Abstract
Background
Sclerosing mesenteritis is a non-neoplastic inflammatory disease that occurs in the bowel mesentery. Distinguishing sclerosing mesenteritis from neoplasms may be difficult because of the clinical and radiographic similarities between the two disease entities.
Case presentation
We report a case of sclerosing mesenteritis mimicking peritoneal metastases of colorectal carcinoma. A 73-year-old man with stage II descending colon adenocarcinoma with poor prognostic features was found to have developed left lower abdominal quadrant masses on computed tomography (CT) 9 months after undergoing radical surgery. These masses were diagnosed as peritoneal metastases because they grew in size and displayed fluorodeoxyglucose (FDG) uptake 3 months later; thus, a laparotomy was performed. The masses, which were localized in the jejunal mesentery, were excised completely via segmental jejunal resection. Histopathological analysis confirmed that the masses were sclerosing mesenteritis. The patient showed no signs of sclerosing mesenteritis or colorectal carcinoma recurrence during follow-up.
Conclusions
In patients suspected of having localized peritoneal metastasis from malignancies, any masses must be sampled by surgical excisional biopsy and subsequently examined to rule out alternative diagnoses, such as sclerosing mesenteritis.
Similar content being viewed by others
Background
Sclerosing mesenteritis is non-specific inflammatory mass-forming lesion in the mesenteric connective tissue that is characterized by variable degrees of fibrosis, chronic inflammation, and fat necrosis [1,2,3,4,5] and mainly affects the mesentery of the small bowel [2,3,4,5,6,7,8,9]. Although the exact etiology of sclerosing mesenteritis has not been elucidated [1,2,3,4,5,6, 9,10,11,12,13,14], it has been reported that the possible risk factors for the disease include malignancy, autoimmune disease, infection, ischemia, trauma, and a history of previous surgery [3, 5, 8, 11, 12]. Sclerosing mesenteritis is sometimes indistinguishable from neoplasms because its manifestations and radiographic findings may be identical to those of malignancies [3, 6, 7]. Despite these similarities, sclerosing mesenteritis is treated very differently than malignancies; thus, it is very important that sclerosing mesenteritis is diagnosed correctly so that the disease can be managed adequately.
Here, we report the case of a patient who underwent surgery for presumed metachronous localized peritoneal metastases from descending colon cancer and was ultimately diagnosed with sclerosing mesenteritis and thus avoided receiving unnecessary chemotherapy.
Case presentation
A 73-year-old man with advanced descending colon carcinoma was referred to our hospital for surgery. The patient had previously undergone a hemithyroidectomy for papillary thyroid carcinoma and an appendectomy for acute appendicitis. He was also receiving medications for the treatment of type 2 diabetes mellitus and the prevention of cerebral infarction. His carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 levels were 4.0 μg/L and 4.7 kU/L, respectively, and were thus each within their normal ranges. Computed tomography (CT) was negative for metastatic lesions. The patient underwent a laparoscopic partial colectomy and regional lymph node dissection. The pathological diagnosis was moderately differentiated tubular adenocarcinoma that had invaded the subserosa (T3) and was without lymph node metastasis (N0) (Fig. 1). The lymph node count was below 12. The patient was considered to have high-risk stage II colorectal cancer but elected not to undergo adjuvant chemotherapy. One month after surgery, he developed an adhesion-related small intestinal obstruction and underwent a partial jejunal resection.
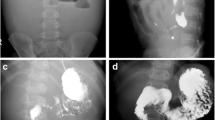
Nine months after the primary surgery, the patient underwent CT, which revealed soft tissue attenuating masses in the left lower abdominal quadrant (Fig. 2a). The patient did not have any symptoms, and his laboratory data, including his tumor marker levels, were within normal limits (his CEA and CA19–9 levels were 3.1 μg/L and 4.4 kU/L, respectively). The masses increased in size during the ensuing 3 months (Fig. 2b) and displayed increased fluorodeoxyglucose (FDG) uptake, with an SUV max of 2.9 on positron emission tomography (PET scan) (Fig. 3). The masses were attributed to peritoneal metastasis; thus, chemotherapy was recommended. However, the patient elected not to undergo chemotherapy because he was unwilling to allow his quality of life to adversely be affected by the therapy. He instead elected to undergo localized tumor excision; thus, a laparotomy was performed. Intraoperatively, the masses were found to be located in the mesentery of the jejunum. Complete excision of these mesenteric masses warranted resection of the affected intestinal segment because the vascular supply to the segment was severely compromised. Grossly, the resected nodules were grayish white, well-demarcated solid masses (Fig. 4a). Microscopically, the masses displayed collagen fiber and fibroblast proliferation and contained a few inflammatory cells. The fibroblasts had infiltrated and surrounded the peripheral fatty tissue. These findings were consistent with sclerosing mesenteritis (Fig. 4b).
