Abstract
Nanotechnology offers the possibility of revolutionizing cancer theranostics in the new era of precision oncology. Extracellular vesicles (EVs)-like biomimetic nanoparticles (EBPs) have recently emerged as a promising platform for targeted cancer drug delivery. Compared with conventional synthetic vehicles, EBPs have several advantages, such as lower immunogenicity, longer circulation time, and better targeting capability. Studies on EBPs as cancer therapeutics are rapidly progressing from in vitro experiments to in vivo animal models and early-stage clinical trials. Here, we describe engineering strategies to further improve EBPs as effective anticancer drug carriers, including genetic manipulation of original cells, fusion with synthetic nanomaterials, and direct modification of EVs. These engineering approaches can improve the anticancer performance of EBPs, especially in terms of tumor targeting effectiveness, stealth property, drug loading capacity, and integration with other therapeutic modalities. Finally, the current obstacles and future perspectives of engineered EBPs as the next-generation delivery platform for anticancer drugs are discussed.
Similar content being viewed by others
Introduction
Cancer poses a serious threat to human health [1]. Chemotherapeutic drugs, one of the principal means of cancer treatment, lack selectivity for cancer cells, leading to toxic side effects such as bone marrow suppression and gastrointestinal issues, as well as the emergence of drug resistance [2]. Targeted drug delivery is an important research direction in the field of precision oncology, which can effectively improve the efficacy of anticancer drugs and reduce their toxicity. Nanoparticle-based drug delivery systems possess some essential features, such as high drug loading capacity, long circulation time, and high bioavailability, which can increase drug concentration in specific organs or tissues, ultimately improving treatment effectiveness and avoiding side effects [3]. Over the past decade, organic and inorganic materials such as polymers, liposomes, and proteins have been developed as nanocarriers to improve the delivery efficiency of therapeutic molecules or genes [4]. However, it is worth noting that after systemic administration, only 0.7% of the injected dose of nanoparticles reaches the tumor site [5]. In addition, recent clinical data indicate that conventional nanoparticles are not very effective in terms of drug specificity and effectiveness, and the incidence of side effects is high. Therefore, novel nanocarriers need to be developed to further improve tumor enrichment of anticancer drugs, thus enhancing their therapeutic efficacy with low toxicity [6].
Extracellular vesicles (EVs) [7], a new class of drug nanocarriers, have gained increasing attention in recent years. According to the definition of the International Society for Extracellular Vesicles (ISEV), EVs are naturally released particles from cells, including exosomes, microvesicles, microparticles, apoptotic bodies, and other non-replicating extracellular vesicle subgroups. Studies have shown that EVs are important mediators of intercellular communication and contain diverse bioactive substances like nucleic acids, proteins, lipids, and metabolites [8,9,10]. These substances play a vital role in physiological and pathological processes by transmitting information through autocrine, paracrine, or endocrine pathways to specific cells. EVs have potential as diagnostic and therapeutic tools for disease detection, prevention, immune system regulation, antitumor therapy, and wound healing [11]. In 2007, Valadi et al. demonstrated that mast cell-derived EVs can deliver functional mRNA [12]. Since then, EVs have been used to transport various therapeutics including siRNAs, miRNAs, small molecule drugs, and proteins. EVs can be derived from various cell lines, primary cells, human plasma, fetal bovine serum, urine, milk, and edible plants, which provides a certain degree of biocompatibility and the ability to evade the immune system [13,14,15,16,17]. In addition, various types of cells are capable of offering diverse biological functions to EVs. However, the clinical application of this approach is constrained by factors including low yield, low cost-effectiveness, insufficient targeting ability, limited drug loading capacity, and inadequate drug release capability. Current isolation methods can only produce a small number of EVs in a short period of time, and inadequate separation techniques can introduce protein contaminants in the final product. This contamination presents challenges in differentiating between EVs and non-EVs for downstream analysis. Furthermore, unmodified EVs may face obstacles in targeting, which may potentially limit their ability to reach target tissues. Additionally, due to the absence of standardized preparation schemes, EVs obtained through different strategies possess varying properties and functions, making large-scale production and clinical application challenging.
Therefore, scientists have attempted to address the aforementioned limitations of EVs through various engineering approaches, including internal loading and external modification, fusion with other membrane components or various types of nanoparticles, and even the disruption of cell membrane structure and recombination of EVs analogues. These unnaturally engineered nanoparticles have undergone a series of manual operations and are no longer suitable for the definition of EVs. Therefore, it is necessary to define such EVs-based nanoparticles, which will facilitate subsequent analysis, research, and the formulation of corresponding standards and specifications. Based on the fact that these engineered nanoparticles have vesicle-like structures similar to EVs as well as biomimetic properties that mimic cell membranes, we refer to them as EV-like biomimetic nanoparticles (EBPs). All artificially engineered nanoparticles based on EVs or EV analogues, as well as those reconstituted with cell membrane components, are classified as EBPs. Table 1 presents the description and comparison of EVs, EBPs, and other non-biomimetic synthetic nanomaterials such as commonly used liposomes and polymers.
EBPs can be invoked as a substitute for EVs to exert their biological function, with simpler preparation methods, higher yield and production efficiency, and more effective combination therapy integration, showing better application prospects. Compared with traditional methods, artificial methods can significantly increase the yield and shorten the preparation time of EBPs [18]. The most common artificial preparation method for EBPs is to extrude vesicles through a membrane filter [19, 20]. When combined with other nanomaterials, the membrane vesicles can be squeezed again to surround the particles. The EBPs prepared by wrapping organic or inorganic synthesized nanoparticles in a cell membrane not only have certain characteristics of synthesized nanoparticles, but also have the natural or modified properties of the source cell [21]. In addition, the combination with other nanocarriers with higher drug loading capacities, such as liposomes and polymeric nanoparticles, also provides a feasible option for more effective drug loading of EBPs. Carboxyfluorescein succinyl ester (CFSE), calcein acetoxymethyl (calcein-AM), and the fluorescent dyes PKH and DiI/O/D/R have been widely used to label EBPs [22,23,24]. Nevertheless, these fluorescent dyes may lead to false positive signals arising from dye-induced micelles, and the resulting fluorescent signal may persist in the tissue beyond the duration of the EBPs themselves. To circumvent the issue of false positive signals, it may be advisable to consider alternative labeling strategies such as merging EBPs with imaging-capable nanomaterials or creating fluorescent membrane proteins through genetic engineering techniques.
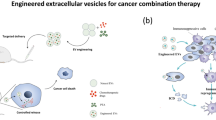
This review aims to summarize the utilization of EBPs as drug delivery vehicles in cancer treatment through different engineering strategies, including biological, physical, and chemical approaches, and discuss the challenges, potential solutions, and future prospects of EBPs in clinical applications. Figure 1 depicts the potential applications of EBPs in the field of targeted cancer therapy, including targeting tumor tissue, improving the tumor immune microenvironment, aiding photothermal and magnetic field therapy, and enhancing drug utilization for safer and more effective treatment. For instance, EBPs can be surface modified with targeted molecules such as antibodies or peptides to recognize and bind specifically to receptors or antigens of tumor cells. This precise targeting facilitates the delivery of therapeutic drugs to tumor tissue, minimizing harm to normal tissues. Additionally, mixing EBPs with synthetic nanoparticles can improve the solubility, stability, and utilization of drugs. The tumor immune microenvironment can suppress the immune response, allowing tumors to evade immune surveillance. EBPs can improve the tumor microenvironment by carrying immunomodulatory molecules or specific cell types, such as dendritic cells or T cells, augmenting the efficiency of immunotherapy. Furthermore, EBPs can combine drug delivery with photothermal and magnetic field therapy to enhance antitumor efficacy. For example, EBPs enable photothermal therapy by carrying photosensitive molecules or thermal agents that, when activated, release heat or generate active substances to destroy tumor cells. Similarly, EBPs carrying magnetic nanoparticles can locate tumor sites under magnetic fields, enabling targeted therapy.
Engineering strategies of EBPs
To improve their scalability and therapeutic outcomes, a range of engineering strategies have been implemented to construct EBPs. The utilization of these strategies endow EBPs with multifunctionality and enhanced antitumor ability. Firstly, through genetic engineering of original cells, it becomes possible to alter the surface properties and confer specific targeting capabilities of EBPs, enabling their precise delivery to the site of the disease. Secondly, the fusion of EVs or cell membrane structures with synthetic nanoparticles can improve the drug loading capacity of the obtained EBPs, enhance their stability and biocompatibility, and thus improve their therapeutic effect. Thirdly, direct modification of EVs can also alter the intracellular delivery mechanism of nanoparticles, further augmenting their therapeutic impact. The integration of these engineering strategies not only expands the application scope of EBPs but also paves the way for the realization of precision medicine.
Genetic engineering of original cells
Genetic engineering is one of the ways to obtain ideal EBPs. Genetic engineering techniques in EBPs display functional ligands on the membrane of donor cells by constructing plasmids and overexpressing proteins. Figure 2 illustrates three examples of genetic engineering modifications. Cheng et al. decorated two distinct antibodies on the surface of the EBPs, one targeting CD3 on T cells and the other targeting epidermal growth factor receptor (EGFR) on triple negative breast cancer (TNBC) cells [25]. Both in vitro and in vivo experiments have demonstrated that the modified SMART-EBPs can induce the specific killing of immune cells against tumors. This suggests that the decoration of EBPs with antibodies can enhance their effectiveness in targeting and eliminating cancer cells. In Fig. 2B, vesicular stomatitis virus glycoprotein (VSV-G) was introduced onto the surface of EVs derived from M1 macrophages and loaded with siPD-L1 [26]. Following administration, EVs can be directed towards tumors through the inherent targeting ability of M1-EVs. In the acidic tumor microenvironment, the pH-responsive VSV-G may undergo a conformational change, resulting in the unfolding of the protein. This, in turn, triggered the fusion of EVs with the cell membrane, enabling them to bypass the intracellular uptake pathway. Therefore, siPD-L1 can be delivered directly into the cytoplasm, triggering the silencing of the PD-L1 gene. At the same time, M1-EVs were found to be effective in repolarizing M2-type tumor-associated macrophages (TAMs) into M1-type macrophages. The combination of siPD-L1 and M1-EVs resulted in a synergistic effect, leading to a potent anticancer immunotherapeutic effect. Cheng et al. designed a hybrid therapeutic nanovesicle that fuses genetically engineered EBPs with heat-sensitive liposomes (TSLs) for cancer therapy in combination with PTT and immunotherapy [27]. First, CT26 cells were transfected to obtain EBPs overexpressing CD47, achieving homologous cell targeting and a long cycling time in vivo. Subsequently, they were fused with TSL to form hybrid nanovesicle hGLVs, which were then loaded with the photothermal agent ICG and immune adjuvant R837 to provide photothermal effects and cytotoxicity. The combination of these technologies may provide enhanced targeting, photothermal effects, and immune stimulation for improved therapeutic outcomes.
