 cm. Compared with the TAISR group, the distance from the anal margin of the tumor was shorter in the PAISR and TPAISR groups, although the distal margin length was similar to that in the TAISR group. PAISR may be safe and suitable for patients with lower rectal cancer, and has advantages in terms of duration of surgery and blood loss.
In the literature, the reported incidence of ISR postoperative complications was 25% on average, of which the incidence of anastomotic leakage was 9.1% and the incidence of anastomotic stenosis was 2.7% [25]. The total complication rate after ISR in this study was 25.1%, including 11.1% in the PAISR group, 29.8% in the TPAISR group, and 26.8% in the TAISR group. No significant differences were noted among the three groups. The anastomotic leakage rates in the three groups (1.8%, 2.8%, and 2.8%, respectively) were lower than some previously reported rates, but similar to the rates reported by Chi et al. [13]. One patient was readmitted for anastomotic leak in the TAISR group. The patient presented only pelvic abscess and could be treated satisfactorily by transperineal US-guided drain without needing a relaparoscopy postoperatively for peritonitis. Although no delayed anastomotic leakage was found in this study, it was reported by Masayoshi et al. [26] so vigilance is still needed during follow-up. In the present study, the anastomotic stenosis rate in all groups was 0.9%, which is lower than previously reported values. The reason may be to mobilize of splenic flexure, remove the irradiated sigmoid colon near the tumor to the greatest extent possible, and descending colon-rectum (anal canal) anastomosis was performed. Only two cases presented with membranous stenosis of the anastomosis, which was improved after regular anal dilation during adjuvant chemotherapy. The rates of surgical site infections (SSI) in this study was low, similar to the results reported by Zheng et al. [27]. In other complications, a significant difference in the incidence of urinary retention was not observed among the three groups, indicating that protection of pelvic floor nerves did not differ significantly among the three ISR approaches.
In the present study, significant differences in the distal resection margin (DRM)-positive rate (1.8%, 1.4%, and 2.8%, respectively, P = 0.758) and circumferential resection margin (CRM)-positive rate (3.5%, 1.4%, and 0%, respectively, P = 0.352) were not observed among the three groups. The overall DRM-positive rate was lower than that of previous reports (2.8–3.6%) [11, 18, 25], and the overall CRM-positive rate was similar to that of previous reports (2.3–6.2%) [19, 25, 28]. However, one patient in the PAISR group presented with a positive DRM. The male patient had a strong anal sphincter and a small pelvis, with a BMI of 24.4 (kg/m2) and LCIT > 50%. The intraoperative abdomen had been separated to an extreme extent, although the pull-through effect was not satisfactory. Therefore, patients with a narrow pelvis, high BMI, and larger tumor may not be suitable for PAISR, and the TPAISR approach should be employed to ensure a negative distal margin. In this study, postoperative pathology of samples from the TAISR group revealed two cases of poorly differentiated adenocarcinoma that possibly resulted from insufficient assessment of the biopsied specimen in the preoperative and postoperative pathological diagnoses.
The local recurrence rate of rectal cancer after ISR ranges from 2 to 13.3% [16, 29]. ISR does not increase the postoperative local recurrence rate of rectal cancer [30]. Previous studies [31,32,33] have reported a 3-year DFS rate of 67–82.6%, a 3-year OS rate of 81–92.8%, and a 3-year LRFS rate of 82.7–87%. In the present study, the median follow-up time was 32 months. No significant differences in 3-year DFS (79%, 83.4%, and 81.8%, respectively), 3-year LRFS (93.7%, 97.7%, and 90%, respectively), 3-year DMFS (84.3%, 86.6%, and 90.9%, respectively), and 3-year OS (97.1%, 89.4%, and 100%, respectively) were observed among the three groups. This study reported similar intermediate-term results for the three surgical approaches. A higher 3-year OS rate was recorded for the PAISR group, possibly because this group included fewer patients. The low DRM-positive rate and better long-term survival outcomes compared with previous reports reflected the importance of accumulated experience, a specialized team, careful patient selection for the operation, and application of neoadjuvant CRT.