Gross and pathological findings (Hematoxylin-Eosion staining) pertaining to the resected specimens. a The mesenteric nodules were grayish white, well-delineated solid masses. b Collagen fibers and fibroblasts had proliferated and infiltrated the peripheral fatty tissues. The scale bar represents 500 μm
The patient suffered from postoperative ileus but recovered with conservative treatment and showed no signs of sclerosing mesenteritis or descending colon cancer recurrence after 6 and 18 months of follow-up, respectively.
Discussion
Sclerosing mesenteritis is an inflammatory process within the mesentery and can be categorized into the following three subgroups, depending on the predominant tissue component: mesenteric panniculitis, which is characterized by chronic inflammation; mesenteric lipodystrophy, which is characterized by fat necrosis; and retractile mesenteritis, which is characterized by fibrosis [2, 8, 10]. Distinguishing sclerosing mesenteritis from neoplasms is sometimes difficult because the disease can mimic lymphoma and digestive or gynecologic organ malignancies both clinically and radiographically [3, 6, 7]. Sclerosing mesenteritis remains asymptomatic in 30–50% of cases [1, 10]. The patient in the present case had no clinical manifestations of disease, nor did his laboratory studies exhibit specific abnormalities. The most common symptoms of the disease include fever, abdominal pain, abdominal mass, and weight loss [1,2,3, 5]; however, these symptoms are nonspecific and are associated with numerous other diseases [4]. Laboratory studies may show an elevated white blood cell count and/or erythrocyte sedimentation rate or anemia but are also unhelpful with respect to making a definitive diagnosis of the disease [4, 5].
The CT findings of sclerosing mesenteritis will vary depending on the predominant features of the disease [2]. Mesenteric panniculitis usually manifests as well-delineated masses composed of heterogeneous fatty tissues with increased densities [2, 11, 13]. In contrast, retractile mesenteritis presents as homogenous masses with a greater proportion of soft tissues [2, 13]. The fat ring sign, which signifies the preservation of a halo of fat around blood vessels, and the presence of a tumoral pseudocapsule consisting of a peripheral band of fatty tissue with soft tissue attenuation that protects normal mesentery from the inflammatory process, are both somewhat specific for mesenteric panniculitis [2, 10]. However, these two CT findings may disappear when mesenteric panniculitis evolves into retractile mesenteritis [2]. There are some reports indicating the superiority of magnetic resonance imaging (MRI) findings for diagnosing sclerosing mesenteritis [15, 16]. If we had performed MRI before laparotomy, the specific MRI findings might be provided. But it was difficult that the patient was diagnosed with no malignancy because the abdominal masses displayed rapid enlargement. The results of recently published studies indicate that PET scan findings enable clinicians to differentiate between benign and neoplastic mesenteric processes [9, 17]. In these studies, all patients whose mesenteric nodules displayed no FDG uptake had no malignant involvement of the mesentery. The authors of these studies concluded that negative PET had high diagnostic accuracy with respect to excluding tumoral mesenteric involvement [9, 17]. On the other hand, Ehrenpreis et al. [14, 18] reported that PET did not appear to be useful to distinguish an inflammatory mesenteric mass due to mesenteric panniculitis from a malignant mesenteric mass. Furthermore, false-positive results have been observed because some phases of the evolution of sclerosing mesenteritis present as hypermetabolic lesions [9, 17]. This was the case for the patient in the present study. If the patient had received chemotherapy for the diagnosis of peritoneal recurrence based on his radiographic findings alone, he would have received unnecessary treatment.
The prognosis of sclerosing mesenteritis is generally regarded as favorable with supportive treatment [1, 6, 7, 12]. In spite of having clinical and radiographic findings similar to those of malignancies, sclerosing mesenteritis is treated differently than malignancies. Radiological examinations, such as CT or PET scan, may be helpful tools for distinguishing sclerosing mesenteritis from alternative diagnoses, especially in patients suspected of having oncologic disease; however, it is necessary to determine whether malignant cells are present or absent to provide appropriate treatment for the disease. As some studies have demonstrated [4, 13], only complete histologic analyses of tissue samples obtained by surgery can rule out malignancy.
In conclusion, it is necessary to excise and examine mesenteric nodules in patients with a history of malignancy to distinguish tumor recurrence from alternative diagnoses, such as sclerosing mesenteritis.
Conclusions
Sclerosing mesenteritis can mimic peritoneal metastasis from malignancies, and it is necessary to excise and examine a mesenteric nodule developing in patients with a history of malignancy to distinguish recurrence and differential diagnosis such as sclerosing mesenteritis.
Abbreviations
- CA19-9:
-
Carbohydrate antigen
- CEA:
-
Carcinoembryonic antigen
- CT:
-
Computed tomography
- FDG:
-
Fluorodeoxyglucose
- MRI:
-
Magnetic resonance imaging
- PET scan:
-
Positron emission tomography
References
Emory TS, Monihan JM, Carr NJ, Sobin LH. Sclerosing mesenteritis, mesenteric panniculitis and mesenteric lipodystrophy: a single entity? Am J Surg Pathol. 1997;21:392–8.