Schematic illustration of the genetically engineered EBPs. A Schematic diagram of the design and generation of αCD3/αEGFR synthetic multivalent antibody-displaying exosomes (SMART-Exos). It was demonstrated that the SMART-Exos, which was designed to simultaneously target CD3 and EGFR, could effectively bind to both T cells and TNBC cells expressing EGFR [25]. Reproduced with permission. Copyright 2018, American Chemical Society. B Schematic illustration of the virus-derived fusogenic protein VSV-G on the surface of M1 EVs loaded with siPD-L1. siRNA@V-M1 EVs can target tumors due to the native targeting capability of M1 EVs. The pH-responsive VSV-G changes to an unfolded fusion state in the acidic tumor microenvironment [26]. Reproduced with permission. Copyright 2020, Wiley. C The design principle of hGLV involves utilizing photothermal therapy (PTT) in combination with immunotherapy to combat tumors [27]. Copyright 2021, Elsevier. TNBC triple-negative breast cancer, EGFR epidermal growth factor receptor, exos exosomes, TSL thermosensitive liposomes, hGLV gene-engineered exosome-thermosensitive liposome hybrid nanovesicles, DCs dendritic cells, SMART-Exos synthetic multivalent antibody-retargeted exosomes, VSV-G vesicular stomatitis virus glycoprotein, M1 EVs M1 macrophage-derived EVs
Genetic engineering approach requires a solid understanding of the physiological conformation of membrane proteins, since the function of many membrane proteins is associated with their complex tertiary structure. In addition to functional proteins, genetic engineering techniques can also introduce noncoding RNA sequences into EBPs, such as miRNA or small interfering RNA (siRNA) [28]. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and its associated protein, Cas9, have emerged as powerful tools for genome editing. By using CRISPR/Cas9, researchers can target specific genomic regions and edit genetic material with high precision and accuracy [29]. CRISPR/Cas9 technology can be used to genetically edit the target protein of original cells to obtain the corresponding engineered EBPs. EBPs can also serve as functional vectors to carry CRISPR-Cas9 for gene editing purposes, leveraging their natural targeting properties and ability to penetrate cell membranes to deliver genetic material with high specificity and efficiency [30]. Overall, this genetic engineering method is easy to operate, but has the limitations of poor specificity and low loading efficiency, and cannot be used to obtain non-genetically coded products. Most experiments involving genetic modifications are designed to improve the ability of EBPs to target tumors and their surrounding sites (Table 2). Engineered EBPs have proven to have useful clinical application potential in various fields, such as cancer, tissue regeneration and repair, cardiovascular and neurological diseases [31,32,33], and all of them generally show better targeting and therapeutic effects than natural EVs. The genetic manipulation approach has brought more standardized products, but also faces some challenges, such as mass production, stability control, storage methods, and quality control. The bioengineering strategy is time-consuming and requires repeating the whole process each time for a new molecule. In addition, due to changes in the bioactivity of EBPs caused by genetic manipulation, it is difficult to control the density and glycosylation state of target proteins, which poses potential safety risks. Researchers can explore ways to optimize and streamline the bioengineering process to reduce the time required for each cycle. This could involve improving cloning techniques, employing automation, or developing more efficient methods for genetic manipulation. In addition, researchers need to monitor and analyze any alterations in bioactivity, immunogenicity, or potential off-target effects resulting from genetic modifications. Close attention to safety concerns and robust experimental validation can help mitigate potential risks.
Fusion of cell membrane nanostructures with synthetic materials
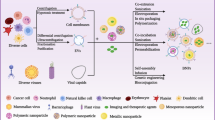
Due to the properties of foreign substances, conventional nanoparticles are rapidly eliminated by the immune system after entering the bloodstream. To achieve superior anticancer effects, an ideal drug nanocarrier should have a long blood circulation time, as well as specific and efficient enrichment in tumor tissue [60]. Using the cytoplasmic membrane as the outer membrane of nanoparticles can reduce the body’s specific rejection to some extent. The cytoplasmic membrane can be obtained from cells through hypertonic treatment or by repeated freeze–thaw processes. Nanocarriers such as liposomes, polymeric micelles, and inorganic nanoparticles can all become the contents of cell membrane-coated EBPs, as shown in Fig. 3. Traditional artificial attachment of functional proteins onto nanocarriers may result in loss of the protein's natural configuration, while directly modified biofilm can avoid damage to the protein's structure and function. The application of cell membrane-coated EBPs can combine the unique properties of original cells and synthetic nanoparticles, thus achieving more effective targeting and intervention effects on cancer cells. It is worth noting that different types of nanomaterial assemblies offer various functions and properties for tumor targeting, in vivo imaging, magnetothermotherapy, photothermotherapy, and drug delivery. In addition, membrane-derived cells can be modified using genetic engineering methods to further enhance the function of cell membranes. Recent studies have demonstrated the potential of membrane-coated EBPs in cancer treatment, and a summary of their applications is provided in Table 3.
Hybrid EBPs that integrate cell membrane nanostructures with synthetic materials. Synthetic nanoparticles can combine with the plasma membrane to create membrane-coated EBPs. These hybrid EBPs not only have the unique characteristics of synthetic nanoparticles but also exhibit the inherent or adapted traits of the parent cells
Inorganic materials such as gold, iron, and silica wrapped in the contents of cell membranes have unique physical, electrical, magnetic, and optical properties that can be used for diagnostic, imaging, and photothermal therapy (PTT) applications [61, 62]. Most inorganic nanoparticles have good biocompatibility and stability. However, their clinical use is restricted due to low solubility and toxicity issues [63]. The use of cell membrane as a shell facilitates long-term blood circulation and targeted delivery of nanoparticles in vivo and is not affected by the properties of the core nanomaterials [64]. Polymeric nanoparticles have favorable biodegradability, water solubility, biocompatibility, mimicability, and storage stability, which are also ideal materials for the targeted delivery of small molecules, biomacromolecules, proteins, and vaccines [65]. By adjusting the composition, stability, reactivity, and surface charge of the particles, the encapsulation and release of drugs can be precisely controlled. Their surfaces can also be easily modified with ligands to deliver drugs, proteins, and genetic material to target tissues. However, only a small number of polymeric nanomaterials have been approved by the US Food and Drug Administration (FDA) for clinical use, due to the agglomeration and non-specific clearance of nanoparticles. On the other hand, the insufficient drug carrying capacity of cell membrane vesicles is one of the reasons limiting their further application in drug delivery. The integration of polymeric nanoparticles with cell membrane not only retain the excellent biocompatibility and targeting ability of the original cell membrane, but also overcome the defect of insufficient drug loading. Lipid nanoparticles are the most common class of nanomedicines approved by the FDA, which have the advantages of simple formulation, self-assembly, biocompatibility, high bioavailability, and high drug loading [66]. Similarly, lipid nanoparticles can be fused with cell membranes to prepare corresponding membrane-coated EBPs. In addition, drug-carrying components from other biological sources can also be encapsulated as the contents of membrane-coated EBPs. For example, albumin is the protein with the highest content in plasma which has good biocompatibility, nonimmunogenicity, easy purification, and good water solubility. Although albumin nanoparticles can efficiently load hydrophobic drugs due to multiple drug binding sites, they may react with proteins in the blood, resulting in reduced uptake of nanoparticles by tumor cells. Xiong et al. showed that the Pt (IV) precursor was able to stably bind to albumin with the aid of lecithin, while the encapsulation of macrophage membranes further enhanced the ability of albumin nanoparticles to actively aggregate at the tumor site and effectively promoted the cellular internalization of drugs, where Pt(IV) precursor was decomposed into toxic Pt(II) drugs under cytoplasmic reduction [67]. This strategy promotes cellular internalization and the conversion of the precursor into its active form, improving the therapeutic potential of the drug. Figure 4 provides a concise overview of the methods employed for the fusion of membrane nanostructures with synthetic materials.
Example of fusion of membrane nanostructures with synthetic materials. A Schematic diagram of the method for preparing the gene-engineered exosome-liposome hybrid nanovesicles (hGLV) [27]. Reprinted with permission. Copyright 2021, Elsevier. B Cationic bovine serum albumin (CBSA)-coupled siS100A4 was coated with exosome membrane by extrusion to form biomimetic nanoparticles [68]. Reprinted with permission. Copyright 2020, Elsevier. C Nano-pathogenoids (NPNs) are prepared by coating Outer membrane vesicles (OMV) on nanoparticles NPs, and NPNs inherit pathogen-associated molecular patterns (PAMPs) from OMV [69]. Reprinted with permission. Copyright 2020, Nature Communications. D DC2.4 was mixed with DOX and extruded with polycarbonate films by a micro-extruder [70]. The obtained EVdox was incubated with HAuCl4·3H2O at 37 °C for 24 h to obtain EVdox@AuNP. Reprinted with permission. Copyright 2019, Elsevier
Direct modification
In addition, direct modification is also an artificial engineering strategy. There are more concerns about direct modification of EBPs than liposomes because they must be modified without compromising the integrity of vesicles. “Click chemistry” reactions can form chemical bonds under very mild conditions and are utilized to covalently bind large biomolecules. This method can also anchor the targeted portion to the cell membrane, controlling the structure and density of the targeted ligand regardless of the cell of origin. In addition, the reaction can be performed during the purification of vesicles, so it is more suitable for clinical applications than the genetic engineering approach. Tian et al. adopted a copper-free click chemistry method to conjugate cyclic (Arg-Gly-Asp-d-Tyr-Lys) peptide [c(RGDyK)] into cerebrovascular endothelial cell-derived EBPs (Fig. 5). EBPs-mediated siPDL1 targeted delivery successfully reversed the expression of radiation-stimulated PD-L1 in tumor cells [91].
Schematic diagram of siRNA-loaded EVs conjugated with c(RGDyK) and their anticancer effects. A A diagram illustrating a two-step reaction that involves conjugating c(RGDyK) and Cy5.5 fluorophore or cholesterol-modified siPDL1 to the amine group of EVs. B Quantification of tumor-associated fluorescence radiance intensity with data presented as the mean ± SEM; **P < 0.01, ***P < 0.001, ****P < 0.0001, by one-way ANOVA. C Kaplan–Meier survival curves are shown (n = 10) [91]. Reprinted with permission.