Anal function was poor in the short term after ISR, but satisfactory in the long term. In this study, the Wexner score improved significantly after 12 months compared with the score recorded in the third month, which was consistent with the study by Ito [31]. Anal function was worse in the TPAISR and PAISR groups than in the TAISR group during the first 6 months after ostomy closure. A potential explanation is that the anastomotic distance was lower than the anal margin in the TPAISR and PAISR groups, less rectal mucosa remained. However, a significant difference in anal function was not observed among the three groups at the 12th month after ostomy closure, possibly due to more time to recovery of the anal sphincter caused by the stoma. Second, after levator ani training and biofeedback treatment, “low anterior resection syndrome” was restored to satisfactory results within 1 year [34]. However, some patients are still unable to tolerate anal incontinence, and undergo permanent colostomy [35]. During the follow-up period, seven patients (3.0%) (five of whom underwent TPAISR) underwent permanent colostomy. Thus, the anal function of patients in the TPAISR group recovers slowly in the early stage, and a small number of patients are not satisfied with long-term anal function. Therefore, TPAISR should be considered only when PAISR is unable to be completed or when TAISR is unable to guarantee a negative distant margin. Figure 6 shows the intraoperative decision flowchart. More careful intraoperative management, biofeedback therapy, and careful patient selection may help improve anal function outcomes [13].
Intraoperative decision flowchart. LAISR laparoscopic intersphincteric resection, TPAISR transabdominal perineal approach for ISR, PAISR transanal pull-through approach for ISR, TAISR transabdominal approach for ISR, APE abdominoperineal excision of the rectum, DRM distal resection margin, BMI body mass index
This investigation is a retrospective study, and selection bias is inevitable. The low CRMI rate cannot be ruled out caused by selection bias. The low rate of SSI may be related to the use of retrospective data, which requires further measurement with a standard SSI evaluation system. In addition, assessing anal function based solely on the Wexner score is another limitation of this retrospective study. Consequently, further follow-up and randomized controlled trials may lead to more scientific conclusions. In this study, a small number of tumors with a distance of 5 cm from the anal verge were included in the TAISR group; therefore, the results of this group may be similar to those of patients with median rectal cancer. Unfortunately, this study failed to evaluate sexual function, which will be reported in future investigations.
Conclusions
In conclusion, LAISR (TAISR, TPAISR, and PAISR) of the three different approaches are all optional surgical methods, each with its own advantages, and there is no significant difference in surgical safety, tumor outcome, and anal function. However, TPAISR requires a longer duration of surgery and results in heavy bleeding and slower recovery of anal function. When the TAISR approach is unable to ensure that the distal margin is negative and the tumor and BMI are small, surgeons should consider switching to PAISR; otherwise, they should switch to TPAISR. Based on the individual characteristics of the patient before surgery, a reasonable ISR surgery strategy can be developed, and the surgical approach can be adjusted immediately according to the intraoperative situation, which will maximize the benefit to the patient.
Availability of data and materials
Some or all data used during the study are available from the corresponding author by request.
Abbreviations
- IRB:
-
Institutional Review Board
- ISR:
-
Intersphincteric resection
- LAISR:
-
Laparoscopic intersphincteric resection
- TAISR:
-
Transabdominal Approach For ISR
- TPAISR:
-
Transabdominal Perineal Approach For ISR
- PAISR:
-
Transanal Pull-through Approach For ISR
- OS:
-
Overall survival
- BMI:
-
Body mass index
- APE:
-
Abdominoperineal excision
- TME:
-
Total mesorectal excision
- ERUS:
-
Endorectal ultrasound
- MRI:
-
Magnetic resonance imaging
- CRT:
-
Chemoradiotherapy
- LCIT:
-
Luminal circumference of the tumor
- DFS:
-
Disease-free survival
- LRFS:
-
Local recurrence-free survival
- DMFS:
-
Distant metastasis-free survival
- IQR:
-
Interquartile range
- SSI:
-
Surgical site infections
References
Marks GJ, Marks JH, Mohiuddin M, Brady L. Radical sphincter preservation surgery with coloanal anastomosis following high-dose external irradiation for the very low lying rectal cancer. Recent Results Cancer Res. 1998;146:161–74.
Mariolis-Sapsakos T, Psathas G, Nikolouzakis TK, et al. An anatomic anal sphincter-saving procedure for rectal cancers located at anorectal junction. World J Surg Oncol. 2019;17:131. https://doi.org/10.1186/s12957-019-1672-y.
Akagi Y, Kinugasa T, Shirouzu K. Intersphincteric resection for very low rectal cancer: a systematic review. Surg Today. 2013;43:838–47. https://doi.org/10.1007/s00595-012-0394-3.
