Abstract
Background
Neoadjuvant concurrent chemoradiotherapy (nCCRT) is one of the standard-of-care options for locally advanced esophageal squamous cell carcinoma (LA-ESqCC). The optimal interval between nCCRT and esophagectomy is unknown.
Methods
We constructed a propensity-score-matched [1:1 for long (8–12 weeks) vs short (4–7 weeks) intervals] cohort of LA-ESqCC patients who were diagnosed from 2011 to 2015 and treated with nCCRT via the Taiwan Cancer Registry and related databases. We compared the hazard ratios (HRs) of death using a robust variance estimator. We also evaluated alternative covariables, outcomes, and interval definitions.
Results
Our study population included 80 patients for each group; groups were balanced with respect to the observed covariables. There was no significant difference for the HR of death [1.22; 95% confidence interval 0.78–1.91, P = 0.39] when the long interval group was compared to the short interval group. There were also no significant differences when alternative covariables, outcomes, or interval definitions were evaluated.
Conclusions
In this population-based study in modern Asia, we found that for LA-ESqCC patients treated with nCCRT and esophagectomy, overall survival was similar for either long or short intervals between nCCRT and esophagectomy. Randomized controlled trials are needed to verify this finding.
Similar content being viewed by others
Background
Esophageal cancer is one of the common causes of cancer mortality worldwide [1]. In contrast to the Western world, where adenocarcinoma is the common histology, squamous cell carcinoma (SqCC) is the predominant histology in Asia [2]. For locally advanced esophageal SqCC (LA-ESqCC), neoadjuvant concurrent chemoradiotherapy (nCCRT) is one of the standard-of-care options [3,4,5,6].
However, the optimal interval between nCCRT and esophagectomy is debated in the literature [7]. In clinical practice, some interval is needed for patients to recover from the side effects of nCCRT, but delayed surgery might lead to tumor growth. In the experience of nCCRT for rectal cancer, a randomized controlled trial (RCT) reported that prolongation was associated with a higher pathological complete response (pCR) [8], a well-known good prognostic factor [9]. In contrast, another RCT reported that prolongation led to a similar pCR but a higher morbidity [10].
Regarding nCCRT for esophageal cancer, a systematic review of non-RCTs published in 2018 reported that a long interval (> 7–8 weeks, vs ≤ 7–8 weeks) was associated with higher pCR rates but worse overall survival (OS), both with statistical significance [7]. However, all Asian studies included in this study were based on patients treated almost a decade ago. In addition, the results of individual studies included in this systematic review were variable. Given the abovementioned geographic variation, controversy in this topic, and lack of RCTs, we aimed to compare the OS of LA-ESqCC treated with nCCRT and esophagectomy in modern Asia with either long or short intervals via a population-based propensity-score-matched analysis.
Methods
Data source
The Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare, database is a set of databases providing complete information regarding the Taiwan Cancer Registry (TCR) (data until 2015), the death registry (data until December 31, 2017), and reimbursement data from the National Health Insurance (NHI) (data until December 31, 2016) for the whole Taiwan population, and it is provided by the Bureau of National Health Insurance [11]. The quality of the TCR was reported in 2019 [12]. The NHI research database has also been used in many population-based studies. All of the HWDC data with personal information were deidentified.
Study population and design
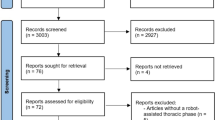
The study flow chart, as suggested in the STROBE statement [13], is depicted in Fig. 1. In this retrospective cohort study, we used the HWDC database to identify LA-ESqCC patients who were diagnosed from 2011 to 2015 and treated with nCCRT (radiotherapy 40–50.4 Gy at a dose per fraction of 1.8–2 Gy) and esophagectomy. nCCRT was defined as concurrent systemic and locoregional therapy with preoperative radiotherapy per the TCR record. Patients with other cancer(s) were excluded. The date of diagnosis was used as the index date. We determined the explanatory variable of interest [interval between nCCRT and esophagectomy (long interval (8–12 weeks) vs short interval (4–7 weeks))] based on the cancer registry data; the primary outcome of interest [OS] and other supplementary outcomes [pCR, 30 and 90 day mortality (since surgery), incidence of local regional recurrence (ILRR), and esophageal cancer mortality (IECM)] were extracted from the TCR or determined via linkage with the death registry. OS was calculated from the date of diagnosis to the date of death or December 31, 2017 (censoring date of the death registry). We also considered other covariables [see the next section] to adjust for potential nonrandomized treatment selection and then constructed a propensity-score (PS)-matched sample (1:1 paired matching) to evaluate the effectiveness of the interval between nCCRT and esophagectomy.