Peptides as tumor cell targeting ligands have lower immunogenicity than antibodies, and researchers can use peptide libraries to screen for different tumor targets. For example, the small molecule polypeptide cNGQGEQc (CC8) that can bind to non-small cell lung cancer is a short peptide consisting of eight amino acid residues, which was first screened by Guo et al. using combinatorial chemical peptide library technology [92]. They added free thiols to both ends of cysteine residues in CC8 to help it modify the membrane through simple incubation [93]. This modification improved the short half-life and poor biological distribution of Imperaline, and played a targeting role in NSCLC cells. This work not only provides a targeted drug delivery system for Imperaline but also offers a new platform for targeting non-small cell lung cancer. Nied et al. produced another type of engineered EBPs for cancer treatment through direct modification. First of all, macrophages were polarized by Mn into anti-tumor M1 macrophages. Then, the azide group was introduced into cholinophospholipids on the cell membrane. The azide-modified M1 EVs were linked with the anti-CD47 antibody (aCD47) and anti-signal regulatory protein α (SIRPα) antibody through dibenzocycloctane (DBCO) and the pH-sensitive benz-imide bond [94]. This resulting M1 type EBPs were able to eliminate the “don’t eat me” signal and improve the phagocytosis ability of macrophages. In addition, the EBPs can effectively restore macrophages in the original tumor from M2 to M1, exerting a synergistic antitumor effect. This engineering strategy can be popularized and adapted to various environments, and the approach is relatively simple and easy to implement.
While modifying the surface of EBPs, precautions must be taken to avoid membrane rupture, surface protein denaturation, and changes in osmotic pressure resulting from exposure to excessive temperature, pressure, solvents, or low or high salt concentrations. Moreover, chemical manipulation may lead to surface protein inactivation or nanoparticle aggregation. Apart from covalent modifications, there are promising noncovalent methods for stable biofilm modification, such as membrane insertion using lipid-binding ligands or hydrophobic drugs, receptor-ligand binding, and transient membrane permeability via electroporation. Figure 6 illustrates the various functional structures that may be directly modified onto the membrane’s surface, including large molecular proteins, antibodies, and small molecular inorganic compounds. In addition, the modification types of EBPs are further diversified through multiple chemical reaction pathways.
Schematic illustration of the direct modification of EBPs on the membrane surface. Examples of direct engineering modification of the surface of EBPs include integrating lipid-conjugated molecules into the lipid bilayer, utilizing click chemistry and affinity interactions to attach target molecules, relying on enzymatic covalent binding through peptides or antibodies, and using biotin-avidin reactions to bind specific molecules. FA folic acid, HA hualuronic acid, Abs antibodies, DBCO dibenzocyclooctyne, PDA polydopamine, Tf transferrin, TfR transferrin receptor, SPIONs superparamagnetic iron oxide nanoparticles
It is a simple and direct method to modify functional molecules with lipophilic molecules and insert them into the cell membrane. DSPE-PEG, as a PEG phospholipid, has good biocompatibility and biodegradability and is often used as a nanoparticle carrier material to assist drug release, which can improve the biocompatibility and stability of the preparations [95, 96]. Functionalized DSPE-PEG can be used to modify proteins, peptides and other materials or small molecules containing sulfhydryl groups and then inserted into the bilayer phospholipid membrane through the DSPE terminal to obtain a new class of targeted EBPs. Lipophilic chemical chains and biofunctional nucleic acid compounds can also be modified onto the surface of EBPs by simple incubation methods. For example, valent-controlled tetrahedral DNA nanostructures (TDNs) were conjugated with DNA aptamers and loaded onto the surface of EBPs via cholesterol anchoring to achieve specific cell targeting [97]. EBPs carrying TLS11a aptamers that bind specifically to HepG2 cells and CRISPR-Cas9 RNA-directed endonucleases (RNPs) led to the downregulation of GFP or WNT10B in specific cells. Cholesterol binds to RNA in a similar manner to DNA. In the study conducted by Zhan et al. cPLA2 siRNA, an inhibitor of energy metabolism, binds to the cell membrane through the cholesterol chain, enabling a straightforward process to form a dual inhibitor with metformin [98]. Due to the active uptake of vesicles by GBM cells, drugs can be selectively concentrated at the tumor site without the need for external targeting design.
In addition, proteins or peptides can also be bound to the membrane surface of EBPs by protein ligases. In the study of Peng et al. the vesicles produced by erythrocytes were linked to the biotin-TRNGL enzyme, and biotin-TRNGL was sequentially coupled to a biotinylated linking peptide with streptavidin tetracer and biotinylated anti-EGFR nanoparticles (α-EGFR-VHH) to functionalize the surface of EBPs, which activated the innate immune system through the RIG-I receptor (RLR) pathway for anticancer treatment [99]. Pham et al. utilized Sortase A and OaAEP1 ligases to establish a permanent covalent bond between vesicles and peptides, and this catalytic reaction occurred at neutral pH. The resulting targeted EBPs significantly inhibited tumor growth by delivering low-dose paclitaxel (10–20 times lower than the clinical equivalent dose) [100]. This simple enzymatic method enables the coupling of peptides and nanoantibodies to the membrane without any genetic or chemical modification of the donor cell.
Gram-negative bacteria-derived outer-membrane vesicles (OMVs) hold significant potential as anticancer therapeutic agents in nanotechnology applications. However, OMVs’ intravenous injection can result in high toxicity and antibody-dependent clearance. To address these challenges, Qing et al. coated the OMVs’ surface with a pH-sensitive calcium phosphate (CaP) shell, which neutralizes the acidic tumor microenvironment [28]. This neutralization provides several advantages, such as improving targeted drug delivery to tumor cells and reducing toxicity to healthy cells. Table 4 provides more details on the direct modification methods of EBPs.
Application of engineered EBPs in cancer therapy
Enhancing tumor targeting capabilities
One common strategy to enhance the tumor targeting of nanoparticles is through modification with tumor-specific ligands. Human epidermal growth factor receptor-2 (HER2) is among the most widely examined breast cancer genes and is a prognostic indicator for clinical treatment monitoring that plays a crucial role in targeted therapy. Shi et al. successfully generated dual-targeted EBPs that targeted T-cell CD3 and tumor HER2 receptors via simultaneous overexpression of anti-human CD3 and anti-human HER2 antibodies (Fig. 7). This technology brought human T cells to HER2-positive breast cancer cells, thereby inducing a highly focused and tumor-specific immune response. [38]. Furthermore, the potential of targeted EBPs was further enhanced by their ability to carry RNA or chemotherapeutic drugs, thus maximizing the therapeutic impact. Targeted EBPs can also be loaded with RNA or chemotherapeutic drugs to further improve the therapeutic effect. EBPs targeting the HER2 receptor and loaded with HChrR6 mRNA in combination with 6-chloro-9-nitro-5-oxo-5H-benzo[a]phenoxazine (CNOB) can specifically kill HER2-positive cells [40].
Schematic of SMART-Exos and their anticancer efficacy. A The design and application of αCD3-αHER2 SMART-Exos as a targeted immunotherapy for breast cancer. B The in vivo antitumor effect of αCD3-αHER2 SMART-Exos. C Representative immunohistofluorescence images of the margin and interior of frozen tumor sections from PBS- and SMART-Exos-treated mice. Blue: nuclei stained with DAPI. Green: CD3 + cells stained with the anti-CD3 antibody. Scale bars: 50 μm. D Quantitative representation of the number of CD3 + cells from each field of view along the margin and interior of each tumor from PBS- and SMART-Exos-treated groups (15 fields of view per region and two mice per group) [38]. Reproduced with permission. Copyright 2020, Elsevier. PDGFR platelet-derived growth factor receptor, TMD transmembrane domain, SMART-Exos synthetic multivalent antibodies retargeted exosome, HER2 human epidermal growth factor receptor 2
Such therapeutic strategies are increasingly being applied to other common tumor targets. The urokinase plasminogen activator receptor (uPAR) is the main receptor of the plasminogen activation system, which is involved in the activation of plasminogen. EBPs overexpressing urokinase-type plasminogen activating peptide (uPA pep) can target uPAR and participate in specific tumor targeting [36]. The combination of uPAR targeting and individual anti-miRNA-loaded nanoagents mediates significant improvements in anticancer therapy’s tumor therapeutic efficacy. The targeted synergy of multiple cell signaling pathways results in a lower therapeutic dose required for each anti-miRNA or chemotherapy agent. Glypican3 (GPC3), a heparan sulfate glycoprotein located on the surface of cell membranes, is a specific antigen associated with cancer. Anti-GPC3 ScFV-modified EBPs deliver miR-26a to GPC3-expressing hepatocellular carcinoma (HCC) cells [37]. These approaches capitalize on the specific characteristics of cancer cells, such as the overexpression of uPAR and the presence of cancer-associated antigens like GPC3, to enable precise and efficient tumor targeting, opening up new avenues for more effective and personalized cancer treatments.
In addition to large molecules such as proteins or antibodies, some small molecules can be used to decorate the surface of the EBPs in a more flexible way. Folic acid receptor (FR) is widely expressed on the surface of a variety of tumor cells. Compared with normal lung tissue, FR is highly expressed in tumor tissue (100 times) and only slightly expressed in normal cells. In addition to the method of binding the DSPE-PEG chain and then inserting it into the cell membrane, folic acid (FA) can also be bound to the cell surface by chemical reaction-mediated covalent binding. The mitochondrial heme synthesis pathway can efficiently convert 5-aminolevulinic acid (5-ALA) into the photosensitizer PpIX. By adopting this principle, Li et al. allowed cells to take up 5-ALA and then converted it into photosensitized agents for packaging into vesicles, which could not only overcome the low drug loading efficiency of the passive encapsulation method but also avoid the destruction of membrane integrity caused by the active encapsulation method [101]. FA-modified macrophage-derived vesicles further realized the targeted delivery of anticancer drugs. Although cancer cell-derived EVs show high tumor targeting and internalization effects, considering the safety of clinical applications, the use of normal cell lines and simulated tumor cell membrane components may be an effective way to improve tumor targeting. Based on the observation of a higher content of phosphatidylcholine (PC) in the EVs of glioma cells, PC molecules were inserted into the membrane lipid layer of reticulocyte-derived EVs using a simple incubation method. It was found that the resulting EBPs significantly promoted the accumulation of loaded drugs in tumor cells [128].
Large proteins such as antibodies can be displayed on the surface of vesicles without genetic engineering. As mentioned earlier, DSPE-PEG chains can not only modify small molecular compounds such as folic acid but also serve as links for proteins and peptides to be inserted into cell membranes. The terminal groups of PEG can be activated and linked to various targeting ligands, such as anti-PD-L1 antibodies, which are subsequently inserted into the outer membrane of EBPs through the lipid accumulation of DSPE [109]. Since anti-PD-1/PD-L1 alone may increase the expression of CD73 on cancer cells, which will induce immunosuppression and reduce the therapeutic effect, the combination of PD-1 antibody and CD73 inhibitor (AB680) co-delivered by EBPs has achieved a synergistic antitumor effect. In another study, Si et al. linked DSPE-PEG-NHS with anti-SSTR2 monoclonal antibodies and anti-CXCR4 monoclonal antibodies to target neuroendocrine tumors, and mPEG-DSPE acted as a stabilizer to improve the circulation stability of EBPs [111].