Sabate JM, Torrubia S, Maideu J, Franquet T, Monill JM, Perez C. Sclerosing mesenteritis: imaging findings in 17 patients. AJR Am J Roentgenol. 1999;172:625–9.
Akram S, Pardi DS, Schaffner JA, Smyrk TC. Sclerosing mesenteritis: clinical features, treatment, and outcome in ninety-two patients. Clin Gastroenterol Hepatol. 2007;5:589–96.
Nobili C, Degrate L, Caprotti R, Franciosi C, Leone BE, Romano F, Dinelli M, Uggeri F. Extensive sclerosing mesenteritis of the rectosigmoid colon associated with erosive colitis. Gastroenterol Res Pract 2009; doi: 10.1155/2009/176793.
Duman M, Kocak O, Fazli O, Kocak C, Atici AE, Duman U. Mesenteric panniculitis patients requiring emergency surgery: report of three cases. Turk J Gastroenterol. 2012;23:181–4.
Scudiere JR, Shi C, Hruban RH, Herman JM, Fishman EK, Schulick RD, Wolfgang CL, Makary MA, Thornton K, Montgomery E, Horton KM. Sclerosing mesenteritis involving the pancreas: a mimicker of pancreatic cancer. Am J Surg Pathol. 2010;34:447–53.
Tierney C, Dinkelspiel HE, Bass AR, Cimic A, Katzen J, Holcomb K. Sclerosing mesenteritis mimics gynecologic malignancy. Gynecol Oncol Rep. 2015;12:49–51.
He H, Zhi M, Zhang M, Su M, Chen H, Kang L, Huang Y, Zhou Z, Gao X, Wang J, Hu P. Sclerosing mesenteritis: multidisciplinary collaboration is essential for diagnosis and treatment. Gastroenterology Res. 2017;10:50–5.
Rincon JO, Regi AR, Hualde AM, Berenguer LR, Moreno LH, Farto JCA. A prospective study to determine the real value of mesenteric 18F-FDG uptake in cancer patients. Inflammatory or tumoral mesenteric paniculitis? Rev Esp Med Nucl Imagen Mol. 2014;33:352–7.
Ferrari TC, Couto CM, Vilaca TS, Xavier MA, Faria LC. An unusual presentation of mesenteric panniculitis. Clinics. 2008;63:843–4.
Daskalogiannaki M, Voloudaki A, Prassopoulos P, Magkanas E, Stefanaki K, Apostolaki E, Gourtsoyiannis N. CT evaluation of mesenteric panniculitis: prevalence and associated diseases. AJR Am J Roentgenol. 2000;174:427–31.
Fujikawa T, Yasuhara H, Matsumi A, Imagawa A. A case of mesenteric panniculitis requiring an operation. BMJ Case Rep 2014; doi: 10.1136/bcr-2014-205028.
Masulovic D, Jovanovic M, Ivanovic A, Stojakov D, Micev M, Stevic R, Filipovic A, Galun D. Sclerosing mesenteritis presenting as a pseudotumor of the greater omentum. Med Princ Pract. 2016;25:93–5.
Ehrenpreis ED, Rao AS, Aki R, Brown H, Pae T, Boiskin I. Normal positron emission tomography-computerized tomogram in a patient with apparent mesenteric panniculitis: biopsy is still the answer. Case Rep Gastroenterol. 2009;3:131–7.
Apostolakis S, Ioannidis A, Tsioga G, Papageorgiou K, Velimezis G. A systematic investigation of sclerosing mesenteritis through CT and MRI. Radiol Case Rep. 2016;11:299–302.
Ghanem N, Pache G, Bley T, Kotter E, Langer M. MR findings in a rare case of sclerosing mesenteritis of the mesocolon. J Magn Reson Imaging. 2005;21:632–6.
Zissin R, Metser U, Hain D, Even-Sapir E. Mesenteric panniculitis in oncologic patients: PET-CT findings. Br J Radiol. 2006;79:37–43.
Ehrenpreis ED, Roginsky G, Gore RM. Clinical significance of mesenteric panniculitis-like abnormalities on abdominal computerized tomography in patients with malignant neoplasms. World J Gastroenterol. 2016;22:10601–8.
Acknowledgements
No additional investigators were involved in this study.
Funding
There was no funding for this case report.
Availability of data and materials
The original data and material are available upon request to the first author.
Author information
Authors and Affiliations
Contributions
TW designed the report, collected the material, analyzed data, and wrote the manuscript. TW and ST performed the surgery. JS and KM carried out the radiographic data analyses. AU evaluated the patient’s pathological data. ST, TT, MT, KM, KA, MK, KM, and KS assisted in preparing the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was approved by the Ethical Committee of Toyama Prefectural Central Hospital (number 5318).
Consent for publication
Written informed consent has been obtained from the patient for the publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Watanabe, T., Terai, S., Tsukada, T. et al. Sclerosing mesenteritis mimicking metachronous peritoneal metastases from descending colon adenocarcinoma. World J Surg Onc 15, 142 (2017). https://doi.org/10.1186/s12957-017-1214-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-017-1214-4