STROBE study flowchart and the number of individuals at each stage of the study. 1: We only included those treated (class 1–2) by any single institution to ensure data consistency. 2: Clinical stage II–III, by the 7th American Joint Committee on Cancer staging. 3: Without missing information in the TCR and death registry
Other explanatory covariables
We identified patient demographic factors [age, gender, residency region], patient characteristics [drinking, betel nut chewing, smoking, body mass index (BMI)], disease characteristics [tumor size, tumor differentiation, tumor location, clinical T-stage and N-stage], diagnosis method [use of positron emission tomography (PET)], and treatment characteristics [number of lymph nodes removed, radiotherapy (RT) delivery method, RT dose] as potential confounders based on our experiences in clinical practice and modified from our TCR/NHI related study [6]. These covariables were defined as follows. Patient residency was classified as northern Taiwan or elsewhere. The drinking, betel nut chewing, smoking, and use of PET variables were classified as yes or no. The number of lymph nodes was classified as < 15 or ≥ 15. Tumor size was dichotomized by tumors having a diameter ≤ 5 or > 5 cm. Tumor differentiation was classified as well/moderately differentiated or poorly/undifferentiated. Tumor location was classified as cervical or not. Clinical stage was classified as T1–T2 vs T3–T4 for T-stage and negative vs positive for N-stage. RT delivery was classified as image-guided radiotherapy (IGRT) or non-IGRT.
Statistical analyses
In the primary analysis (PA), we used the propensity score method as advocated in the literature to balance the measured potential confounders [14, 15]. We used a logistic regression model based on all covariables [see the above subsection “Other explanatory covariables”] to evaluate the probability with a long interval [vs a short interval]. Patients were matched on the logit of the propensity score using a caliper of 0.25 standard deviations of the logit of the propensity score via a greedy match algorithm as used in the literature [16]. The standardized difference (SDif) was used to assess the balance of the covariates [17, 18]. We used a robust variance estimator to compare the hazard ratio (HR) of death between PS-matched groups during the entire follow-up period [15] and evaluated the effect of potential unmeasured confounding factor(s) via the E value [19]. Binary outcomes (pCR) within the matched pairs were compared using McNemar’s test. We adopted the subdistribution HR via the clustered Fine–Gray model to evaluate ILRR and IECM [20]. Because of the vague [7–8 weeks] cutoff point used in the recent systematic review [7], we used alternative definitions [(1) 4–8 weeks vs 8–12 weeks; (2) 4–7 weeks vs 7–12 weeks] for the interval between nCCRT and esophagectomy to compare the OS as the first and second supplementary analyses (SA-1, SA-2) via separate PS matching. In the third SA (SA-3), we considered additional covariables [including site patient volume [21, 22] plus number of positive lymph node] and outcome [R0 resection], by constructing another PS-matched population for comparison. Although optimal interval was not specified in the recent treatment guideline [3], 4~6 weeks were commonly used in the RCT [23, 24]. Therefore, we performed the fourth SA (SA-4) by constructing additional PS-matched population to only compare 4~6 weeks vs 6~8 weeks. SAS v.9.4 software (SAS Institute, Cary, NC, USA) was used for statistical analyses.
Results
Study population
As shown in Fig. 1, we identified 160 eligible PS-matched patients treated with nCCRT and esophagectomy between 2011 and 2015 from 7908 esophageal cancer patients (65% locally advanced) as our primary study population and divided them into two groups [long interval group (n = 80) vs short interval group (n = 80)]. All covariates were balanced [SDif < 0.25] after matching (Table 1), though some were not balanced before matching.
Primary analysis
After a median follow-up of 30 months [range 4–81] (median 41 and range 24–81 for the survivors), 83 deaths were recorded (39 and 44 in the short and long interval groups, respectively). The Kaplan–Meier OS curve is shown in Fig. 2. The 1/2/3/4/5-year OS rates [in %] for the short and long interval groups were 89/83, 68/59, 56/51, 45/39, 45/35, respectively. There was no significant difference for HR [1.22; 95% confidence interval (95% CI) 0.78–1.91, P = 0.39] when the long interval group was compared to the short interval group. Our result may be due to an unmeasured confounding variable associated with both treatment selection and survival by a risk ratio of 1.56 [E value] fold each, but weaker confounding could not do so. The results of the HR for ILRR (HR = 1.44, P = 0.29) and IECM (HR = 1.18, P = 0.48) were similar. The pCR rates (55% vs 54% for the short vs long interval groups, P = 1), 30-day mortality (P = 0.06, exact numbers not reported per HWDC policy due to few events), and 90-day mortality (4% vs 9%, P = 0.19) were also not significantly different between the two groups.