In addition to tumor cells, immune cells surrounding tumors are also popular targets for drug delivery. For example, mannose and mannose receptor (MR) pairs can recognize and capture antigen molecules, promote antigen presentation, and regulate the maturation and differentiation of immune cells. Given that both tumor cells and macrophages overexpress MR on their surfaces, mannose modification or mannosylation is an effective dual targeting strategy that can be involved in the design of specific nanomaterials. OMV comes from microorganisms, which are more easily recognized and phagocytosed by macrophages. In a study conducted by Guo et al. they demonstrated that OMVs modified with mannose exhibited excellent targeting ability towards a specific subtype of macrophages called M2-type macrophages. These M2-type macrophages are known to be present in the tumor microenvironment and have been implicated in promoting tumor progression [125]. When OMVs were modified with mannose, they specifically targeted and interacted with M2-type macrophages. This interaction led to the downregulation of Redd1. Additionally, the mannose-modified OMVs increased the levels of glycolysis, a metabolic process that provides energy to cells. This alteration in the metabolic phenotype of macrophages was crucial in transitioning their function from promoting tumor progression to inhibiting tumor progression. By modifying OMVs with specific molecules such as mannose, they can be engineered to selectively target specific cell types, such as M2-type macrophages. This targeted delivery approach holds great promise for harnessing the immune system to combat tumor growth and offers new avenues for developing innovative and effective cancer treatments.
Magnetic nanoparticles, together with an external magnetic field and/or magnetized implants, can deliver and anchor the particles to the target area, enabling local and sustained drug release. Hyperthermia refers to placing superparamagnetic ferric oxide in an AC electromagnetic field, which can make the magnetic direction randomly change between parallel and antiparallel so that magnetic energy can be transferred to particles in the form of heat, a property that can be utilized in the body to destroy sick cells. Iron oxide particles not only have magnetic responsiveness but also regulate the tumor microenvironment and trigger T-cell recruitment when combined with thermochemotherapy. Bose et al. successfully wrapped gold-iron oxide nanoparticles (GION) into EBPs derived from tumor cells to deliver therapeutic anti-Mir-21 and doxorubicin (DOX) drugs to cancer cells, achieving superior anticancer effects [71]. DOX was loaded into EBPs by fusing the positively charged liposomes carrying DOX with cell membrane vesicles. In addition, these EBPs can also be used as contrast agents for magnetic resonance imaging (MRI) and computed tomography (CT). Shen et al. also recently demonstrated that iron oxide nanoparticles wrapped in cell membranes show promising tumor targeting and stimulate T-cell infiltration with the help of magnetic fields, effectively inhibiting tumor growth [72].
Improving anticancer efficacy
In addition to enhancing targeting capabilities, the engineering strategies may also improve the anticancer effects of EBPs. Tumor necrosis factor-associated apoptosis inducing ligand (TRAIL) is a membrane protein that can induce tumor cell apoptosis with tumor targeting. Shi et al. developed EBPs using amphiphilic poly(lactic-co-glycolic acid) (PLGA) carrying oxaliplatin (OXA) and hydroxychloroquine as the core, and the cell outer membrane with TRAIL as the shell [49]. These EBPs demonstrated good antitumor therapeutic effects for both in situ and metastatic hepatocellular carcinoma. To address the toxicity issues associated with the systemic administration of TNF-α, Zhuang et al. fused the cell penetrating peptide with TNF-α to form a fusion protein that anchored TNF-α in the cell membrane. This approach produced TNF-α presenting vesicles that can kill cancer cells under the magnetic targeting of superparamagnetic iron oxide nanoparticles [50]. These studies collectively demonstrate the potential of EBPs for targeted cancer therapy [51].
The antitumor properties of EBPs can be activated or promoted by the application of external light or magnetic fields. For example, thermosensitive and photosensitive EBPs are promising nanocarriers that effectively utilize the dual advantages of EBPs and thermotherapy/photodynamic therapy (PDT) to improve therapeutic efficacy and reduce side effects. In addition, the increase in temperature can be combined with chemodynamic therapy (CDT), which is an emerging therapeutic approach that utilizes Fenton-type reactions to produce highly cytotoxic hydroxyl radicals that can resist the anoxic environment of tumors. The hydrogen peroxide generated by the reaction of glucose oxidase (GOx) with glucose can enhance the Fenton reaction at the tumor site. Photothermally sensitive EBPs can be used to produce photothermal effects under irradiation, and the increase in temperature can further enhance the activity of GOx (Fig. 8), while laser irradiation can also enhance the targeting ability of EBPs [87].
The combined application of two cascade treatment strategies based on engineered EBPs. A Schematic illustration of the structural design and therapeutic mechanism of photothermal EBPs combined with CDT. B The size of H22 tumors in mice following various treatments is depicted in terms of tumor volume. C The survival curve of H22 tumor-bearing mice after receiving different treatments [87]. Reproduced with permission. Copyright 2022, Elsevier. PTSL photothermal sensitive liposomes, PEL platelet exosomes with photothermal sensitive liposomes, FAC ferric ammonium. Gox glucose oxidase
EBPs carrying a photosensitizer also act as photosensitive nanocarriers. Ce6 is usually highly efficient at producing singlet oxygen because it is suitable for the development of PDT for tumors. However, like most photosensitizers to date, Ce6 is hydrophobic and tends to aggregate in solution, so its practical application has encountered some difficulties. Hydrophobic Ce6 loaded into the lipid bilayer can provide photoacoustic imaging and PDT functions. CPPO is another type of chemical cold light source with high brightness and high efficiency of chemiluminescence. Wang et al. used these two hydrophobic agents (chemical excitation source CPPO and photosensitizing agent Ce6) to functionalize EBPs derived from M1 macrophages [115]. The intrinsic properties of M1-type macrophages allow EBPs to penetrate the blood–brain barrier and regulate the immunosuppressive tumor microenvironment through the conversion of M2-to-M1 polarization. The hydrogen peroxide produced in the process can be utilized to activate Ce6 and generate a large number of reactive oxygen species to achieve CDT. The exacerbation of tumor hypoxia also leads to the conversion of the nontoxic hydrophilic anoxic-activating prodrug AQ4N into toxic AQ4 for chemotherapy. Therefore, the EBPs achieved effective synergy between immunomodulation, CDT, and hypoxia-activated chemotherapy, exerting a powerful therapeutic effect on GBM.
Similar to photosensitizers, gold nanoparticles are also photoresponsive. The MSC membrane encapsulated hollow gold nanoparticles with a near-infrared (NIR) response, which showed enhanced nanoparticle delivery efficiency and tumor killing ability compared to conventional metal nanoparticles. Compared with traditional metal semiconductor quantum dots and organic dyes, carbon quantum dots not only maintain excellent fluorescence properties but also overcome the shortcomings of poor optical stability, high toxicity, and complex preparation processes. Cao et al. produced ultrasmall two-dimensional vanadium carbide quantum dots to show a strong photothermal effect at NIR light, and the EBPs prepared by wrapping them in tumor cell membranes exhibited good fluorescence imaging ability and photothermal effects in cancer treatment. In addition to being wrapped in the interior of EBPs, gold nanoparticles can also gather on the surface of EBPs to achieve photothermal conversion and kill tumors [80]. The use of VCQDs and AuNPs within the EBPs offers a multifunctional approach, combining fluorescence imaging, photothermal effects, and tumor targeting capabilities.
Reshaping the immunosuppressive tumor microenvironment
Activating the antitumor immune response at the tumor site and improving the immune tolerance microenvironment are also hot research directions in cancer treatment. EBPs derived from tumor cells overexpressing the high-affinity variant human PD-1 protein (havPD-1) can block the function of highly expressed PD-L1 in tumors, induce tumor cell apoptosis, and activate cytotoxic T cells in combination with low-dose PARP inhibitors, thereby effectively treating homotypic tumors [52]. Liu et al. found that adenovirus infection promoted the presentation of MHC-I binding antigens on DCs and simultaneously increased the overexpression of anti-PD1 antibodies and B7 costimulatory molecules, so that EBPs derived from DC cells could activate the immune response and break immune tolerance [53]. In addition to delivering drugs, targeting tumor cells can also bring immune cells to the tumor site. EBPs derived from Expi293F cells expressing both CD3 and EGFR were able to bring CD3-expressing T cells to TNBC cells overexpressing EGFR [25]. The tumor antigen human papillomavirus type 16 early protein E7 (HPV16E7) is a major driving factor in the development and progression of cervical cancer. HPV16E7 was expressed in the membrane vesicles of Escherichia coli by genetic engineering technology for subcutaneous immunotherapy to induce an E7 antigen-specific cellular immune response [54].
In addition to directly activating immune cells, the high molecular weight hyaluronic acid (HA) around tumor tissues is also a barrier to the infiltration of immune cells. As a membrane protein, hyaluronase itself has the inherent ability to be expressed on the surface of cell membranes and can be simply modified to express on the surface of cell membranes. Considering that hyaluronidase can degrade high molecular weight HA to low molecular weight HA (LMW-HA), some studies have used this feature to polarize macrophages into the M1 phenotype by LMW-HA and reduce the number of associated immunosuppressive cells, thus changing the immune microenvironment from immunosuppressive to immunosupportive, reshaping the tumor microenvironment, and improving the efficacy of chemotherapy [57]. Prostaglandin F2 receptor negative regulatory factor (PTGFRN) can enhance drug delivery to tumor-associated macrophages (TAMs), and the PTGFRN-engineered EBPs effectively delivered STAT6-targeting ASO to TAMs and reprogrammed them into a proinflammatory M1 phenotype, which is beneficial for cancer treatment [58]. This approach shows promise in the field of cancer treatment by leveraging the immune response within the tumor microenvironment. By enhancing drug delivery to TAMs, it becomes possible to target and modulate their functions, with the goal of shifting them towards a proinflammatory M1 phenotype that is more beneficial for cancer treatment.
EBPs derived from bacteria have immunomodulatory activity, showing great potential for the development of anticancer drugs and nanotechnology applications. The outer membrane vesicles (OMVs) released by genetically engineered bacteria can serve as vaccine carriers to break autoimmune tolerance and induce the production of autoantibodies for cancer therapy. The BFGF antibody can effectively inhibit angiogenesis and tumor growth. The use of BFGF-modified OMVs (BFGF-OMVs) as an antitumor vaccine can induce high levels of anti-BFGF autoantibodies, which can inhibit angiogenesis, promote tumor cell apoptosis, and reverse tumor immunosuppression [55]. Virus-derived proteins also inherit some of the characteristics of the virus itself. Vesicular stomatitis virus glycoprotein (VSV-G), a fusion protein derived from the virus, can induce cell membrane fusion. The researchers expressed this protein on the surface of M1 macrophage-derived extracellular vesicles (M1 EVs) and then functionalized the EVs by loading siPD-L1. The resulting siRNA@V-M1 EVs can be targeted to tumors due to the natural targeting ability of M1 EVs. In the acidic tumor microenvironment, the pH-responsive VSV-G changed to an unfolded fusion state and then induced EVs to fuse with the targeted cell membrane, thus bypassing the endocytic uptake pathway [26].