Dimitriou N, Michail O, Moris D, Griniatsos J. Low rectal cancer: sphincter preserving techniques-selection of patients, techniques and outcomes. World J Gastrointest Oncol. 2015;7:55–70. https://doi.org/10.4251/wjgo.v7.i7.55.
Matsuhashi N, Takahashi T, Tanahashi T, et al. Safety and feasibility of laparoscopic intersphincteric resection for a lower rectal tumor. Oncol Lett. 2017;14:4142–50. https://doi.org/10.3892/ol.2017.6664.
Klose J, Tarantino I, Kulu Y, et al. Sphincter-preserving surgery for low rectal cancer: do we overshoot the mark? J Gastrointest Surg. 2017;21:885–91. https://doi.org/10.1007/s11605-016-3339-0.
Chen H, Ma B, Gao P, et al. Laparoscopic intersphincteric resection versus an open approach for low rectal cancer: a meta-analysis. World J Surg Oncol. 2017;15:229. https://doi.org/10.1186/s12957-017-1304-3.
Yu S, Deng J, Peng X, et al. Study of postoperative anorectal dynamics in ultra-low rectal cancer patients undergoing laparoscopic intersphincteric resection. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19:928–32.
Zhou H, Ruan C, Wang Z, Hu Z. Laparoscopic-assisted modified intersphincter resection for ultralow rectal cancer. Ann Surg Oncol. 2018;25:947–8. https://doi.org/10.1245/s10434-017-6280-x.
Zheng H, Li Z, Zheng S, Li J, Zhao E. A retrospective study on the feasibility of completely laparoscopic transabdominal approach partial intersphincteric resection. J Laparoendosc Adv Surg Tech A. 2021. https://doi.org/10.1089/lap.2021.0544.
Fujimoto Y, Akiyoshi T, Kuroyanagi H, et al. Safety and feasibility of laparoscopic intersphincteric resection for very low rectal cancer. J Gastrointest Surg. 2010;14:645–50. https://doi.org/10.1007/s11605-009-1150-x.
Kawada K, Hida K, Hasegawa S, Sakai Y. A comparison of the long-term anorectal function between laparoscopic intersphincteric resection and low anterior resection for low rectal cancer. Surg Today. 2018;48:921–7. https://doi.org/10.1007/s00595-018-1669-0.
Chi P, Huang SH, Lin HM, et al. Laparoscopic transabdominal approach partial intersphincteric resection for low rectal cancer: surgical feasibility and intermediate-term outcome. Ann Surg Oncol. 2015;22:944–51. https://doi.org/10.1245/s10434-014-4085-8.
Park SY, Choi GS, Park JS, et al. Robotic-assisted transabdominal intersphincteric resection: a technique involving a completely abdominal approach and coloanal anastomosis. Surg Laparosc Endosc Percutan Tech. 2013;23:e5–10. https://doi.org/10.1097/SLE.0b013e318275b27a.
Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP. Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc. 2013;27:48–55. https://doi.org/10.1007/s00464-012-2405-2.
Fu CG, Gao XH, Wang H, et al. Treatment for early ultralow rectal cancer: pull-through intersphincteric stapled transection and anastomosis (PISTA) versus low anterior resection. Tech Coloproctol. 2013;17:283–91. https://doi.org/10.1007/s10151-012-0919-1.
Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study Group of Rectal Cancer. Surgery. 2010;147:339–51. https://doi.org/10.1016/j.surg.2009.10.012.
Kuo LJ, Hung CS, Wang W, et al. Intersphincteric resection for very low rectal cancer: clinical outcomes of open versus laparoscopic approach and multidimensional analysis of the learning curve for laparoscopic surgery. J Surg Res. 2013;183:524–30. https://doi.org/10.1016/j.jss.2013.01.049.
Park JS, Choi GS, Jun SH, Hasegawa S, Sakai Y. Laparoscopic versus open intersphincteric resection and coloanal anastomosis for low rectal cancer: intermediate-term oncologic outcomes. Ann Surg. 2011;254:941–6. https://doi.org/10.1097/SLA.0b013e318236c448.
Zhang B, Zhao K, Liu Q, et al. Clinical and functional results of laparoscopic intersphincteric resection for ultralow rectal cancer: is there a distinction between the three types of hand-sewn Colo-anal anastomosis? Int J Color Dis. 2017;32:587–90. https://doi.org/10.1007/s00384-016-2724-1.