Supplementary analysis (SA)
When alternative definitions of the interval between nCCRT and esophagectomy were used, we were still able to construct balanced study populations (Table 2). The results were not significantly different [SA-1: HR for death 1.08, P = 0.71; SA-2: HR for death 1.32, P = 0.10]. In SA-3, we constructed another balanced study population (Table 3) and found that the results were not significantly different [HR for death 1.22, P = 0.35]. There were also no statistically significant differences in the distribution of R0 resection [P = 0.07, exact proportion not reported per HWDC policy due to the small number of events]. In SA-4, we constructed additional balanced study population (Table 4) and found that the results were not significantly different [HR for death 1.01, P = 0.98].
Discussion
In our analysis of LA-ESqCC treated with nCCRT and esophagectomy in this population-based study from modern Asia, we found that OS was similar for long and short intervals between nCCRT and esophagectomy.
We searched the literature up to May 2019 by using the same strategy as used in the recent systematic review [7] to see if there were other modern studies and found two population-based studies from North America and another two single-institution studies from Asia [25,26,27,28]. Azab et al. utilized the American National Cancer Database (NCDB) to identify more than 5000 patients (81% adenocarcinoma) and found that SqCC groups had similar OS across interval lengths [25]. Franko and McAvoy used the same NCDB specifically for SqCC and found that OS was not affected by the interval length [26]. Furukawa et al. identified 134 patients from a Japanese hospital and reported that OS survival rates did not significantly differ between the two groups (≤ 8 or > 8 weeks) [27]. Roh et al. identified 348 Korean patients and found no significant difference in OS between the groups [P = 0.101] [28]. Our results were similar to the results of these four studies in that the OS between different interval length groups was similar.
However, there were inherent limitations in our analysis. As in all nonrandomized studies, our results were prone to potential unmeasured confounder(s), although we used PS matching to balance observed covariables. There was a risk of unmeasured confounders (such as surgical techniques or systemic therapy details), so we reported the E value, as suggested in the literature [19]. For example, a transthoracic approach has been reported to lead to a trend of favorable long-term outcomes [29] and taxane has been used in modern neoadjuvant trials with excellent results [23]. Besides, the importance of the anastomotic sites or the surgical fields was debated in the literatures [30]. However, these factors were not considered in our study due to the data not being available. Some potential pathological factors like extranodal extension, perineural invasion, or lymphovascular invasion were also not included due to the same data limitation. Therefore, phase III RCTs are needed to clarify the findings from our study and other studies. However, when we searched the clinical trial registry [https://clinicaltrials.gov/] in March 2019 using the keywords “esophagectomy | Interventional Studies | Esophagus Cancer | Phase 3”, we found no relevant studies. Therefore, we believe that our study provides useful information until higher-level data are available.
Conclusions
In this population-based study from modern Asia, we found that for LA-ESqCC patients treated with nCCRT and esophagectomy, OS was similar for long and short intervals between nCCRT and esophagectomy. Randomized controlled trials are needed to clarify this finding.
Availability of data and materials
The data that support the findings of this study are available from the Taiwan Cancer Registry, but restrictions apply to the availability of these data, which were used under license for the current study, and so they are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the Taiwan Cancer Registry.
Abbreviations
- 95% CI:
-
95% confidence interval
- BMI:
-
Body mass index
- HR:
-
Hazard ratio
- HWDC:
-
Health and Welfare Data Science Center
- IECM:
-
Incidence of esophageal cancer mortality
- IGRT:
-
Image-guided radiotherapy
- ILRR:
-
Incidence of local regional recurrence
- LA-ESqCC:
-
Locally advanced esophageal SqCC
- nCCRT:
-
Neoadjuvant concurrent chemoradiotherapy
- NHI:
-
National Health Insurance
- OS:
-
Overall survival
- PA:
-
Primary analysis
- pCR:
-
Pathological complete response
- PET:
-
Positron emission tomography
- PS:
-
Propensity score
- RCT:
-
Randomized controlled trial
- RT:
-
Radiotherapy
- SA:
-
Supplementary analyses
- SDif:
-
Standardized difference
- SqCC:
-
Squamous cell carcinoma
- TCR:
-
Taiwan Cancer Registry
References
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–509.
Chien CR, Lin CY, Chen CY. Re: Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2009;101:1428 author reply 1429.
National Comprehensive Cancer Network Guidelines for Esophageal and Esophagogastric Junction Cancers, version 1. 2019 [free registration required]. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed 30 Mar 2019.
Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan esophageal society. Esophagus. 2015;12:1–30.
Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D, ESMO guidelines committee. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50–7.