PTT induces immunogenic cell death (ICD) through NIR-mediated photothermal ablation and release of tumor-associated antigen (TAA), which subsequently activates the immune response in vivo. Tan et al. selected dipalmitoyl phosphatidylcholine (DPPC) as a thermosensitive lipid component to construct thermosensitive lipid nanoparticles, incorporated the PD-1/PD-L1 inhibitor BMS202 into NIR-responsive EVs, and finally constructed thermosensitive EBPs by membrane fusion that can target both primary tumors and lung metastases [90]. Under laser irradiation, thermosensitive EBPs can influence the extracellular matrix in the tumor microenvironment and tumor-associated fibroblasts, thereby increasing the infiltration of tumor-infiltrating lymphocytes. Meanwhile, NIR radiation can trigger the reactive release of PD-1/PD-L1 inhibitors, block the PD-1/PD-L1 pathway, and reverse tumor immunosuppression. Exposed cationic lipid nanoparticles can capture tumor-associated antigens, thereby enhancing the maturation and antigen presentation of DCs and activating cytotoxic T lymphocytes to kill tumor cells. Therefore, the combined EBPs synergistically exerted an antitumor immune response to inhibit the growth and metastasis of primary tumors.
Improving the utilization rate of drugs and reducing their toxic effects
Polymeric nanoparticles are commonly used drug as carriers in cancer treatment. The utilization of pH, thermal, ultrasonic, enzyme, and light-sensitive block copolymers allows for controlled micellar dissociation, triggering drug release. This approach not only enhances the specificity and effectiveness of micellar drug delivery but also provides insights into the development of smart drug delivery systems. By integrating EBPs with these industrial products, drug delivery systems with improved stability, higher drug-carrying capacity, and slower release kinetics can be achieved.
Chen et al. employed a novel polymerized non-ionic surfactant, Pluronic F-127, to encapsulate the drug Tegafol [75]. The stability of the micelles in the OMV coating was stronger, whereas the uncoated F127 micelles showed easy removal in an aqueous solution. The encapsulated F127 micelles exhibit spherical, dense nanostructures and can maintain a constant particle size in high serum and salt solutions. Encapsulated drugs may be rapidly released under acidic conditions, such as tumors or endosomal microenvironments. They further modified it with polyethylene glycol and RGD peptide to enhance its circulation and tumor targeting capabilities. This enabled its combination with bacterial immunotherapy and chemotherapy. PLGA [Poly(lactic-co-glycolic acid)] stands as the most representative type of nano-carrier system, consisting of lactide and glycolide copolymers [129]. It possesses excellent biocompatibility and biodegradability. At physiological pH levels, the hydrophobic core of PLGA contains an array of peptides and proteins, allowing encapsulated vesicles to bind and interact with the cell membrane. This ensures that peptides and proteins enter the cells through endocytosis without significant degradation. Li et al. demonstrated that OMV-encapsulated PEG-b-PLGA nanoparticles possess a typical core–shell structure and exhibit stability in a biological environment [69].
Dendrimers are capable of loading various types of drugs, including insoluble ones, in large quantities. Current research mainly focuses on nucleic acid and small molecule delivery. Both polymer types protect loaded therapeutic RNA, bind to the vesicle shell with targeted functionality, and exhibit effective tumor tissue killing effects. In the study by Wang et al. natural killer cell-derived exosomes (NKEXO) were utilized to encapsulate dendritic macromolecular cores for loading therapeutic miRNAs. Natural killer cells exhibit specific aggregation towards tumors without exerting cytotoxic effects on normal tissues. Simultaneously, the acidic tumor microenvironment facilitates the accumulation of these nanoparticles, thereby enhancing the biocompatibility and targeting capabilities of PAMAM dendrimers. Polyethylenimine (PEI), another widely used organic macromolecule, possesses a high cationic charge density and protonated amino nitrogen atoms. This facilitates the binding of negatively charged nucleic acid molecules, forming positively charged complex particles. Zhupanyn et al. developed an extracellular vesicle-modified PEI/siRNA complex. Upon intravenous administration in tumor-bearing mice, this complex showcased specific tumor inhibition through siRNA [76]. The modification of the PEI complex by extracellular vesicles altered key physicochemical and biological properties, enhancing nanoparticle functionality.
It is widely accepted that inorganic non-metallic nanoparticles are generally considered to have better biosafety compared to metal nanoparticles, along with higher drug loading capabilities. Among them, porous silicon nanoparticles exhibit excellent drug loading abilities, high biocompatibility, and biodegradability. In a study conducted by Wu et al. glutathione-responsive biodegradable silica nanoparticles were prepared to encapsulate catalase and ICG (Indocyanine Green). This fusion preparation not only enhances the efficacy of acousodynamic therapy but also maintains low toxicity to normal tissue [84]. Furthermore, engineered EBPs (extracellular vesicle-based particles) that wrap macrophage-derived EVs on their surface demonstrate effective penetration of the blood–brain barrier and accumulation at the tumor site.
Metal–organic frameworks (MOFs) are a type of organic–inorganic hybrid material composed of organic ligands and metal ions or clusters, self-assembled through coordination bonds to form intramolecular pores. MOF-protein (MP) nanoparticles possess a large internal surface area, high non-covalent affinity, and excellent drug loading capabilities. The pH-responsive metal–ligand bonds within MP nanoparticles, upon cellular internalization, can release proteins in acidic endosomes and lysosomes. This protects the proteins from protease digestion and immune system clearance and enables selective targeting of homologous tumor sites [81].
Challenges in clinical translations
EBPs used as drug delivery systems in clinical trials need to fulfill the same basic requirements as traditional synthetic carriers, including simplicity, efficiency, and scalability [130]. During the large-scale production process, several factors need to be considered, such as the selection of production cell lines and media, whether to add serum or other treatments, routine or hypoxic environments, and two-dimensional or three-dimensional culture supports. It is preferable to use stable and safe cell sources with high secretion capacity, such as mesenchymal stem cells (MSCs) and HEK293 cells, to help reduce production costs and improve efficiency. Umbilical cord-derived multifunctional mesenchymal cells possess abundant clinical study data and high safety, making them the preferred choice for the production of EBPs. In addition, it is necessary to conduct a comprehensive composition analysis for all the EBPs used in clinical studies. Due to the potential inaccuracies in particle determination, we recommend the measurement of vesicle particle number or total protein mass as a dosing standard and the quantification of total RNA or miRNA and cytokines as predictors of therapeutic effect.
It has been reported that the combination of tangential flow filtration (TFF) technology and ultra-high-speed centrifugation is a convenient and efficient method for enriching EVs. Lorenzini et al. have developed a large-scale cell culture method, in combination with TFF, that can support cell survival and EV production for at least 216 h in both EV-free human platelet lysate (hPL) and fetal calf serum (FCS) environments [131]. This method provides technical support for creating a cell culture environment without contamination from external EVs. Previous studies have indicated that the use of phosphate-buffered saline (PBS) as a dissolving agent can significantly reduce EVs recovery. However, it has recently been found that by using serum albumin and trehalose (PBS-HAT) as an alternative to PBS, EVs products can be stored at -80 °C for long periods of time [132]. In addition, the efficiency and loss of vesicles can be minimized by optimizing centrifugation conditions, including sample pretreatment, adjustments in centrifugation speed and time, and the incorporation of other purification techniques based on downstream requirements.
Currently, there are several approaches to increasing yield. One approach is to expand cell culture, such as by utilizing 3D culture or hollow fiber culture, to involve a larger number of cells in the production of vesicles. Another approach is to enhance the production of vesicles through chemical or physical stimulation of cells or direct disruption of reconstituted EV-like nanostructures [133]. Achieving stable and efficient drug encapsulating is a challenge faced by various drug carriers. Hydrophilic macromolecules present the greatest difficulty for membrane loading, while lipophilic compounds offer a simpler and more efficient drug loading process. When dealing with highly soluble compounds, increasing the drug concentration in solution or encapulating the drug in synthetic nanoparticles before wrapping the cell membrane are viable options. Studies have shown that lipid nanoparticles (LNP) are the optimal carriers for nucleic acid drug delivery. EBPs formed by the fusion of LNPs and EVs can reduce the inherent toxicity of LNPs, enhance the functionalities of EVs or modified EVs, and synergistically improve therapeutic efficacy. Furthermore, EBPs obtained by coating polymeric nanoparticles with cell membranes can overcome the limitations of the membrane structure for drug loading and enable controlled drug release triggered by external stimuli such as heat, light, ultrasound, or specific tumor tissue characteristics such as a low pH or high hyaluronic acid environment.
Perspectives
In this review, various modification and engineering strategies of EBPs for efficient drug delivery in targeted cancer therapy are introduced. Through biomimetic technology, different cell membranes can be fused to produce hybrid EBPs that maintain the unique characteristics of the originating cells, thereby integrating the functions of various cell types into biomimetic nanoparticles. The coating of nanomaterials with cell membranes, such as erythrocyte-derived vesicles, is typically effective in prolonging blood circulation and reducing systemic clearance. Moreover, EBPs derived from immune cells can regulate and mediate immune responses to tumors, while inheriting the targeting abilities of the original cells to tumor tissues. Tumor cells, on the other hand, can be utilized to extract and produce EBPs due to their innate ability to target their own tumors while also possessing active immunity to the same.
In recent years, combination therapy has emerged as a crucial trend in cancer intervention. Extensive research has demonstrated that EBPs represent a new and promising delivery platform for cancer combination therapy. Therefore, comprehending the various engineering strategies of EBPs is crucial for accelerating their clinical application and reducing obstacles in cancer combination therapy. As compared to traditional EVs, EBPs produced through artificial means offer greater efficiency and simplicity in drug delivery, as well as increased feasibility in combining EBPs with other therapeutic modalities to treat tumors. In this regard, some researchers are exploring the possibility of de novo synthesis to achieve a completely artificial synthesis of EBPs. As artificial products can achieve composition control and safety, they may better meet regulatory requirements and aid mass production. Hybrid EBPs based on cell membrane components may be a potential breakthrough in clinical applications. These hybrid EBPs may exhibit better delivery efficiency than conventional synthetic nanoparticles and greater stability than conventional EVs, thus demonstrating high applicability.