Fukunaga M, Kidokoro A, Iba T, et al. Laparoscopy-assisted low anterior resection with a prolapsing technique for low rectal cancer. Surg Today. 2005;35:598–602. https://doi.org/10.1007/s00595-004-2984-1.
Kwok SP, Lau WY, Leung KL, Liew CT, Li AK. Prospective analysis of the distal margin of clearance in anterior resection for rectal carcinoma. Br J Surg. 1996;83:969–72.
Andreola S, Leo E, Belli F, et al. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis Colon Rectum. 1997;40:25–9.
Bujko K, Rutkowski A, Chang GJ, et al. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Indian J Surg Oncol. 2012;3:139–46. https://doi.org/10.1007/s13193-012-0158-y.
Martin ST, Heneghan HM, Winter DC. Systematic review of outcomes after intersphincteric resection for low rectal cancer. Br J Surg. 2012;99:603–12. https://doi.org/10.1002/bjs.8677.
Iwamoto M, Kawada K, Hida K, Hasegawa S, Sakai Y. Delayed anastomotic leakage following laparoscopic intersphincteric resection for lower rectal cancer: report of four cases and literature review. World J Surg Oncol. 2017;15:143. https://doi.org/10.1186/s12957-017-1208-2.
Liu Z, Efetov S, Guan X, et al. A multicenter study evaluating natural orifice specimen extraction surgery for rectal cancer. J Surg Res. 2019;243:236–41. https://doi.org/10.1016/j.jss.2019.05.034.
Kim CH, Lee SY, Kim HR, Kim YJ. Factors associated with oncologic outcomes following abdominoperineal or intersphincteric resection in patients treated with preoperative chemoradiotherapy: a propensity score analysis. Medicine (Baltimore). 2015;94:e2060. https://doi.org/10.1097/MD.0000000000002060.
Tokoro T, Okuno K, Hida J, et al. Analysis of the clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. World J Surg Oncol. 2013;11:24. https://doi.org/10.1186/1477-7819-11-24.
Saito N, Sugito M, Ito M, et al. Oncologic outcome of intersphincteric resection for very low rectal cancer. World J Surg. 2009;33:1750–6. https://doi.org/10.1007/s00268-009-0079-2.
Ito M, Saito N, Sugito M, et al. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis Colon Rectum. 2009;52:64–70. https://doi.org/10.1007/DCR.0b013e31819739a0.
Lim SW, Huh JW, Kim YJ, Kim HR. Laparoscopic intersphincteric resection for low rectal cancer. World J Surg. 2011;35:2811–7. https://doi.org/10.1007/s00268-011-1277-2.
Abdel-Gawad W, Zaghloul A, Fakhr I, et al. Evaluation of the frequency and pattern of local recurrence following intersphincteric resection for ultra-low rectal cancer. J Egypt Natl Canc Inst. 2014;26:87–92. https://doi.org/10.1016/j.jnci.2014.02.001.
Weiser MR, Quah HM, Shia J, et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236–42. https://doi.org/10.1097/SLA.0b013e318195e17c.
Barisic G, Markovic V, Popovic M, et al. Function after intersphincteric resection for low rectal cancer and its influence on quality of life. Color Dis. 2011;13:638–43. https://doi.org/10.1111/j.1463-1318.2010.02244.x.
Acknowledgements
The authors thank Dr. Shoufeng Li and Dr. Weizhong of the Jiang Department of Colorectal Surgery, Affiliated Union Hospital of Fujian Medical University, for their advice on this article. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This study was supported by the Fujian Medical University Professor Academic Development Fund (JS11006).
Author information
Authors and Affiliations
Contributions
Conception and design: GG, WO, XL, and XW. Development of the methodology: WO, XW, and JZ. Acquisition of data: YY, WO, and YZ. Analysis and interpretation of data: XL and JZ. Writing, review and/or revision of the manuscript: WO, XW, GG, and XL. Study supervision: WO and XW. All authors have revised the manuscript and approved the final version for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of Fujian Medical University Union Hospital (2013051). We confirm that the study was conducted in accordance with the relevant guidelines/regulations, and all participants and/or their legal guardians provided informed consent.
Consent for publication
Informed consent for research purpose with patient data and images were obtained for all patients.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ou, W., Wu, X., Zhuang, J. et al. Clinical efficacy of different approaches for laparoscopic intersphincteric resection of low rectal cancer: a comparison study. World J Surg Onc 20, 43 (2022). https://doi.org/10.1186/s12957-022-02521-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02521-5