Chen CY, Li CC, Chien CR. Neoadjuvant vs definitive concurrent chemoradiotherapy in locally advanced esophageal squamous cell carcinoma patients. World J Surg Oncol. 2018;16:141.
Qin Q, Xu H, Liu J, Zhang C, Xu L, Di X, et al. Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A meta-analysis. Int J Surg. 2018;59:11–8.
Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396.
Chen WT, Ke TW, Li CC, Chien CR. Questionable role of adjuvant chemotherapy in rectal cancer patients who had reached pathological complete response after neoadjuvant concurrent chemoradiotherapy: no matter in the east or in the west. J Cancer Res Clin Oncol. 2014;140:1495–6.
Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. 2016;34:3773–80.
The Health and Welfare Data Science Center Database [in Chinese]. http://dep.mohw.gov.tw/DOS/np-2497-113.html. Accessed 12 Mar 2019.
Chiang CJ, Wang YW, Lee WC. Taiwan’s Nationwide Cancer registry system of 40 years: past, present, and future. J Formos Med Assoc. 2019;118:856–8.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9.
Jagsi R, Bekelman JE, Chen A, Chen RC, Hoffman K, Shih YC. Considerations for observational research using large data sets in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90:11–24.
Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–58.
Austin PC, Chiu M, Ko DT, et al. Propensity score matching for estimating treatment effects. In: Faries DE, Leon AC, Haro JM, Obenchain RL, editors. Analysis of observational health care data using SAS®. Cary: SAS Institute Inc.; 2010. p. 51–84.
Ali MS, Groenwold RH, Belitser SV, Pestman WR, Hoes AW, Roes KC, et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol. 2015;68:112–21.
Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701–20.
Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321:602–3.
Austin PC, Fine JP. Propensity-score matching with competing risks in survival analysis. Stat Med. 2019;38:751–77.
Gockel I, Ahlbrand CJ, Arras M, Schreiber EM, Lang H. Quality management and key performance indicators in oncologic esophageal surgery. Dig Dis Sci. 2015;60:3536–44.
Metzger R, Bollschweiler E, Vallböhmer D, Maish M, DeMeester TR, Hölscher AH. High volume centers for esophagectomy: what is the number needed to achieve low postoperative mortality? Dis Esophagus. 2004;17:310–4.
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8.
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36:2796–803.
Azab B, Amundson JR, Picado O, Ripat C, Macedo FI, Franceschi D, et al. Impact of chemoradiation-to-surgery interval on pathological complete response and short- and long-term overall survival in esophageal cancer patients. Ann Surg Oncol. 2019;26:861–8.
Franko J, McAvoy S. Timing of esophagectomy after neoadjuvant chemoradiation treatment in squamous cell carcinoma. Surgery. 2018;164:455–9.
Furukawa T, Hamai Y, Hihara J, Emi M, Yamakita I, Ibuki Y, et al. Impact of interval between neoadjuvant chemoradiation and surgery upon morbidity and survival of patients with squamous cell carcinoma of thoracic esophagus. Anticancer Res. 2018;38:5239–45.
Roh S, Iannettoni MD, Keech J, Arshava EV, Swatek A, Zimmerman MB, et al. Timing of esophagectomy after neoadjuvant chemoradiation therapy affects the incidence of anastomotic leaks. Korean J Thorac Cardiovasc Surg. 2019;52:1–8.
Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–9.
Cuesta MA, van der Peet DL, Gisbertz SS, Straatman J. Mediastinal lymphadenectomy for esophageal cancer: differences between two countries, Japan and the Netherlands. Ann Gastroenterol Surg. 2018;2:176–81.
Acknowledgements
The data analyzed in this study were provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. We are grateful to the Health Data Science Center at the China Medical University Hospital for providing administrative, technical, and funding support. Part of this manuscript has been reported in Dr. Kuo’s master’s thesis. The authors thank “American Journal Experts” for editorial assistance.
Funding
None.
Author information
Authors and Affiliations
Contributions
Y-HK, Y-WC, P-RC, and C-LF participated in the conceptualization and design of the study, interpretation of the data, and drafting of the manuscript. C-CL participated in the conceptualization and design of the study, analysis of the data, and drafting of the manuscript. C-RC participated in the conceptualization and design of the study, collection of the related studies, analysis and interpretation of the data, and drafting of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee, National Health Research Institutes [CMUH 104-REC-003].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kuo, YH., Chien, YW., Chen, PR. et al. Impact of the interval between neoadjuvant concurrent chemoradiotherapy and esophagectomy in the modern era: a population-based propensity-score-matched retrospective cohort study in Asia. World J Surg Onc 17, 222 (2019). https://doi.org/10.1186/s12957-019-1712-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-019-1712-7