Multiple clinical trials have demonstrated the potential efficacy of EBPs. However, several challenges must be addressed before their successful translation from the laboratory to clinical application. Research efforts must be directed towards a deeper understanding of the biological function of EBPs, scale-up production, the development of standardized purification protocols, and evaluation methods. Moreover, the quantitative determination of the physicochemical characterization of EBPs is essential to ensure drug loading, quality control, and stable storage. In order to achieve these goals, it is vital to develop large-scale bioreactors, standardized and reproducible culture conditions, and production programs. Optimization of extraction techniques to obtain sufficient cell membranes and identification and quantification of by-products from the production process are also necessary. To successfully translate EBPs from laboratory research to clinical application, it is essential to improve the purity and storage life of the final product. Manufacturing processes must be conducted under aseptic conditions to ensure high recovery rates of therapeutic EBPs under storage conditions without excessive damage to the biological content and particulate matter. The future of EBPs holds immense promise. These remarkable nanoparticles, inspired by the natural properties of extracellular vesicles (EVs) derived from cells, have the potential to revolutionize drug delivery and targeted therapy. By mimicking the surface characteristics and cargo-loading capabilities of EVs, EBPs can effectively navigate the complexities of the human body, reaching specific target sites with remarkable precision. The use of EBPs opens up new possibilities in personalized medicine, enabling tailored treatments that harness the body’s own natural processes. Furthermore, these nanoparticles demonstrate enhanced stability, biocompatibility, and prolonged circulation time, further augmenting their therapeutic potential. As research advances and technology evolves, we can eagerly anticipate the continued exploration and development of these innovative nanoparticles, bringing us closer to a future where precise and effective therapeutic interventions become a reality.
Availability of data and materials
Not applicable.
Abbreviations
- EV:
-
Extracellular vesicle
- EBP:
-
Cell-generated EV-biomimetic nanoparticles
- ISEV:
-
The International Society for Extracellular Vesicles
- TLR:
-
Toll-like receptor
- LPS:
-
Lipopolysaccharide
- TEV:
-
Tumor cell-derived EV
- CRISPR:
-
Clustered regularly scattered short palindromic repeating sequence
- Cas9:
-
CRISPR-associated proteins 9
- PD1:
-
Programmed cell death protein 1
- VSV-G:
-
Vesicular stomatitis virus g-glycoprotein
- CXCR4:
-
C-X-C motif chemokine receptor 4
- scFv:
-
Single-chain fragment variable
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- ICG:
-
Indocyanine green
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- PLGA:
-
Poly (lactic-co-glycolic acid)
- GPC3:
-
Glypican 3
- HER2:
-
Human epidermal growth factor receptor 2
- EGF:
-
Epidermal growth factor
- DOX:
-
Doxorubicin
- 5-FU:
-
5-Fluorouracil
- CAR:
-
Chimeric antigen receptor
- LAMP2b:
-
Lysosome-associated membrane glycoprotein 2b
- DARPin:
-
Designed ankyrin repeat protein
- PSMA:
-
Prostate-specific membrane antigen
- PSA:
-
Prostate-specific antigen
- TNF-α:
-
Tumour necrosis factor alpha
- MHC-I:
-
Major histocompatibility class I
- PARP:
-
Poly (ADP-ribose) polymerase
- EGFR:
-
Epidermal growth factor receptor
- BFGF:
-
Basic fibroblast growth factor
- PD-L1:
-
Programed-cell death ligand 1
- ASO:
-
Antisense oligonucleotide
- NP:
-
Nano particle
- SPION:
-
Superparamagnetic iron oxide nanoparticles
- MRI:
-
Magnetic resonance imaging
- DSPE-PEG:
-
1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol)
- MOF:
-
Metal–organic framework
- DPPC:
-
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine
- CLT:
-
Celastrol
- MSPC:
-
1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine
- Tf:
-
Transferrin
- TfR:
-
Transferrin receptor
- DBCO:
-
Dibenzocyclooctyne
- PS:
-
Phosphatidylserine
- HA:
-
Hyaluronic acid
- PDA:
-
Polydopamine
- GFP:
-
Green fluorescent protein
- RBC:
-
Red blood cell
- PTX:
-
Paclitaxel
- FA:
-
Folic acid
- OMV:
-
Outer membrane vesicles
- MNP:
-
Metal-nanoparticle
- NHS:
-
n-hydroxysuccinimde
- EDC:
-
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
- GBM:
-
Glioblastoma
- BBB:
-
Blood–brain barrier
- ROS:
-
Reactive oxygen species
- SIRP-α:
-
Signal-regulatory protein
- SSTR:
-
Somatostatin receptor
- AA:
-
Aminoethylanisamide
- 5-ALA:
-
5-Aminolevulinic acid
- PpIX:
-
Protoporphyrin IX
- TAM:
-
Tumor-associated macrophages
- NMR:
-
Nuclear magnetic resonance
- TPL:
-
Triptolide
- CDT:
-
Chemokinetic therapy
- Ce6:
-
Chlorin e6
- MSC:
-
Mesenchymal stem cell
- TNBC:
-
Triple negative breast cancer
- ICD:
-
Immunogenic cell death
- PTT:
-
Photothermal therapy
- TAA:
-
Tumor-associated antigen
- PAMAM:
-
Polyamine-amine dendrimers
References
Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, Wang S, Sun K, Matsuda T, Bray F, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21(7):e342–9.
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers. 2014;6(3):1769–92.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71.
Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014.
Yong T, Wang D, Li X, Yan Y, Hu J, Gan L, Yang X. Extracellular vesicles for tumor targeting delivery based on five features principle. J Control Release. 2020;322:555–65.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180(5):3081–90.
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6.
Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS ONE. 2009;4(9):e7140.
Song S, Zhu L, Wang C, Yang Y. In vitro diagnostic technologies for the detection of extracellular vesicles: current status and future directions. VIEW. 2023;4(2):20220011.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Lewis JM, Vyas AD, Qiu Y, Messer KS, White R, Heller MJ. Integrated analysis of exosomal protein biomarkers on alternating current electrokinetic chips enables rapid detection of pancreatic cancer in patient blood. ACS Nano. 2018;12(4):3311–20.
Cavallaro S, Horak J, Hååg P, Gupta D, Stiller C, Sahu SS, Görgens A, Gatty HK, Viktorsson K, El Andaloussi S, et al. Label-free surface protein profiling of extracellular vesicles by an electrokinetic sensor. ACS Sens. 2019;4(5):1399–408.
Yan Z, Dutta S, Liu Z, Yu X, Mesgarzadeh N, Ji F, Bitan G, Xie YH. A label-free platform for identification of exosomes from different sources. ACS Sens. 2019;4(2):488–97.
Zhang L, He F, Gao L, Cong M, Sun J, Xu J, Wang Y, Hu Y, Asghar S, Hu L, et al. engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int J Nanomed. 2021;16:1575–86.
Kandimalla R, Aqil F, Alhakeem SS, Jeyabalan J, Tyagi N, Agrawal A, Yan J, Spencer W, Bondada S, Gupta RC. Targeted oral delivery of paclitaxel using colostrum-derived exosomes. Cancers. 2021;13(15):3700.
Tan S, Wu T, Zhang D, Zhang Z. Cell or cell membrane-based drug delivery systems. Theranostics. 2015;5(8):863–81.
Guo P, Busatto S, Huang J, Morad G, Moses MA. A facile magnetic extrusion method for preparing endosome-derived vesicles for cancer drug delivery. Adv Funct Mater. 2021;31(44):2008326.
Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, Fang Y, Xia Y, Cheng G, He X, et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018;78(3):798–808.
Gong YK, Winnik FM. Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale. 2012;4(2):360–8.
Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14(11):1891–900.
Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, Xiao ZD. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289(32):22258–67.
Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H, Du JL. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27(7):882–97.
Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, Zhang Y. Reprogramming exosomes as nanoscale controllers of cellular immunity. J Am Chem Soc. 2018;140(48):16413–7.
Liu H, Huang L, Mao M, Ding J, Wu G, Fan W, Yang T, Zhang M, Huang Y, Xie H-Y. Viral protein-pseudotyped and siRNA-electroporated extracellular vesicles for cancer immunotherapy. Adv Func Mater. 2020;30(52):2006515.
Cheng L, Zhang X, Tang J, Lv Q, Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275:120964.
Qing S, Lyu C, Zhu L, Pan C, Wang S, Li F, Wang J, Yue H, Gao X, Jia R, et al. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater. 2020;32(47):2002085.
Liu W, Li L, Jiang J, Wu M, Lin P. Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precis Clin Med. 2021;4(3):179–91.
Xu Q, Zhang Z, Zhao L, Qin Y, Cai H, Geng Z, Zhu X, Zhang W, Zhang Y, Tan J, et al. Tropism-facilitated delivery of CRISPR/Cas9 system with chimeric antigen receptor-extracellular vesicles against B-cell malignancies. J Control Release. 2020;326:455–67.
Wang Y, Cao Z, Wei Q, Ma K, Hu W, Huang Q, Su J, Li H, Zhang C, Fu X. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022;147:342–55.
Song X, Xu L, Zhang W. Biomimetic synthesis and optimization of extracellular vesicles for bone regeneration. J Control Release. 2023;355:18–41.
Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y, Zheng Y. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11(18):8926–44.
Du J, Wan Z, Wang C, Lu F, Wei M, Wang D, Hao Q. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics. 2021;11(17):8185–96.
Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J, Zhou J, Liu B, Liu J. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci. 2020;7(18):2000515.
Bose RJ, Kumar US, Garcia-Marques F, Zeng Y, Habte F, McCarthy JR, Pitteri S, Massoud TF, Paulmurugan R. Engineered cell-derived vesicles displaying targeting peptide and functionalized with nanocarriers for therapeutic microrna delivery to triple-negative breast cancer in mice. Adv Healthc Mater. 2022;11(5):e2101387.
Mahati S, Fu X, Ma X, Zhang H, Xiao L. Delivery of miR-26a using an exosomes-based nanosystem inhibited proliferation of hepatocellular carcinoma. Front Mol Biosci. 2021;8:738219.
Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, Epstein AL, Lenz HJ, Zhang Y. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol Ther. 2020;28(2):536–47.
Wang L, Zhou X, Zou W, Wu Y, Zhao J, Chen X, Zhou GG. Exosomes containing miRNAs targeting HER2 synthesis and engineered to adhere to HER2 on tumor cells surface exhibit enhanced antitumor activity. J Nanobiotechnology. 2020;18(1):153.
Wang JH, Forterre AV, Zhao J, Frimannsson DO, Delcayre A, Antes TJ, Efron B, Jeffrey SS, Pegram MD, Matin AC. Anti-HER2 scFv-directed extracellular vesicle-mediated mRNA-based gene delivery inhibits growth of HER2-positive human breast tumor xenografts by prodrug activation. Mol Cancer Ther. 2018;17(5):1133–42.
Zhang P, Zhang L, Qin Z, Hua S, Guo Z, Chu C, Lin H, Zhang Y, Li W, Zhang X, et al. Genetically engineered liposome-like nanovesicles as active targeted transport platform. Adv Mater. 2018;30(7):1705350.
Gomari H, Forouzandeh Moghadam M, Soleimani M, Ghavami M, Khodashenas S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int J Nanomed. 2019;14:5679–90.
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, Sun B, Chen B, Xiao Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18(1):10.
Ma G, Severic M, Barker M, Pereira S, Ruiz A, Cheung CCL, Al-Jamal WT. Dually targeted bioinspired nanovesicle delays advanced prostate cancer tumour growth in vivo. Acta Biomater. 2021;134:559–75.
Wang K, Kumar US, Sadeghipour N, Massoud TF, Paulmurugan R. A microfluidics-based scalable approach to generate extracellular vesicles with enhanced therapeutic microRNA loading for intranasal delivery to mouse glioblastomas. ACS Nano. 2021;15(11):18327–46.
Xu S, Liu B, Fan J, Xue C, Lu Y, Li C, Cui D. Engineered mesenchymal stem cell-derived exosomes with high CXCR4 levels for targeted siRNA gene therapy against cancer. Nanoscale. 2022;14(11):4098–113.
Kim G, Kim M, Lee Y, Byun JW, Hwang DW, Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Control Release. 2020;317:273–81.
Han S, Li G, Jia M, Zhao Y, He C, Huang M, Jiang L, Wu M, Yang J, Ji X, et al. Delivery of anti-miRNA-221 for colorectal carcinoma therapy using modified cord blood mesenchymal stem cells-derived exosomes. Front Mol Biosci. 2021;8:743013.
Shi Y, Lin G, Zheng H, Mu D, Chen H, Lu Z, He P, Zhang Y, Liu C, Lin Z, et al. Biomimetic nanoparticles blocking autophagy for enhanced chemotherapy and metastasis inhibition via reversing focal adhesion disassembly. J Nanobiotechnology. 2021;19(1):447.
Jiang L, Gu Y, Du Y, Tang X, Wu X, Liu J. Engineering exosomes endowed with targeted delivery of triptolide for malignant melanoma therapy. ACS Appl Mater Interfaces. 2021;13(36):42411–28.
Zhuang M, Chen X, Du D, Shi J, Deng M, Long Q, Yin X, Wang Y, Rao L. SPION decorated exosome delivery of TNF-α to cancer cell membranes through magnetism. Nanoscale. 2020;12(1):173–88.
Chen Y, Wang L, Zheng M, Zhu C, Wang G, Xia Y, Blumenthal EJ, Mao W, Wan Y. Engineered extracellular vesicles for concurrent Anti-PDL1 immunotherapy and chemotherapy. Bioact Mater. 2022;9:251–65.
Liu C, Liu X, Xiang X, Pang X, Chen S, Zhang Y, Ren E, Zhang L, Liu X, Lv P, et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat Nanotechnol. 2022;17(5):531–40.
Wang S, Huang W, Li K, Yao Y, Yang X, Bai H, Sun W, Liu C, Ma Y. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int J Nanomed. 2017;12:6813–25.
Huang W, Shu C, Hua L, Zhao Y, Xie H, Qi J, Gao F, Gao R, Chen Y, Zhang Q, et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020;108:300–12.
Huang L, Rong Y, Tang X, Yi K, Qi P, Hou J, Liu W, He Y, Gao X, Yuan C, et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol Cancer. 2022;21(1):45.
Feng C, Xiong Z, Wang C, Xiao W, Xiao H, Xie K, Chen K, Liang H, Zhang X, Yang H. Folic acid-modified exosome-PH20 enhances the efficiency of therapy via modulation of the tumor microenvironment and directly inhibits tumor cell metastasis. Bioact Mater. 2021;6(4):963–74.
Kamerkar S, Leng C, Burenkova O, Jang SC, McCoy C, Zhang K, Dooley K, Kasera S, Zi T, Sisó S, et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci Adv. 2022;8(7):eabj7002.
Zhou W, Xu M, Wang Z, Yang M. Engineered exosomes loaded with miR-449a selectively inhibit the growth of homologous non-small cell lung cancer. Cancer Cell Int. 2021;21(1):485.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51.
El-Boubbou K. Magnetic iron oxide nanoparticles as drug carriers: clinical relevance. Nanomedicine. 2018;13(8):953–71.
Wang J, Potocny AM, Rosenthal J, Day ES. Gold nanoshell-linear tetrapyrrole conjugates for near infrared-activated dual photodynamic and photothermal therapies. ACS Omega. 2020;5(1):926–40.
Bayda S, Hadla M, Palazzolo S, Riello P, Corona G, Toffoli G, Rizzolio F. Inorganic nanoparticles for cancer therapy: a transition from lab to clinic. Curr Med Chem. 2018;25(34):4269–303.
Liu Y, Luo J, Chen X, Liu W, Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nanomicro Lett. 2019;11(1):100.
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.
Sedighi M, Sieber S, Rahimi F, Shahbazi MA, Rezayan AH, Huwyler J, Witzigmann D. Rapid optimization of liposome characteristics using a combined microfluidics and design-of-experiment approach. Drug Deliv Transl Res. 2019;9(1):404–13.
Xiong F, Ling X, Chen X, Chen J, Tan J, Cao W, Ge L, Ma M, Wu J. Pursuing specific chemotherapy of orthotopic breast cancer with lung metastasis from docking nanoparticles driven by bioinspired exosomes. Nano Lett. 2019;19(5):3256–66.
Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15.
Li M, Li S, Zhou H, Tang X, Wu Y, Jiang W, Tian Z, Zhou X, Yang X, Wang Y. Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat Commun. 2020;11(1):1126.
Zhang D, Qin X, Wu T, Qiao Q, Song Q, Zhang Z. Extracellular vesicles based self-grown gold nanopopcorn for combinatorial chemo-photothermal therapy. Biomaterials. 2019;197:220–8.
Bose RJC, Uday Kumar S, Zeng Y, Afjei R, Robinson E, Lau K, Bermudez A, Habte F, Pitteri SJ, Sinclair R, et al. Tumor cell-derived extracellular vesicle-coated nanocarriers: an efficient theranostic platform for the cancer-specific delivery of anti-miR-21 and imaging agents. ACS Nano. 2018;12(11):10817–32.
Shen WT, Hsu RS, Fang JH, Hu PF, Chiang CS, Hu SH. Marginative delivery-mediated extracellular leakiness and T cell infiltration in lung metastasis by a biomimetic nanoraspberry. Nano Lett. 2021;21(3):1375–83.
Jia G, Han Y, An Y, Ding Y, He C, Wang X, Tang Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–16.
Wang G, Hu W, Chen H, Shou X, Ye T, Xu Y. Cocktail strategy based on NK cell-derived exosomes and their biomimetic nanoparticles for dual tumor therapy. Cancers. 2019;11(10):1560.
Chen Q, Bai H, Wu W, Huang G, Li Y, Wu M, Tang G, Ping Y. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. 2020;20(1):11–21.
Zhupanyn P, Ewe A, Büch T, Malek A, Rademacher P, Müller C, Reinert A, Jaimes Y, Aigner A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J Control Release. 2020;319:63–76.
Li S, Wu Y, Ding F, Yang J, Li J, Gao X, Zhang C, Feng J. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale. 2020;12(19):10854–62.
Wang D, Yao Y, He J, Zhong X, Li B, Rao S, Yu H, He S, Feng X, Xu T, et al. Engineered cell-derived microparticles Bi(2)Se(3)/DOX@MPs for imaging guided synergistic photothermal/low-dose chemotherapy of cancer. Adv Sci. 2020;7(3):1901293.
Sancho-Albero M, Encinas-Giménez M, Sebastián V, Pérez E, Luján L, Santamaría J, Martin-Duque P. Transfer of photothermal nanoparticles using stem cell derived small extracellular vesicles for in vivo treatment of primary and multinodular tumours. J Extracell Vesicles. 2022;11(3):e12193.
Cao Y, Wu T, Zhang K, Meng X, Dai W, Wang D, Dong H, Zhang X. Engineered exosome-mediated near-infrared-II region V(2)C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano. 2019;13(2):1499–510.
Cheng G, Li W, Ha L, Han X, Hao S, Wan Y, Wang Z, Dong F, Zou X, Mao Y, et al. Self-assembly of extracellular vesicle-like metal-organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins. J Am Chem Soc. 2018;140(23):7282–91.
Tian R, Wang Z, Niu R, Wang H, Guan W, Chang J. tumor exosome mimicking nanoparticles for tumor combinatorial chemo-photothermal therapy. Front Bioeng Biotechnol. 2020;8:1010.
Nie D, Dai Z, Li J, Yang Y, Xi Z, Wang J, Zhang W, Qian K, Guo S, Zhu C, et al. Cancer-cell-membrane-coated nanoparticles with a yolk-shell structure augment cancer chemotherapy. Nano Lett. 2020;20(2):936–46.
Wu T, Liu Y, Cao Y, Liu Z. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv Mater. 2022;34(15):e2110364.
Cao X, Tan T, Zhu D, Yu H, Liu Y, Zhou H, Jin Y, Xia Q. Paclitaxel-loaded macrophage membrane camouflaged albumin nanoparticles for targeted cancer therapy. Int J Nanomedicine. 2020;15:1915–28.
Jiang Q, Liu Y, Guo R, Yao X, Sung S, Pang Z, Yang W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308.
Ma Y, Zhang Y, Han R, Li Y, Zhai Y, Qian Z, Gu Y, Li S. A cascade synergetic strategy induced by photothermal effect based on platelet exosome nanoparticles for tumor therapy. Biomaterials. 2022;282:121384.
Zhou X, Miao Y, Wang Y, He S, Guo L, Mao J, Chen M, Yang Y, Zhang X, Gan Y. Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J Extracell Vesicles. 2022;11(3):e12198.
Deng L, Zhang H, Zhang Y, Luo S, Du Z, Lin Q, Zhang Z, Zhang L. An exosome-mimicking membrane hybrid nanoplatform for targeted treatment toward Kras-mutant pancreatic carcinoma. Biomater Sci. 2021;9(16):5599–611.
Tan YN, Huang JD, Li YP, Li SS, Luo M, Luo J, Lee AW, Fu L, Hu FQ, Guan XY. Near-infrared responsive membrane nanovesicles amplify homologous targeting delivery of anti-PD immunotherapy against metastatic tumors. Adv Healthc Mater. 2022;11(6):e2101496.
Tian T, Liang R, Erel-Akbaba G, Saad L, Obeid PJ, Gao J, Chiocca EA, Weissleder R, Tannous BA. Immune checkpoint inhibition in GBM primed with radiation by engineered extracellular vesicles. ACS Nano. 2022;16(2):1940–53.
Guo L, Guo Y, Lau DHM, Xu YC. A novel specific small molecule peptide for non-small cell lung cancer cell A549. Progress Biochem Biophys. 2007;34:1080–5.
Lin Q, Qu M, Zhou B, Patra HK, Sun Z, Luo Q, Yang W, Wu Y, Zhang Y, Li L, et al. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J Control Release. 2019;311–312:104–16.
Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, Zhang Y, Liu Y, Li J, Pu K, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020;59(5):2018–22.
Wu H, Zhu L, Torchilin VP. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials. 2013;34(4):1213–22.
de Guimarães MS, Cachumba JJM, Bueno CZ, Torres-Obreque KM, Lara GVR, Monteiro G, Barbosa LRS, Pessoa A Jr, Rangel-Yagui CO. Peg-grafted liposomes for l-asparaginase encapsulation. Pharmaceutics. 2022;14(9):1819.
Zhuang J, Tan J, Wu C, Zhang J, Liu T, Fan C, Li J, Zhang Y. Extracellular vesicles engineered with valency-controlled DNA nanostructures deliver CRISPR/Cas9 system for gene therapy. Nucleic Acids Res. 2020;48(16):8870–82.
Zhan Q, Yi K, Cui X, Li X, Yang S, Wang Q, Fang C, Tan Y, Li L, Xu C, et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro Oncol. 2022;24(11):1871–83.
Peng B, Nguyen TM, Jayasinghe MK, Gao C, Pham TT, Vu LT, Yeo EYM, Yap G, Wang L, Goh BC, et al. Robust delivery of RIG-I agonists using extracellular vesicles for anti-cancer immunotherapy. J Extracell Vesicles. 2022;11(4):e12187.
Pham TC, Jayasinghe MK, Pham TT, Yang Y, Wei L, Usman WM, Chen H, Pirisinu M, Gong J, Kim S, et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J Extracell Vesicles. 2021;10(4):e12057.
Li R, Gong X, Hong C, Wang H, Chen Y, Tan K, Liu X, Wang F. An efficient photochemotherapy nanoplatform based on the endogenous biosynthesis of photosensitizer in macrophage-derived extracellular vesicles. Biomaterials. 2021;279:121234.
Zheng Z, Li Z, Xu C, Guo B, Guo P. Folate-displaying exosome mediated cytosolic delivery of siRNA avoiding endosome trapping. J Control Release. 2019;311–312:43–9.
Kwon SH, Faruque HA, Kee H, Kim E, Park S. Exosome-based hybrid nanostructures for enhanced tumor targeting and hyperthermia therapy. Colloids Surf B Biointerfaces. 2021;205:111915.
Nguyen Cao TG, Kang JH, You JY, Kang HC, Rhee WJ, Ko YT, Shim MS. Safe and targeted sonodynamic cancer therapy using biocompatible exosome-based nanosonosensitizers. ACS Appl Mater Interfaces. 2021;13(22):25575–88.
Cheng H, Fan JH, Zhao LP, Fan GL, Zheng RR, Qiu XZ, Yu XY, Li SY, Zhang XZ. Chimeric peptide engineered exosomes for dual-stage light guided plasma membrane and nucleus targeted photodynamic therapy. Biomaterials. 2019;211:14–24.
Cao H, Wang H, He X, Tan T, Hu H, Wang Z, Wang J, Li J, Zhang Z, Li Y. Bioengineered macrophages can responsively transform into nanovesicles to target lung metastasis. Nano Lett. 2018;18(8):4762–70.
Ye Z, Zhang T, He W, Jin H, Liu C, Yang Z, Ren J. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl Mater Interfaces. 2018;10(15):12341–50.
Li L, He D, Guo Q, Zhang Z, Ru D, Wang L, Gong K, Liu F, Duan Y, Li H. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnol. 2022;20(1):50.
Zhou Q, Ding W, Qian Z, Zhu Q, Sun C, Yu Q, Tai Z, Xu K. Immunotherapy strategy targeting programmed cell death ligand 1 and CD73 with macrophage-derived mimetic nanovesicles to treat bladder cancer. Mol Pharm. 2021;18(11):4015–28.
Forterre AV, Wang JH, Delcayre A, Kim K, Green C, Pegram MD, Jeffrey SS, Matin AC. Extracellular vesicle-mediated in vitro transcribed mRNA delivery for treatment of HER2(+) breast cancer xenografts in mice by prodrug CB1954 without general toxicity. Mol Cancer Ther. 2020;19(3):858–67.
Si Y, Guan J, Xu Y, Chen K, Kim S, Zhou L, Jaskula-Sztul R, Liu XM. Dual-targeted extracellular vesicles to facilitate combined therapies for neuroendocrine cancer treatment. Pharmaceutics. 2020;12(11):1079.
Fan M, Liu H, Yan H, Che R, Jin Y, Yang X, Zhou X, Yang H, Ge K, Liang XJ, et al. A CAR T-inspiring platform based on antibody-engineered exosomes from antigen-feeding dendritic cells for precise solid tumor therapy. Biomaterials. 2022;282:121424.
Chen K, Si Y, Guan JS, Zhou Z, Kim S, Kim T, Shan L, Willey CD, Zhou L, Liu X. Targeted extracellular vesicles delivered verrucarin A to treat glioblastoma. Biomedicines. 2022;10(1):130.
Jang Y, Kim H, Yoon S, Lee H, Hwang J, Jung J, Chang JH, Choi J, Kim H. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J Control Release. 2021;330:293–304.
Wang X, Ding H, Li Z, Peng Y, Tan H, Wang C, Huang G, Li W, Ma G, Wei W. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduct Target Ther. 2022;7(1):74.
Ding J, Lu G, Nie W, Huang LL, Zhang Y, Fan W, Wu G, Liu H, Xie HY. Self-activatable photo-extracellular vesicle for synergistic trimodal anticancer therapy. Adv Mater. 2021;33(7):e2005562.
Lara P, Huis In’t Veld RV, Jorquera-Cordero C, Chan AB, Ossendorp F, Cruz LJ. Zinc-phthalocyanine-loaded extracellular vesicles increase efficacy and selectivity of photodynamic therapy in co-culture and preclinical models of colon cancer. Pharmaceutics. 2021;13(10):1547.
Zhan Q, Yi K, Qi H, Li S, Li X, Wang Q, Wang Y, Liu C, Qiu M, Yuan X, et al. Engineering blood exosomes for tumor-targeting efficient gene/chemo combination therapy. Theranostics. 2020;10(17):7889–905.
Luo C, Hu X, Peng R, Huang H, Liu Q, Tan W. Biomimetic carriers based on giant membrane vesicles for targeted drug delivery and photodynamic/photothermal synergistic therapy. ACS Appl Mater Interfaces. 2019;11(47):43811–9.
Wang J, Chen P, Dong Y, Xie H, Wang Y, Soto F, Ma P, Feng X, Du W, Liu BF. Designer exosomes enabling tumor targeted efficient chemo/gene/photothermal therapy. Biomaterials. 2021;276:121056.
Zhang J, Ji C, Zhang H, Shi H, Mao F, Qian H, Xu W, Wang D, Pan J, Fang X, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022;8(2):eabj8207.
Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, Zhang Y, Liu P, Zhang Y, Li C, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546.
Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X, Dai W, Zhang Q. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9(1):1806444.
Ma C, He D, Tian P, Wang Y, He Y, Wu Q, Jia Z, Zhang X, Zhang P, Ying H, et al. miR-182 targeting reprograms tumor-associated macrophages and limits breast cancer progression. Proc Natl Acad Sci USA. 2022;119(6):e2114006119.
Guo Q, Li X, Zhou W, Chu Y, Chen Q, Zhang Y, Li C, Chen H, Liu P, Zhao Z, et al. Sequentially triggered bacterial outer membrane vesicles for macrophage metabolism modulation and tumor metastasis suppression. ACS Nano. 2021;15(8):13826–38.
Lee H, Park H, Noh GJ, Lee ES. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr Polym. 2018;202:323–33.
Fan Z, Xiao K, Lin J, Liao Y, Huang X. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15(47):e1903761.
Zhan Q, Yi K, Li X, Cui X, Yang E, Chen N, Yuan X, Zhao J, Hou X, Kang C. Phosphatidylcholine-engineered exosomes for enhanced tumor cell uptake and intracellular antitumor drug delivery. Macromol Biosci. 2021;21(8):e2100042.
Pandita D, Kumar S, Lather V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: design and delivery prospectives. Drug Discov Today. 2015;20(1):95–104.
Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: a systemic analysis of clinical trials. Cytotherapy. 2017;19(12):1351–82.
Lorenzini B, Peltzer J, Goulinet S, Rival B, Lataillade JJ, Uzan G, Banzet S, Mauduit P. Producing vesicle-free cell culture additive for human cells extracellular vesicles manufacturing. J Control Release. 2023;355:501–14.
Görgens A, Corso G, Hagey DW, Jawad Wiklander R, Gustafsson MO, Felldin U, Lee Y, Bostancioglu RB, Sork H, Liang X, et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J Extracell Vesicles. 2022;11(6):e12238.
Grangier A, Branchu J, Volatron J, Piffoux M, Gazeau F, Wilhelm C, Silva AKA. Technological advances towards extracellular vesicles mass production. Adv Drug Deliv Rev. 2021;176:113843.
Acknowledgements
We would like to acknowledge Qiyi Feng from the laboratory of Precision Cancer Therapeutics in the Precision Medicine Research Center for her assistance. This work was supported by the National Natural Science Foundation of China (81572617), Sichuan Science and Technology Program (2021ZYD0097), the Fundamental Research Funds for the Central Universities (SCU2022D025), International Cooperation Project of Chengdu Science and Technology Bureau (2022-GH02-00023-HZ), Innovation Spark Project of Sichuan University (2019SCUH0015), Talent Training Fund for Medical-engineering Integration of Western China Hospital—University of Electronic Science and Technology (HXDZ22012), 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18026), and 1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2020HXFH023). Figures 1, 3, and 6 were created using BioRender.com under a publication license.
Funding
This work was supported by the National Natural Science Foundation of China (81630101), the Fundamental Research Funds for the Central Universities (SCU2022D025), the Sichuan Science and Technology Program (2021ZYD0097), the International Cooperation Project of Chengdu Science and Technology Bureau (2022-GH02-00023-HZ), the Innovation Spark Project of Sichuan University (2019SCUH0015), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18026), and the 1·3·5 project for disciplines of excellenceClinical Research Incubation Project, West China Hospital, Sichuan University (2020HXFH023).
Author information
Authors and Affiliations
Contributions
Conceptualization, XL, and KX; data collection, XL, and CX; data collation, XL, writing—original draft preparation, XL; writing—review and editing, KX; supervision, KX; funding acquisition, KX. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We have included 3 figures from previously published literature with copyright permission. We have appropriate references in the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, X., Xiao, C. & Xiao, K. Engineered extracellular vesicles-like biomimetic nanoparticles as an emerging platform for targeted cancer therapy. J Nanobiotechnol 21, 287 (2023). https://doi.org/10.1186/s12951-023-02064-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-02064-1